Abstract
Bacteria of the genus Corynebacterium are important primary and opportunistic pathogens. Many are zoonotic agents. In this report, phenotypic (API Coryne analysis), genetic (rpoB and 16S rRNA gene sequencing), and physical methods (MS) were used to distinguish the closely related diphtheroid species Corynebacterium ulcerans and Corynebacterium pseudotuberculosis, and to definitively diagnose Corynebacterium renale from cephalic implants of rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques used in cognitive neuroscience research. Throat and cephalic implant cultures yielded 85 isolates from 43 macaques. Identification by API Coryne yielded C. ulcerans (n = 74), Corynebacterium pseudotuberculosis (n = 2), C. renale or most closely related to C. renale (n = 3), and commensals and opportunists (n = 6). The two isolates identified as C. pseudotuberculosis by API Coryne required genetic and MS analysis for accurate characterization as C. ulcerans. Of three isolates identified as C. renale by 16S rRNA gene sequencing, only one could be confirmed as such by API Coryne, rpoB gene sequencing and MS. This study emphasizes the importance of adjunct methods in identification of coryneforms and is the first isolation of C. renale from cephalic implants in macaques.
Introduction
Reports of coryneform bacteria in the literature are becoming more prevalent, most notably due to the taxonomic changes in phylogenetic groupings (Funke et al., 1997) and an increase in the number of invasive treatments and procedures performed on immunocompromised patients. The genus Corynebacterium is one of the largest in the coryneform group of bacteria, containing more than 60 species, 40 which are medically relevant (Funke et al., 1997; Khamis et al., 2005). They are Gram-positive, non-motile, facultative anaerobes, characterized as having the appearance of straight or slightly curved slender rods with tapered or clubbed ends (Funke et al., 1997). Corynebacterium species cause opportunistic infections in both humans and domestic animals. Corynebacterium diphtheriae, the most widely known bacterium in the genus, is the causative agent of human diphtheria, a highly contagious upper respiratory tract infection which is still implicated in outbreaks worldwide. C. diphtheriae also causes cutaneous infections, endocarditis, septicaemia and osteomyelitis (Aubel et al., 1997). Hall et al. (2010) recently identified a novel C. diphtheriae isolate from the ears of two domestic cats in West Virginia, but found no evidence of zoonotic transmission.
The non-diphtheroid species, specifically Corynebacterium ulcerans and Corynebacterium pseudotuberculosis, have been shown to produce a variety of both animal and human diseases, and are important zoonotic pathogens. C. pseudotuberculosis is best known as the causative agent of caseous lymphadenitis in ruminants and ulcerative lymphangitis in horses (Dorella et al., 2006; Pacheco et al., 2007). Human infections with these bacteria, although uncommon, have been documented, with the majority of cases resulting from occupational exposure (Dorella et al., 2006). C. ulcerans was first isolated in 1926 by Gilbert and Stewart from human pharyngeal cultures (Funke et al., 1997). It has been reported as a cause of pharyngitis, granulomatous pneumonia, and less commonly diphtheria, in humans (Funke et al., 1997). The traditional association of C. ulcerans infection of humans is zoonotic transmission from cattle or the consumption of raw milk from infected cattle (Lartigue et al., 2005). It is now the most common cause of diphtheria in the UK (Wagner et al., 2010). Recent literature also suggests that C. ulcerans isolated from domestic pigs (Schuhegger et al., 2009), domestic cats (De Zoysa et al., 2005) and dogs (Lartigue et al., 2005; Katsukawa et al., 2009) may be a reservoir for human infection. We have previously described C. ulcerans from a case of mastitis in a bonnet macaque and as a frequent contaminant of cephalic implants from macaques used in cognitive neuroscience (Fox & Frost, 1974; Bergin et al., 2000).
Corynebacterium species are potentially zoonotic, so rapid and accurate discrimination of these organisms is crucial. In diagnostic laboratories, Analytical Profiling Index (API) is a common, rapid and inexpensive method used to identify closely related bacteria. For Corynebacterium species, the API Coryne test is fairly reliable, citing 97.71 % of the strains being correctly identified (with or without supplementary tests), 1.28 % of the strains not identified, and 1.01 % of the strains misidentified [personal communication from bioMérieux (or http://www.biomerieux.com/servlet/srt/bio/portail/home)]. However, the test can be subjective, can only detect known coryneforms, requires bacterial suspensions of adequate turbidity, and may not discriminate between closely related species.
Historically, the 16S rRNA gene sequence has been considered the gold standard for determination of the phylogenetic relationship among bacteria. Unfortunately, the 16S rRNA gene sometimes lacks the high intra-genus polymorphism that is needed for precise taxonomic analysis and species discrimination (Khamis et al., 2004). The percentage similarity in the 16S rRNA gene sequence between C. diphtheriae and C. ulcerans has been reported to be 98.5; between C. diphtheriae and C. pseudotuberculosis, 98.5; and between C. ulcerans and C. pseudotuberculosis, 99.7 % (Khamis et al., 2004).The RNA polymerase beta subunit-encoding gene (rpoB) is a universal gene that has been used in the phylogenetic analysis of a variety of bacteria, and has been highly beneficial in distinguishing among closely related isolates (Adékambi et al., 2009). This method has been used in the past to demonstrate the variability among species, isolates, serotypes and biotypes for Escherichia coli, Salmonella enterica, Vibrio cholerae and Haemophilus influenzae (Adékambi et al., 2009). Furthermore, sequencing of the hypervariable region of the rpoB gene has allowed for the identification of unknown isolates in the bacterial orders Aquificales and Rhizobiales (Adékambi et al., 2009). Khamis et al. (2004) obtained almost complete rpoB sequences of several isolates of Corynebacterium species and identified an area with a high degree of polymorphism (hypervariable region) for subsequent primer design. With complete sequencing of the rpoB gene, the percentage similarity between C. ulcerans and C. pseudotuberculosis drops to 93.6 %, and the percentage similarity between C. diphtheriae and C. ulcerans drops to 86 % (Khamis et al., 2004). The corresponding similarity between and among these species using 16S rRNA gene sequence analysis was over 98.5 %. They also demonstrate that two Corynebacterium isolates belong to the same species if they show 95 % or greater similarity, and argue that by using the rpoB gene sequencing analysis, a more discriminatory characterization of isolates can be obtained (Khamis et al., 2005).
To further define the status of Corynebacterium species in macaques housed in our vivarium, we have now collected samples from the cephalic implants and oropharynges of all implanted non-human primates. We initially characterized coryneform isolates from non-human primates using API testing and 16S rRNA gene sequencing analysis; however, discrepancies among selected samples were discovered. Isolates identified as C. pseudotuberculosis on API testing were characterized as C. ulcerans by 16S rRNA analysis. As both analytical methods used for confirming Corynebacterium species have potential limitations, we assessed two additional methods of identification, rpoB gene sequencing analysis and matrix-assisted laser desorption/ionization-time of flight MS (MALDI-TOF MS), in an effort to resolve discordant results and provide diagnostic adjuncts to 16S rRNA sequencing for definitive diagnosis.
Methods
Animals.
Macaques were singly or pair housed in stainless steel quadrangles of four units with individual cage dimensions of 31×29×64 inches (78.7×73.7×163 cm). Animals were fed ad libitum with commercial primate chow (Lab Diet 5038, PMI Nutrition International) and daily fruits, vegetables and treats. All animals were used in cognitive neuroscience research and, in accordance with Institutional Animal Care and Use Committee (IACUC)-approved protocols, had periodic limited restriction of access to water. Animals that were off-study had water available ad libitum. Macaques were housed in an AAALAC International-accredited animal facility with 10–15 complete air changes an hour, a 12 h light : dark cycle, and temperature and humidity levels of 72–78 °F (22–26 °C) and 30–70 %, respectively.
Microbiology.
Isolates were cultured from oropharynx and cephalic implants of rhesus monkeys (Macaca mulatta, 32 males and 9 females) and cynomolgus monkeys (Macaca fascicularis, n = 2 males) over a period of approximately 4 years. An individual sterile bacterial transport culturette (Venturi Transystem Transport Swab, Copan Diagnostics) was swabbed across the oropharynx, interior of the cephalic recording chamber, or skin/implant interface of implants on each animal. Swabs were streaked onto 5 % sheep blood, MacConkey and chocolate agar plates as previously described (Bergin et al., 2000). Swabs were also placed in trypticase soy broth for enrichment; plates and broth were incubated for 18–24 h at 37 °C and 5 % CO2. A blood agar plate was also incubated at room temperature to prevent overgrowth by non-coryneforms. Aliquots of broth were subsequently subcultured onto blood, MacConkey and chocolate agar plates (Bergin et al., 2000).
Pure cultures were obtained by restreaking single colonies onto blood agar. These were subsequently characterized by Gram staining and coryneforms were then speciated using the API Coryne strip system (bioMérieux) according to instructions from the manufacturer. Plates with optimum bacterial growth were used for DNA extraction and collected in freeze medium (Brucella broth and 20 %, v/v, glycerol) for storage at −70 °C. Coryneforms identified as C. pseudotuberculosis or Corynebacterium renale were tested by a second laboratory [Centers for Disease Control and Prevention (CDC), Atlanta, GA] using the API Coryne strip system.
DNA extraction.
The High Pure PCR Template Preparation kit (Roche Molecular Biochemicals) was used to extract DNA from bacterial pellets, as described previously (Fox et al., 2009).
16S rRNA gene sequencing.
The sequences of the 16S rRNA genes of 17 selected isolates were performed as described by Dewhirst et al. (2010). These isolates had been presumptively identified by our laboratory using the API Coryne strip system as C. pseudotuberculosis (n = 14) or C. renale (n = 3). Briefly, primers F24 (positions 9–27 in the forward direction) and F25 (positions 1525–1541 in the reverse direction) were used to amplify the 16S rRNA genes. The PCR product was concentrated and purified with QIAquick PCR purification kits (Qiagen). Purified DNA was sequenced with an ABI Prism cycle sequencing kit (BigDye Terminator Cycle Sequencing kit) on an ABI 3100 genetic analyser (Applied Biosystems). The sequencing primers (Dewhirst et al., 2010) were used in quarter-dye reactions, according to the manufacturer’s instructions. The 16S rRNA gene sequences were entered into RNA, a program for analysis of 16S rRNA gene data, and were aligned as described elsewhere (Jukes & Cantor, 1969; Paster & Dewhirst, 1988). The aligned sequences were exported and analysed using mega5 (Tamura et al., 2011). The evolutionary history was inferred using the neighbour-joining method (Saitou & Nei, 1987). Bootstrapping was performed with 1000 replicates.
rpoB gene sequencing.
The sequences of the partial rpoB gene were obtained for the 17 isolates originally identified as C. pseudotuberculosis or C. renale by API Coryne. The conserved primers C2700F and C3130R from the rpoB gene were used to amplify the PCR products, as described by Khamis et al. (2004). We detected PCR products of a size similar to those reported by Khamis et al. (2004). The amplicons were purified and directly sequenced using an ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems). The sequences were aligned using clustal w and analysed using mega5 (Tamura et al., 2011). The evolutionary history was inferred using the neighbor-joining method (Saitou & Nei, 1987). Bootstrapping was performed with 1000 replicates.
tox gene.
PCR amplification of the diphtheria toxin (tox) gene was performed on all isolates and used specific primers against the A and B subunits, as described by Schuhegger et al. (2008). NCTC 10648 was used as the positive control and NCTC 10356 as the negative control.
pld gene.
PCR amplification of the phospholipase D (pld) gene was performed on the 17 isolates originally identified as C. pseudotuberculosis or C. renale by API Coryne. The primers used were derived from the C. ulcerans (pld) sequence (GenBank accession no. L16585). The sequence of the forward primer (PLD-1) was 5′-TGTTTCACATGACGCAGCTT-3′; the sequence of the reverse primer (PLD-2) was 5′-AAGATCATTCCGTCTACATGA-3′. Reagents without DNA were used as a negative control; 720 bp PCR products from the isolates were sequenced and confirmed to have 98–99 % homology with the C. ulcerans pld gene. Conditions were as described previously (Bergin et al., 2000).
Elek toxigenicity test.
All coryneform isolates were tested for the production of diphtheria toxin using the conventional Elek assay as described by Engler et al. (1997). Briefly, two test strains and three control strains (positive control: NCTC 10648, C. diphtheriae gravis; negative control: NCTC 10356, C. diphtheriae belfanti; weak positive control: NCTC 3984, C. diphtheriae gravis) were inoculated in straight lines across each plate of Elek medium. A filter paper strip containing 500 IU diphtheria antitoxin ml−1 was placed across the agar surface and perpendicular to the inoculation lines. Plates were incubated in air at 37 °C for 48 h and examined for precipitin lines after 24 and 48 h. Non-toxigenic strains produced no precipitin lines.
MALDI-TOF MS.
The 17 isolates originally identified by API Coryne as C. pseudotuberculosis or C. renale were analysed by MALDI-TOF MS to determine their protein patterns. The data were searched within the Bruker BioTyper database using the Bruker BioTyper software (Bruker Daltonics). Isolates were prepared using the ethanol/formic acid extraction procedure recommended by the manufacturer, spotted on the target, allowed to dry, and overlaid with alpha-cyano-4-hydroxycinnamic acid (HCCA) matrix (Bruker, 255344). The MALDI-TOF MS analysis was performed using a Bruker Ultraflex III mass spectrometer operated in positive linear mode. The instrument was calibrated before the analysis using Bruker bacterial test standards (255343).
Results
Microbiology
Eighty-five isolates were obtained from 43 macaques: eight from throat cultures and 77 from implants. The isolates were identified by API Coryne strips as C. ulcerans (n = 60), C. pseudotuberculosis (n = 14) and C. renale (n = 3). Of the isolates obtained from throat cultures, one (07-2012) was identified as C. renale and the others were identified as C. ulcerans. Isolates identified as C. pseudotuberculosis and C. renale (n = 17) at our laboratory (MIT) were evaluated by a separate laboratory (CDC) using the same method. Twelve of 14 were interpreted to be C. ulcerans (likelihood 99.8 %), 2/14 were interpreted to be C. pseudotuberculosis (likelihood greater than 97.6 %), and 1/3 closely matched C. renale (likelihood 99.8 %). The remaining two isolates originally classified as C. renale produced API codes that were less closely matched but which were certainly members of the genus Corynebacterium (Table 1).
Table 1. Identification of isolates using API Coryne, MALDI-TOF MS and gene sequencing modalities.
Percentages after the API identification refer to confidence limits. Percentages after the gene sequencing results refer to percentage identities with respect to a reference strain.
| Accession | CDC API code | CDC API interpretation | CDC MALDI-TOF MS | 16S rRNA | rpoB |
| 05-1536 | 0110326 | C. ulcerans (85.6 %) | C. ulcerans | C. ulcerans (99.6 %) | C. ulcerans (99.5 %) |
| 06-0265 | 0111326 | C. ulcerans (99.7 %) | C. ulcerans | C. ulcerans (99.5 %) | C. ulcerans (99.5 %) |
| 07-0051 | 0111326 | C. ulcerans (99.7 %) | C. ulcerans | C. ulcerans (99.7 %) | C. ulcerans (99.5 %) |
| 06-0572 | 0111326 | C. ulcerans (99.7 %) | C. ulcerans | C. ulcerans (99.6 %) | C. ulcerans (99.5 %) |
| 07-1734 | 0111326 | C. ulcerans (99.7 %) | C. ulcerans | C. ulcerans (99.6 %) | C. ulcerans (99.5 %) |
| 07-1845 | 0111326 | C. ulcerans (99.7 %) | C. ulcerans | C. ulcerans (99.7 %) | C. ulcerans (99.5 %) |
| 07-1845 | 0111326 | C. ulcerans (99.7 %) | C. ulcerans | C. ulcerans (99.7 %) | C. ulcerans (99.5 %) |
| 07-0690 | 0111326 | C. ulcerans (99.7 %) | C. ulcerans | C. ulcerans (99.7 %) | C. ulcerans (99.5 %) |
| 07-1694 | 2001304 | C. renale group (90.1 %, doubtful profile) | Aromatoleum aromaticum | C. renale (98.8 %) | C. renale (92.3 %) |
| 07-2012 | 2001304 | C. renale group (90.1 %, doubtful profile) | No identification | C. renale (98.8 %) | C. renale (92.3 %) |
| 07-2044 | 2201304 | C. renale group (99.8 %) | C. renale | C. renale (99.2 %) | C. renale (98.5 %) |
| 07-2017 | 0111326 | C. ulcerans (99.7 %) | C. ulcerans | C. ulcerans (99.6 %) | C. ulcerans (99.5 %) |
| 07-2027 | 0101304 | C. pseudotuberculosis (97.6 %) | C. ulcerans | C. ulcerans (99.6 %) | C. ulcerans (99.5 %) |
| 07-7334 | 0111326 | C. ulcerans (99.7 %) | C. ulcerans | C. ulcerans (99.6 %) | C. ulcerans (99.5 %) |
| 07-7343 | 0111326 | C. ulcerans (99.7 %) | C. ulcerans | C. ulcerans (99.6 %) | C. ulcerans (99.5 %) |
| 07-7343 | 0111304 | C. pseudotuberculosis (99.3 %) | C. ulcerans | C. ulcerans (99.6 %) | C. ulcerans (99.5 %) |
| 08-0538 | 0111326 | C. ulcerans (99.7 %) | C. ulcerans | C. ulcerans (99.6 %) | C. ulcerans (99.5 %) |
A number of additional coryneforms were isolated from throat swabs and identified by API Coryne as Corynebacterium propinquum (n = 4), Corynebacterium auris (n = 1) and Corynebacterium minitissimum (n = 1). Two isolates of Corynebacterium striatum/amycolatum were isolated from cephalic implants. These organisms were not further characterized.
16S rRNA gene and rpoB gene sequencing
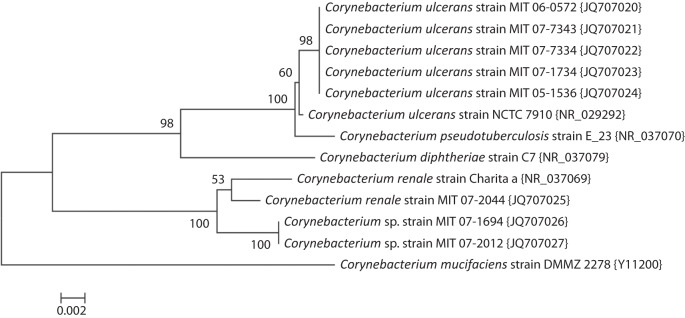
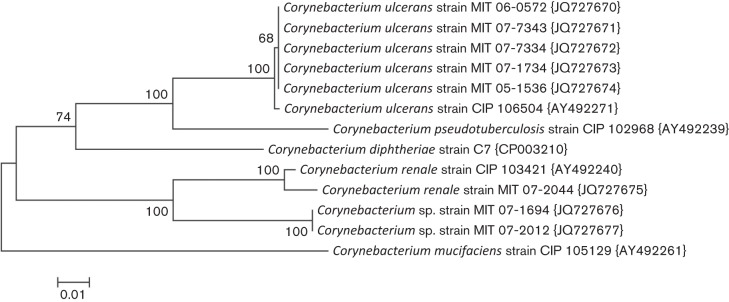
Full-length 16S rRNA sequences were obtained for the 17 strains identified by API at the second laboratory as C. ulcerans, C. pseudotuberculosis or C. renale. A phylogenetic tree for representative isolates is shown in Fig. 1. All of the C. ulcerans or C. pseudotuberculosis strains were identified as closest to the sequence for the type strain of C. ulcerans, but also very close to C. pseudotuberculosis. By blastn analysis, the 16S rRNA sequences of the three remaining strains were closest to C. renale. Strain 07-2044 had 99.2 % sequence similarity and strains 07-1694 and 07-2012 had 98.8 % similarity to C. renale. The lack of clear species separation by 16S rRNA for some taxa within the genus Corynebacterium is apparent in the tree. The partial rpoB gene sequences obtained for these strains and the phylogenetic tree for representative isolates is shown in Fig. 2. The strains identified by API as C. ulcerans or C. pseudotuberculosis all cluster with the type strain of C. ulcerans (0.5 % divergent), and C. pseudotuberculosis is well resolved as a separate taxon (8.5 % divergent). The rpoB sequence for strain 07-2044 is highly similar to that of C. renale (1.5 % divergent), while those of strains 07-1694 and 07-2012 diverged by 8.2 % from C. renale. The latter two isolates are therefore unlikely to be C. renale, as the limit for species identity with rpoB is 95 % and both genetic and physical methods indicate another species. rpoB gene sequencing in this instance was in general agreement with the phenotypic characterization of the API Coryne code generated at the second laboratory (90.1 % likelihood). It is clear from comparing the 16S rRNA and the rpoB trees that rpoB has superior ability to resolve the phylogeny of Corynebacterium spp.
Fig. 1.
Evolutionary relationships of Corynebacterium based on 16S rRNA sequences. The evolutionary history was inferred using the neighbor-joining method (Saitou & Nei, 1987). The optimal tree with the sum of branch length = 0.09719690 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches (Felsenstein, 1985). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Jukes–Cantor method (Jukes & Cantor, 1969) and are in the units of the number of base substitutions per site. The analysis involved 13 nt sequences. All ambiguous positions were removed for each sequence pair. There were a total of 1494 positions in the final dataset. Evolutionary analyses were conducted in mega5 (Tamura et al., 2011).
Fig. 2.
Evolutionary relationships of Corynebacterium based on the rpoB sequences. The evolutionary history was inferred using the neighbor-joining method (Saitou & Nei, 1987). The optimal tree with the sum of branch length = 0.44347215 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches (Felsenstein, 1985). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Jukes–Cantor method (Jukes & Cantor, 1969) and are in the units of the number of base substitutions per site. The analysis involved 13 nt sequences. All ambiguous positions were removed for each sequence pair. There were a total of 369 positions in the final dataset. Evolutionary analyses were conducted in mega5 (Tamura et al., 2011).
Elek toxigenicity test and tox gene
Neither toxin activity nor the tox gene was detected by the Elek test and PCR amplification, respectively.
pld gene
Sixteen of 17 isolates were positive by pld PCR.
MALDI-TOF MS
The 14 isolates identified as C. ulcerans or C. pseudotuberculosis by API were identified in the CDC MALDI-TOF MS database as C. ulcerans. Strain 07-2044, identified as C. renale by API, was similarly identified by CDC MALDI-TOF MS. The two novel Corynebacterium spp. strains were either not identified or misidentified (as Aromatolecum aromaticum, an unrelated organism from the phylum Proteobacteria), as the novel organism is not in the CDC MALDI-TOF MS database.
Discussion
In this study, we used phenotypic, biochemical, genetic and physical methods for identification of coryneforms from clinical specimens. We initially used API to identify C. ulcerans, C. renale and C. pseudotuberculosis from the cephalic implants and oropharynx of macaques. C ulcerans had been previously identified in our macaque population; the isolation of two additional species of Corynebacteria motivated efforts to confirm the identity of these latter isolates. Corynebacteria that were not identified as C. ulcerans were then evaluated by API at CDC, and some discordant results were generated. These 17 isolates were subjected to additional analysis (MALDI-TOF MS, 16S rRNA and rpoB gene sequencing), and 14 isolates were confirmed by all three methods to be C. ulcerans. API testing is a rapid method frequently used in diagnostic settings, but may not be adequately discriminatory to distinguish between closely related Corynebacterium species. This conclusion is in agreement with Contzen et al. (2011), who found that API could not identify unequivocally two isolates of C. ulcerans from wild boars. API interpretations can be subjective or require ancillary tests; moreover, expression of phenotypic characters may vary depending upon environmental pressures such as antibiotic administration (Drancourt et al., 2000). The critical difference between C. ulcerans and C. pseudotuberculosis on API Coryne is glycogen fermentation. Positive tests in maltose and glycogen fermentation generate an API code of 0111326, signifying 99.7 % confidence in an identification of C. ulcerans. Negative tests for fermentation of these substrates generates a code of 0111304 and 99.3 % confidence in a diagnosis of C. pseudotuberculosis. Ancillary chemotaxonomic methods for distinguishing between these organisms include trehalose fermentation (C. ulcerans +; C. pseudotuberculosis −), amylase activity (C. ulcerans +; C. pseudotuberculosis −), and 4-methylumbelliferyl-N-acetyl-β-d-glucosamide hydrolysis (60 % of C. ulcerans isolates +; C. pseudotuberculosis −) (Contzen et al., 2011; Kämpfer, 1992). Contzen et al. (2011) demonstrated that addition of trehalose fermentation to the reactions contained in API Coryne allowed 100 % discrimination of isolates of C. pseudotuberculosis and C. ulcerans from multiple host species. More variable characteristics that have been noted include alkaline phosphatase (C. ulcerans +; C. pseudotuberculosis variable but often −) and nitrate reduction (C. ulcerans −; C. pseudotuberculosis variable) (Funke et al., 1997). Cellular polar lipid profiles and fatty acid profiles can also be useful in distinguishing closely related species (Frischmann et al., 2011).
MALDI-TOF MS uses the protein composition of abundant protein species in bacterial cells for identification of isolates. The spectrum generated by the MS process is compared with reference spectra and a specific identification can be made if the isolate is a species included in the reference database. In a study of 116 Corynebacterium species isolates submitted to the German Consiliary Laboratory, MALDI-TOF MS showed agreement with rpoB gene sequencing for 115 of 116 isolates (99.1 %) (Konrad et al., 2010). The only isolate for which MALDI-TOF MS was accurate only to the genus level was one identified by rpoB as Corynebacterium tuberculostearicum. In contrast, API Coryne results were ambiguous at the species level for 12 isolates (11.2 %). API Coryne did, however, identify correctly and congruently all isolates of the coryneforms C. diphtheriae, C. ulcerans and C. pseudotuberculosis. MALDI-TOF MS can complement traditional phenotypic and taxonomic methods in a high-throughput fashion with little sample preparation if dealing with organisms in the reference database.
As 16S rRNA gene sequencing has low intra-genus polymorphism for Corynebacterium species, we evaluated selected isolates by rpoB gene sequencing. Studies by Khamis et al. (2004, 2005) had demonstrated that high degrees of similarity in 16S rRNA gene sequencing among isolates did not correlate with degree of similarity in rpoB gene sequencing. They further showed that the hypervariable region sequence of between 434 and 452 base pairs of the rpoB gene was superior to 16S rRNA gene sequencing in discrimination of closely related species. In this study, complete gene sequencing of the 16S rRNA gene was concordant with that of the hypervariable region of rpoB. In two of our 74 isolates (2.7 %) identified by API Coryne as either C. ulcerans or C. pseudotuberculosis, molecular genotyping was required for accurate identification.
In macaques, skin erosion and necrosis, as well as the generation of exuberant granulation tissue at the skin–implant interface, are potential clinical sequelae of cephalic implant placement. C. ulcerans may have been associated with bilateral chronic skin ulcers in a Brazilian woman with pulmonary infection from whom the organism was isolated in a bronchoalveolar lavage (BAL) sample (Mattos-Guaraldi et al., 2008). While C. ulcerans was not cultured specifically from the ulcers, the lesions regressed during antimicrobial therapy directed at the BAL isolate. In another report, a 71-year-old man with chronic non-healing ulcers of the right leg was diagnosed with toxigenic C. ulcerans (Wagner et al., 2001). The lesions resembled those of cutaneous diphtheria, a well-recognized clinical entity caused by non-toxigenic C. diphtheriae (Lowe et al., 2011). Similar skin lesions have been reported in the C. pseudotuberculosis-associated diseases, oedematous skin disease of buffalo and ulcerative lymphangitis of horses (Selim, 2001). Clinical features of infection by C. ulcerans and highly related organisms suggest a potential aetiological role for C. ulcerans in these outcomes in macaques. The prevalence of mixed infections in affected macaques and the lack of demonstration of PLD toxin elaboration from the isolates reported here make unequivocal association difficult, however.
We report for the first time the isolation of C. renale from the cephalic implant of a macaque, a finding confirmed by rpoB gene sequencing analysis. The performance of the identification modalities regarding the three putative C. renale isolates reflects the difficulty in definitive diagnosis of some isolates. Two of the three isolates did not reach the 95 % identity threshold recommended by Khamis et al. (2005) for species identity in rpoB gene sequencing. API Coryne and MALDI-TOF MS analysis were in concordance with that of rpoB, while both disagreed with the results of 16S rRNA gene sequencing. C. renale is the most common causative agent of urogenital disease in ruminants (Funke et al., 1997) and has been previously identified in laboratory animals. Stevens et al. (2007) reported the isolation of the organism from the urinary bladder of a rhesus monkey with necrohaemorrhagic cystitis. This finding, however, was based solely on microbial culture and Gram staining, without confirmation by molecular methods. There have been cases of spontaneous urinary calculi in young laboratory rats reportedly caused by C. renale (Osanai et al., 1994; Takahashi et al., 1995). The clinical impact of C. renale on cephalic implants of macaques is uncertain.
In this study, we have isolated an additional species of Corynebacterium from cephalic implants of macaques, affirmed the difficulty of distinguishing among closely related coryneforms, and demonstrated the use of rpoB gene sequencing and MALDI-TOF MS as discriminatory tests for identification of closely related isolates. Although the 16S rRNA and rpoB gene sequencing methods yielded identical results, rpoB did not require sequencing of the entire gene. MALDI-TOF MS analysis, though fast and accurate for species in its database, is currently restricted to large diagnostic centres and reference laboratories. Finally, Pacheco and co-workers, working with clinical isolates of C. pseudotuberculosis from small ruminants with caseous lymphadenitis, were able to distinguish between C. pseudotuberculosis and C. ulcerans using a multiplex PCR capable of detecting the 16S rRNA, rpoB and pld genes (Pacheco et al., 2007).
ACKNOWLEDGEMENTS
This study was funded by NIH grant(s): DE106937 (F. E. D.); T32-RR032307 (J. G. F.).
Abbreviations:
- MALDI-TOF MS
matrix-assisted laser desorption/ionization-time of flight MS
References
- Adékambi T., Drancourt M., Raoult D. (2009). The rpoB gene as a tool for clinical microbiologists. Trends Microbiol 17, 37–45 10.1016/j.tim.2008.09.008 [DOI] [PubMed] [Google Scholar]
- Aubel D., Renaud F., Freney J. (1997). Genomic diversity of several Corynebacterium species identified by amplification of the 16S–23S rRNA gene spacer region. Int J Syst Bact 47, 767–772 10.1099/00207713-47-3-767 [DOI] [Google Scholar]
- Bergin I. L., Chien C.-C., Marini R. P., Fox J. G. (2000). Isolation and characterization of Corynebacterium ulcerans from cephalic implants in macaques. Comp Med 50, 530–535 [PubMed] [Google Scholar]
- Contzen M., Sting R., Blazey B., Rau J. (2011). Corynebacterium ulcerans from diseased wild boars. Zoonoses Public Health 58, 479–488 10.1111/j.1863-2378.2011.01396.x [DOI] [PubMed] [Google Scholar]
- De Zoysa A., Hawkey P. M., Engler K., George R., Mann G., Reilly W., Taylor D., Efstratiou A. (2005). Characterization of toxigenic Corynebacterium ulcerans strains isolated from humans and domestic cats in the United Kingdom. J Clin Microbiol 43, 4377–4381 10.1128/JCM.43.9.4377-4381.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst F. E., Chen T., Izard J., Paster B. J., Tanner A. C. R., Yu W.-H., Lakshmanan A., Wade W. G. (2010). The human oral microbiome. J Bacteriol 192, 5002–5017 10.1128/JB.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorella F. A., Pacheco L. G. C., Oliveira S. C., Miyoshi A., Azevedo V. (2006). Corynebacterium pseudotuberculosis: microbiology, biochemical properties, pathogenesis and molecular studies of virulence. Vet Res 37, 201–218 10.1051/vetres:2005056 [DOI] [PubMed] [Google Scholar]
- Drancourt M., Bollet C., Carlioz A., Martelin R., Gayral J.-P., Raoult D. (2000). 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J Clin Microbiol 38, 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler K. H., Glushkevich T., Mazurova I. K., George R. C., Efstratiou A. (1997). A modified Elek test for detection of toxigenic corynebacteria in the diagnostic laboratory. J Clin Microbiol 35, 495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791 10.2307/2408678 [DOI] [PubMed] [Google Scholar]
- Fox J. G., Frost W. W. (1974). Corynebacterium ulcerans mastitis in a bonnet macaque (Macaca radiata). Lab Anim Sci 24, 820–822 [PubMed] [Google Scholar]
- Fox J. G., Shen Z., Muthupalani S., Rogers A. R., Kirchain S. M., Dewhirst F. E. (2009). Chronic hepatitis, hepatic dysplasia, fibrosis, and biliary hyperplasia in hamsters naturally infected with a novel Helicobacter classified in the H. bilis cluster. J Clin Microbiol 47, 3673–3681 10.1128/JCM.00879-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischmann A., Knoll A., Hilbert F., Zasada A. A., Kämpfer P., Busse H.-J. (2011). Corynebacterium epidermicanis sp. nov., isolated from a dog’s skin. Int J Syst Evol Microbiol In Press. E-publication November 11, 2011. [Google Scholar]
- Funke G., von Graevenitz A., Clarridge J. E., III, Bernard K. A. (1997). Clinical microbiology of coryneform bacteria. Clin Microbiol Rev 10, 125–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. J., Cassiday P. K., Bernard K. A., Bolt F., Steigerwalt A. G., Bixler D., Pawloski L. C., Whitney A. M., Iwaki M. & other authors (2010). Novel Corynebacterium diphtheriae in domestic cats. Emerg Infect Dis 16, 688–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes T. H., Cantor C. R. (1969). Evolution of protein molecules. In Mammalian Protein Metabolism, pp. 21–132 Edited by Munro H. N. New York: Academic Press [Google Scholar]
- Kämpfer P. (1992). Differentiation of Corynebacterium spp., Listeria spp., and related organisms by using fluorogenic substrates. J Clin Microbiol 30, 1067–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsukawa C., Kawahara R., Inoue K., Ishii A., Yamagishi H., Kida K., Nishino S., Nagahama S., Komiya T. & other authors (2009). Toxigenic Corynebacterium ulcerans isolated from the domestic dog for the first time in Japan. Jpn J Infect Dis 62, 171–172 [PubMed] [Google Scholar]
- Khamis A., Raoult D., La Scola B. (2004). rpoB gene sequencing for identification of Corynebacterium species. J Clin Microbiol 42, 3925–3931 10.1128/JCM.42.9.3925-3931.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamis A., Raoult D., La Scola B. (2005). Comparison between rpoB and 16S rRNA gene sequencing for molecular identification of 168 clinical isolates of Corynebacterium. J Clin Microbiol 43, 1934–1936 10.1128/JCM.43.4.1934-1936.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad R., Berger A., Huber I., Boschert V., Hormansdorfer S., Busch U., Hogardt M., Schubert S., Sing A. (2010). Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectroscopy as a tool for rapid diagnosis of potentially toxigenic Corynebacterium species in the laboratory management of diphtheria-associated bacteria. Euro Surveill 15, 1–5 [DOI] [PubMed] [Google Scholar]
- Lartigue M. F., Monnet X., Le Flèche A., Grimont P. A. D., Benet J. J., Durrbach A., Fabre M., Nordmann P. (2005). Corynebacterium ulcerans in an immunocompromised patient with diphtheria and her dog. J Clin Microbiol 43, 999–1001 10.1128/JCM.43.2.999-1001.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C. F., Bernard K. A., Romney M. G. (2011). Cutaneous diphtheria in the urban poor population of Vancouver, British Columbia, Canada: a 10-year review. J Clin Microbiol 49, 2664–2666 10.1128/JCM.00362-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos-Guaraldi A. L., Sampaio J. L. M., Santos C. S., Pimenta F. P., Pereira G. A., Pacheco L. G. C., Miyoshi A., Azevedo V., Moreira L. O. & other authors (2008). First detection of Corynebacterium ulcerans producing a diphtheria-like toxin in a case of human with pulmonary infection in the Rio de Janeiro metropolitan area, Brazil. Mem Inst Oswaldo Cruz 103, 396–400 10.1590/S0074-02762008000400014 [DOI] [PubMed] [Google Scholar]
- Osanai T., Miyoshi I., Hiramune T., Kasai N. (1994). Spontaneous urinary calculus in young LEW rats caused by Corynebacterium renale. J Urol 152, 1002–1004 [DOI] [PubMed] [Google Scholar]
- Pacheco L. G. C., Pena R. R., Castro T. L. P., Dorella F. A., Bahia R. C., Carminati R., Frota M. N. L., Oliveira S. C., Meyer R. & other authors (2007). Multiplex PCR assay for identification of Corynebacterium pseudotuberculosis from pure cultures and for rapid detection of this pathogen in clinical samples. J Med Microbiol 56, 480–486 10.1099/jmm.0.46997-0 [DOI] [PubMed] [Google Scholar]
- Paster B. J., Dewhirst F. E. (1988). Phylogeny of campylobacters, wolinellas, Bacteroides gracilis, and Bacteroides urealyticus by 16S ribosomal ribonucleic acid sequencing. Int J Syst Bacteriol 38, 56–62 10.1099/00207713-38-1-56 [DOI] [Google Scholar]
- Saitou N., Nei M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4, 406–425 [DOI] [PubMed] [Google Scholar]
- Schuhegger R., Lindermayer M., Kugler R., Heesemann J., Busch U., Sing A. (2008). Detection of toxigenic Corynebacterium diphtheriae and Corynebacterium ulcerans strains by a novel real-time PCR. J Clin Microbiol 46, 2822–2823 10.1128/JCM.01010-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhegger R., Schoerner C., Dlugaiczyk J., Lichtenfeld I., Trouillier A., Zeller-Peronnet V., Busch U., Berger A., Kugler R. & other authors (2009). Pigs as source for toxigenic Corynebacterium ulcerans. Emerg Infect Dis 15, 1314–1315 10.3201/eid1508.081568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim S. A. (2001). Review of oedematous skin disease of buffalo in Egypt. J Vet Med B 48, 241–258 10.1046/j.1439-0450.2001.00451.x [DOI] [PubMed] [Google Scholar]
- Stevens E. L., Twenhafel N. A., MacLarty A. M., Kreiselmeier N. (2007). Corynebacterial necrohemorrhagic cystitis in two female macaques. J Am Assoc Lab Anim Sci 46, 65–69 [PubMed] [Google Scholar]
- Takahashi T., Tsuji M., Kikuchi N., Ishihara C., Osanai T., Kasai N., Yanagawa R., Hiramune T. (1995). Assignment of the bacterial agent of urinary calculus in young rats by the comparative sequence analysis of the 16S rRNA genes of corynebacteria. J Vet Med Sci 57, 515–517 10.1292/jvms.57.515 [DOI] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). mega5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28, 2731–2739 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J., Ignatius R., Voss S., Höpfner V., Ehlers S., Funke G., Weber U., Hahn H. (2001). Infection of the skin caused by Corynebacterium ulcerans and mimicking classical cutaneous diphtheria. Clin Infect Dis 33, 1598–1600 10.1086/322969 [DOI] [PubMed] [Google Scholar]
- Wagner K. S., White J. M., Crowcroft N. S., De Martin S., Mann G., Efstratiou A. (2010). Diphtheria in the United Kingdom, 1986–2008: the increasing role of Corynebacterium ulcerans. Epidemiol Infect 138, 1519–1530 10.1017/S0950268810001895 [DOI] [PubMed] [Google Scholar]