Abstract
The mechanisms that allow Streptococcus pyogenes to survive and persist in the human host, often in spite of antibiotic therapy, remain poorly characterized. Therefore, the determination of culture conditions for long-term studies is crucial to advancement in this field. Stationary cultures of S. pyogenes strain NZ131 and its spontaneous small-colony variant OK171 were found to survive in rich medium for less than 2 weeks, and this inability to survive resulted from the acidification of the medium to below pH 5.5, which the cells did not tolerate for longer than 6–7 days. The growth of NZ131 resulted in acidification of the culture to below pH 5.5 by the onset of stationary phase, and the loss of viability occurred in a linear fashion. These results were also found to be true for M49 strain CS101 and for M1 strain SF370. The S. pyogenes strains could be protected from killing by the addition of a buffer that stabilized the pH of the medium at pH 6.5, ensuring bacterial survival to at least 70 days. By contrast, increasing the glucose added to the medium accelerated the loss of culture viability in strain NZ131 but not OK171, suggesting that the small-colony variant is altered in glucose uptake or metabolism. Similarly, acidification of the medium prior to inoculation or at the middle of exponential phase resulted in growth inhibition of all strains. These results suggest that control of the pH is crucial for establishing long-term cultures of S. pyogenes.
Introduction
Streptococcus pyogenes (group A streptococcus; GAS) is an important human pathogen that produces a plethora of diseases, ranging from mild suppurative throat and skin infections such as pharyngitis and pyoderma to life-threatening invasive diseases such as streptococcal toxic shock syndrome and necrotizing fasciitis. Further, debilitating nonsuppurative sequelae such as acute post-streptococcal glomerulonephritis (APSGN) and rheumatic heart disease can follow uncomplicated throat or skin infections (Cunningham, 2000; Hynes, 2004). Although generally viewed as extracellular pathogens, group A streptococci have been shown to invade epithelial and pharyngeal cells (Fluckiger et al., 1998; Cleary & Cue, 2000; Courtney et al., 2002; Molinari & Chhatwal, 1999). They can induce lysis of their host, but following a different route they can also establish themselves as an intracellular, non-proliferating latent form that can be defined as the streptococcal carrier state (Facinelli et al., 2001; Fluckiger et al., 1998; Österlund & Engstrand, 1997; Österlund et al., 1997).
Due to the lack of an adequate animal model for studying different aspects of S. pyogenes biology, most experiments have been performed in vitro, i.e. using rich (complex) or defined growth media. In the case of studying long-term persistence of streptococcal cultures it is logical to propose that the stationary phase of an in vitro culture should physiologically bear a close resemblance to the environment experienced by the bacteria during their intracellular phase. In both cases bacteria experience a dramatic environmental change that is countered by a metabolic response characteristic of the latent lifestyle (Wood et al., 2005). Thus, monitoring long-term survival of S. pyogenes in stationary phase in rich media could serve as an approximation of events taking place inside infected cells.
Slow-growing mutants with altered colonial morphology occasionally appear in populations of S. pyogenes (Cleary et al., 1998; Leonard et al., 1998; McCarty, 1966; Schmitt-Slomska et al., 1972). Two studies have examined the possible role of small, dry colonies of S. pyogenes with suppressed hyaluronic acid capsule operon (has), M1 virulence protein (emm) and NAD glycohydrolase (nga) mRNAs in the invasion of eukaryotic cells and subsequent intracellular phase of these streptococci (Cleary et al., 1998; Leonard et al., 1998). S. pyogenes strain OK171 is a small-colony morphology variant that arose spontaneously from M49 genome strain NZ131 (McShan et al., 2008) in our laboratory, and as presented below, OK171 occasionally reverts to wild-type (WT) large-colony morphology. One of the interesting questions concerning S. pyogenes is the survival and persistence of this pathogen in the human host, often in spite of antibiotic therapy (Kaplan et al., 2007). Therefore, it was of interest to determine any differences that existed between NZ131 and OK171 in their abilities to adapt to long-term culture in rich media.
Recent studies had reported conditions used to maintain GAS in long-term culture for over a year (Wood et al., 2005, 2009) and provided the starting point for our investigations. Here we report that S. pyogenes does not survive extended stationary phase in unbuffered rich media for longer than 6–8 days, the cause of cell death being the acidification of the medium. Further, we show that a spontaneous small-colony variant of strain NZ131, OK171, exhibited an altered growth pattern that increased the survival period in extended culture, and that this survival was the result of delayed acidification of the medium. The results presented here suggest that in long-term survival of S. pyogenes cultures, either a mechanism must be present to stabilize the pH such as the human host buffering system, or a mutation must be present in the starting cells to prevent lethal acidification of the culture, which occurs by the end of exponential growth.
Methods
Bacterial strains, growth and media.
GAS strains used in this study were NZ131 (M49), SF370 (M1) and CS101 (M49). Of these strains, the complete genome sequences of NZ131 and SF370 have been determined (Ferretti et al., 2001; McShan et al., 2008). Strain CS101 has been used in a number of published studies concerning environmental adaptation (Dmitriev et al., 2008; Eberhard et al., 2001; Haanes et al., 1992; Wood et al., 2005, 2009) and was obtained from Michael S. Chaussee, University of South Dakota. Strain OK171 is a spontaneous small-colony variant derivative of NZ131, while OK172 is a spontaneous large-colony revertant of OK171. Strains were grown on Todd–Hewitt medium (TH) or in TH broth supplemented with 0.2 % yeast extract (THY). Media were purchased from Difco. In some studies, TH and THY media were enriched by the addition of 0.2 or 0.8 % (v/v) glucose. THYB medium is THY buffered with 10 mM HEPES (pH 7.4). Where needed, THY plates (THY plus 1.5 % agar) and sheep blood plates (BP) were used. During growth of long-term cultures, samples were plated onto BP plates to monitor continuously for possible contamination during the course of the experiment. In all experiments the cultures were kept static and were gently shaken only when samples were taken.
Lactate assay.
The presence of lactate in streptococcal cultures was determined using a commercial kit and following the manufacturer’s recommended protocol (Eton Bioscience). Overnight cultures of strains NZ131 or OK171 were diluted into fresh THY broth to a final OD600 of 0.02 and grown at 37 °C. Growth was monitored by OD600, and beginning when the cultures reached OD600 0.1, samples were removed every 30 min, the cells were removed by centrifugation, and the culture media were stored at −20 °C. Once all samples had been collected, each sample was sequentially diluted, and the concentration of lactate (mM) was determined as directed by the manufacturer. Each assay was performed three times to determine reproducibility.
Survival of bacterial culture.
Overnight cultures were diluted to OD600 0.02 in fresh medium and incubated at 37 °C. Most experiments were carried out in both a regular atmosphere and in 5 % CO2. Bacteria were incubated either with no shaking in a water bath (with gentle mixing of the culture when samples were taken) or as a thin layer in serum bottles in a CO2 incubator. The ability of cells to survive in liquid cultures was monitored by measuring c.f.u., which were determined by making appropriate serial dilutions in the same media used for cultivating bacteria. For each sample, 0.1 ml diluted culture was plated in triplicate onto THY plates, and colonies were counted after approximately 18–20 h of incubation at 37 °C in the regular or CO2 incubator. In the later phases of extended culture experiments that were at low titres, larger volumes (up to 0.3 ml) of undiluted samples were plated on both THY and BP plates. At each point when samples were taken to assess survival, the acidity of cultures was also measured with a pH meter. Before measurement of pH values, the aliquots of cultures were centrifuged and supernatants were passed through 0.2 µm pore-size Millipore filters. Growth of cultures through exponential phase until the onset of stationary phase was also monitored by OD600.
Fluctuation test.
The determination of the spontaneous mutation rate for the reversion by OK171 to large-colony morphology was done by a modification of previous methods (Rosche & Foster, 2000; Scott et al., 2008). The number of bacteria in individual colonies of OK171 was determined by mixing three individual colonies picked up by a Pasteur pipette, their suspension by intense vortexing and determining the titre as described above. The obtained titre values were used for calculating the titre to start a 100 ml culture from a fresh colony to give approximately 10–50 cells ml−1, as recommended for fluctuation tests (Rosche & Foster, 2000). The culture was used to establish 30–40 parallel 1 ml cultures. The cultures were incubated for >18 h at 37 °C, and the titre was determined by serial dilution from a mixture combining 0.1 ml samples from five independent cultures. From each culture, 0.1 ml was spread in triplicate onto THY agar plates and incubated for 48 h at 37 °C. The distinct, large revertant colonies appearing over the bacterial background were counted, and the total population size and frequency of appearance of revertants were used to calculate the number of mutations per generation using the software package ft (Shaver & Sniegowski, 2003).
Electron microscopy.
Strains NZ131 and OK171 were grown overnight in 5 ml THY broth at 37 °C. Samples (3 µl) of each were allowed to settle for 2.5 min on glow-discharged Formvar-coated 400 copper mesh grids. Excess liquid was wicked off the grid using filter paper. The grids were then washed with 0.5 % uranyl acetate in double-distilled H2O and immediately the stain was wicked off. The samples were then stained with 0.5 % uranyl acetate for 5 s and again the excess stain was wicked off. The grids were allowed to air-dry completely before viewing with a Hitachi H-7600 transmission electron microscope. Microscopy was done at the Oklahoma Medical Research Foundation Imaging Core Facility.
Results
Survival of strains NZ131 and OK171 after prolonged culture
The growth and survival of the M49 strain NZ131 and its small-colony spontaneous derivative OK171 were observed over extended culture in THY media. The exponential and early stationary phases of growth of strain NZ131 and its slow-growing mutant OK171 are illustrated in Fig. 1. Strain NZ131 rapidly acidified the medium during exponential growth, and the pH dropped below pH 6.0 during the first day of culture when the culture density reached OD600 values between 0.62 and 0.70 (beginning of stationary phase). By contrast, strain OK171 entered slowly into stationary phase and its pH stayed almost at the initial value of 7.5 over the same time period (Fig. 1a). Interestingly, the number of c.f.u. ml−1 in OK171 was almost the same as that in NZ131, even though the optical density of the culture was half that of the WT strain (Fig. 1b). Under the microscope the cell agglomerates of NZ131 and OK171 appeared the same, so the observed difference between the OD600 and c.f.u. could be the result of easier disruption of OK171 clumps during spreading on plates (results not shown). The drop in culture pH was mirrored by the release of lactate into the culture media following metabolism of glucose (Fig. 2). The appearance of lactate in the NZ131 culture was essentially collinear with the increase in cell density, and maximum production was achieved by the onset of stationary phase. By contrast, strain OK171 produced little lactate during the first 20 h, and maximum production occurred between 1 and 2 days of incubation.
Fig. 1.
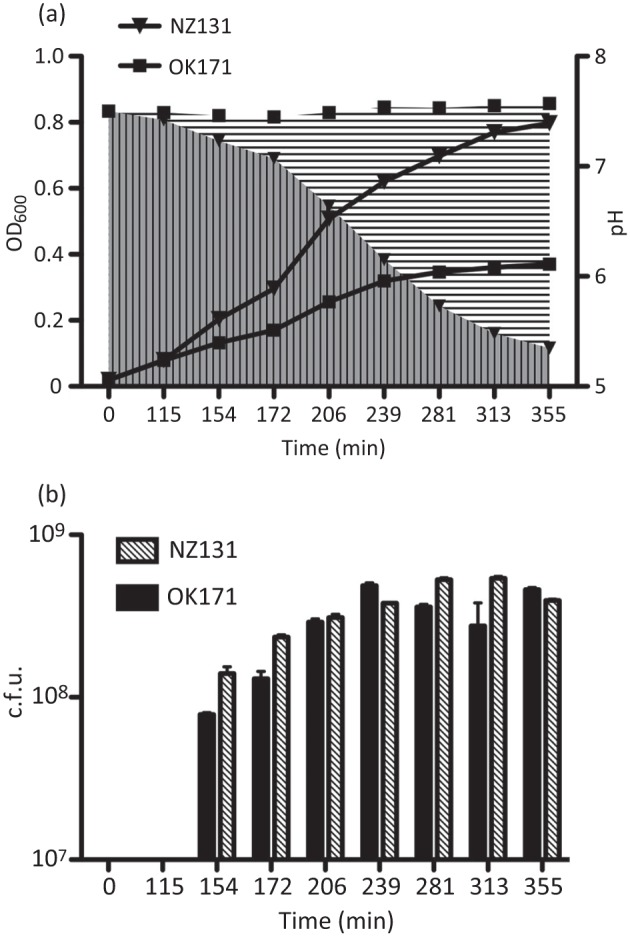
Growth of S. pyogenes strains NZ131 and OK171. The growth of NZ131 and its small-colony variant OK171 in THY broth culture was monitored by OD600 measurement. At each time point, the pH of the culture and the titre (c.f.u.) were determined. (a) OD600 and pH of the cultures are shown. The lines are the OD600 values for the cultures, and the areas filled with vertical and horizontal lines indicate the pH values (NZ131 and OK171, respectively). (b) NZ131 and OK171 titres at each time point are indicated. Even though the cultures differed in optical density, with NZ131 achieving a higher density, the c.f.u. values were essentially the same. For both experiments, the data (OD600, pH and c.f.u.) are the average and standard error of two independent experiments for each strain (not all error bars are visible).
Fig. 2.
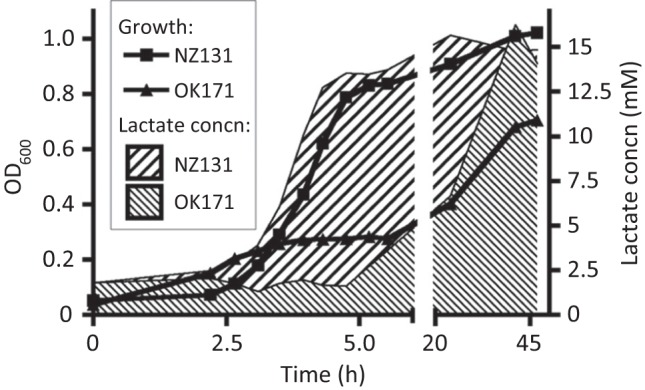
Production of lactate during growth of strains NZ131 and OK171. The growth of NZ131 and OK171 in THY broth culture was monitored by OD600. At each time point, the concentration of lactate released into the culture medium was determined. In contrast to NZ131, strain OK171 delayed its production of lactate, and maximum levels did not appear until after nearly 2 days of incubation. The lines indicate the growth of the cultures as measured by OD600, and the filled areas indicate the associated production of lactate during this growth. Data (OD600, lactate production) are the average and standard error of two independent experiments for each strain; error bars are too small to be displayed.
The cultures then were incubated for an extended period at 37 °C. The first measurements were performed the next day after an additional 16–18 h of incubation. Afterward, the titres and pH values of each sample were monitored on a daily basis. The results presented in Fig. 3 demonstrate that slow-growing mutant OK171, after overnight incubation, reached high titre values without substantially increasing the acidity of the medium. Further incubation showed a steady fall in bacterial titres and pH values in both cultures but with different dynamics (Fig. 3). In both cultures the pH value never dropped below pH 5.3. However, in contrast to OK171, NZ131 lost viability sooner, which was possibly due to the faster increase of acidity in the medium, and its titre reached zero significantly sooner than the OK171 culture (Fig. 3). The parallel experiment in TH medium, lacking yeast extract, with both strains produced virtually the same results, including the duration of cell survival and the final culture pH (results not shown). The analysis also included strains SF370 and CS101 used in an earlier report (Wood et al., 2005). As compared with NZ131, they demonstrated a slightly steeper fall in cell numbers that always resulted in culture death 1 day earlier in both THY and TH media (results not shown). The large colony-forming revertant of OK171, strain OK172, was also included in the experiments and demonstrated almost the same profile of growth and survival as NZ131 (data not shown). At the end of all experiments, 5 ml of all five cultures was transferred into either 100 ml fresh THY broth or THY broth enriched with 0.2 % glucose to detect the presence of any viable cells. Growth could not be detected after 3 days of incubation at 37 °C in any of the tested cultures (NZ131, OK171, SF370 and CS101, OK172), nor were any colonies detected after the cells were concentrated by centrifugation and spread onto THY or BP plates and incubated for 48 h at 37 °C.
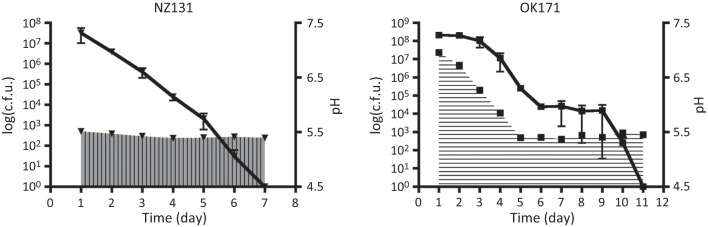
Fig. 3.
Extended culture of NZ131 and OK171 in unbuffered medium. The cultures of NZ131 and OK171 shown in Fig. 1 were allowed to continue growth, and the changes in c.f.u. and pH of each were monitored from day 2 to the point when the cultures died. The first measurements were taken 18–20 h after the last sample of the previous day had been analysed (Fig. 1). The lines show the c.f.u. values for the cultures, and the areas filled with vertical and horizontal lines indicate the pH (NZ131 and OK171, respectively). As in Fig. 1, the data (pH and c.f.u.) are the average and standard error of two independent experiments for each strain.
To confirm that the acidity of the medium affects the growth of NZ131 we performed two additional experiments. In the first, the overnight culture was diluted to OD600 0.02 in fresh THY medium already acidified with lactic acid to pH 6.36 and 5.30. In this experiment, the growth curve of the first culture almost overlapped with the curve of the control culture from OD600 0.02 to OD600 0.480, after which the growth was reduced and finally levelled off at OD600 0.660. On the other hand, the second culture, after being diluted (pH 5.3), failed to show any growth for the duration of the experiment (Fig. 4a). In the second experiment, an overnight culture was diluted to OD600 0.02 in fresh THY medium and grown to OD600 0.3, at which time the pH of the culture was lowered to 5.41 by the addition of lactic acid. In contrast to the control culture, which continued full growth, the acidified culture displayed drastically slowed growth and after about 1.5 h growth levelled off at OD600 ~0.4 (titre value 2.7×108) (Fig. 4b). The results presented here, as well as the results presented below, are of the experiments performed in a normal atmosphere. However, all survival experiments were also carried out several times in a CO2 atmosphere; they produced virtually the same data and are not presented.
Fig. 4.
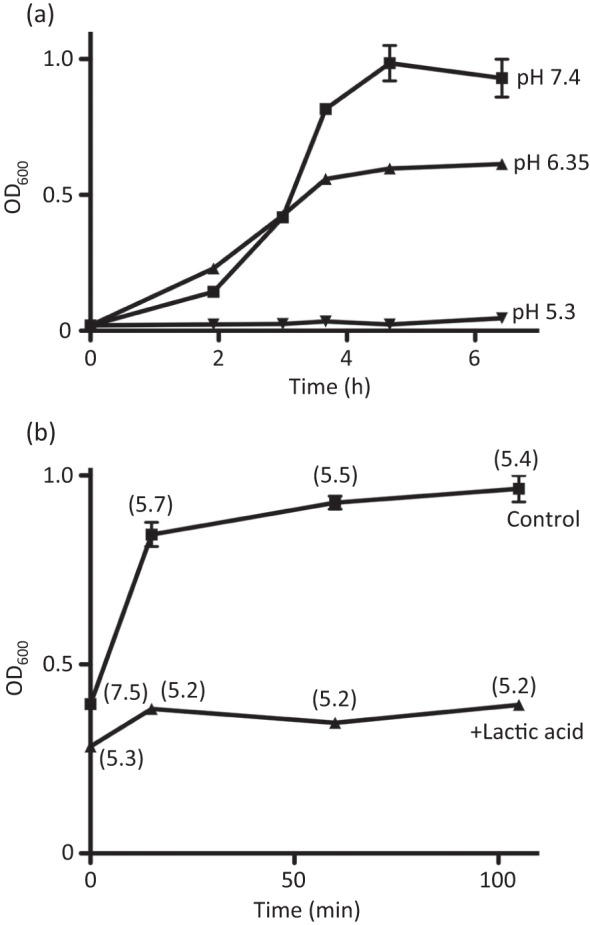
(a) Effect of lactic acid on NZ131 during the lag and exponential phases of growth. (a) A bacterial culture grown overnight in THY was diluted to OD600 ~0.02 in fresh THY medium or in THY medium acidified with 1 M lactic acid to a pH of either 6.35 or 5.3. Samples were taken 2 h after the start of the culture, every 1 h during the exponential phase and for 2 h after the end of the exponential phase. Each time, OD600 values were determined. The initial pH values are given at the end of each curve. (b) Effect of acidification of a NZ131 culture at the beginning of the exponential phase. An overnight bacterial culture was diluted to OD600 ~0.02 and grown to OD600 0.3. At that point, the culture was split into two and one half was acidified to pH 5.41 with 1 M lactic acid. Samples from both cultures were taken every 15 min or at multiples of 15 min, and OD600 and pH values (numbers in parentheses) were determined.
The presence of glucose expedites death of streptococcal cultures
To confirm the assumption that the increased acidity of the medium affects the longevity of streptococcal cultures, we grew strains OK171 and NZ131 in THY medium enriched with glucose to give final concentrations of 0.4 or 1 %, which is twice or four times the amount present in typical TH broth formulations, respectively (Atlas, 2004). The obtained data for strain NZ131 (Fig. 5) confirmed the earlier report of Wood and colleagues that an increased concentration of glucose accelerates the death of the culture (Wood et al., 2005). No difference was noticed in the kinetics of dying of NZ131 incubated in 0.4 and 1 % glucose (Fig. 5; data of the experiment with 1 % glucose are not presented). On the other hand, the change in pH values and accompanying decline in viability for the slow-growing strain OK171 were almost the same as in the experiments with medium without added glucose (Figs 3 and 5). Some decline in viability, in parallel with a steeper increase in acidity, was observed when OK171 was incubated in 1 % glucose [the culture died at day 9 or 10 as compared with day 11 when cells were grown in 0.2 and 0.4 % glucose (results not shown)]. The fast-growing revertant of OK171, strain OK172, was also used in the experiments with additional glucose and behaved the same as NZ131 (data not shown).
Fig. 5.
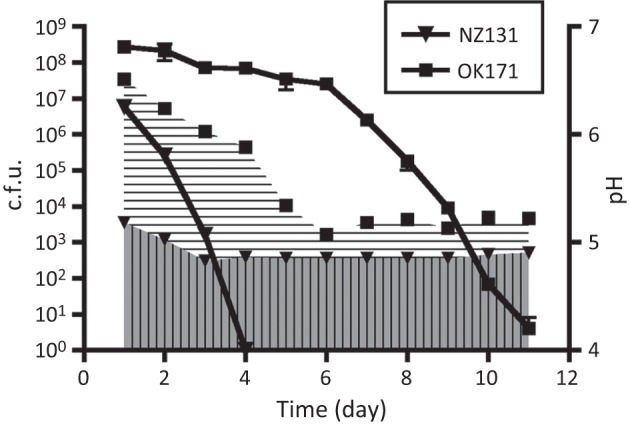
Survival of strains NZ131 and OK171 in medium enriched with 0.2 % glucose (cumulative concentration 0.4 %). Values shown of c.f.u. and culture pH are the mean and standard deviation of two independent experiments. The lines show the c.f.u. values for the cultures, and the areas filled with vertical and horizontal lines indicate the pH (NZ131 and OK171, respectively).
Survival of S. pyogenes in buffered medium
To verify that the acidity of the medium affects cell viability and early death of the culture (Figs 3 and 5), we performed an identical experiment in buffered medium (THY medium with 10 mM HEPES, pH 7.4). In contrast to the previous experiment (Fig. 3), the cultures grown in buffered medium demonstrated a quite different survival pattern (Fig. 6). After growth during the previous 24 h (results not presented), the stationary culture of NZ131 reached a titre (2.2×108) higher than that of OK171 (6.7×107), possibly due to the lack of rapid acidification of the buffered medium with NZ131 (Figs 1 and 3). The pH values at that point were 6.75 (NZ131) and 7.32 (OK171). Over the next 3 days the pH of the NZ131 culture decreased to 6.51. To reach approximately the same pH value (6.54) it took the OK171 culture 7 days (Fig. 6), and these pH values stayed stable for both cultures for the rest of the experiment. On the other hand, the titres of both cultures decreased in parallel until day 11, when they attained a value of approximately 2–3×105 c.f.u. (Fig. 6). This titre remained stable with some variations for 2 months and 10 days, when the experiment was discontinued. Interestingly, the results of Wood et al. (2005) showed similar titre values after 12 weeks of incubation in regular, unbuffered TH medium.
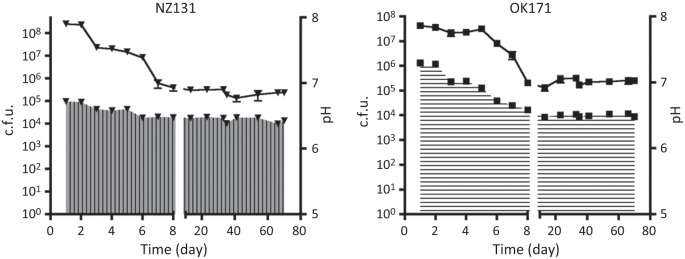
Fig. 6.
Survival of NZ131 and OK171 in buffered medium. The strains were grown in THY medium buffered with 10 mM HEPES buffer, pH 7.4. After the first week of incubation, the pH of both cultures stabilized at pH ~6.5 and remained at this value for the remainder of the experiment. Similarly, both populations stabilized at ~105 cells ml−1, a drop of about 1000-fold from the maximum cell density. The experiment was discontinued after 70 days. The lines show the c.f.u. values for the cultures, and the areas filled with vertical and horizontal lines indicate the pH (NZ131 and OK171, respectively).
Small-colony variant OK171 reverts to the WT colonial morphology by a mutational event
In the studies of small-colony variants described by Leonard et al. (1998), it was reported that the small, dry colonies that they isolated reverted to the original large and smooth form by an epigenetic event, although no further clarification or speculation on the underlying mechanism was offered. In contrast to these epigenetic transitional forms, OK171 isolated in our laboratory differs from its progenitor NZ131 only by the small size of its colonies (Fig. 6a). It produces hasA mRNA (our unpublished results), which is involved in the synthesis of streptococcal capsule, and the colonies have an appearance as glossy as that of the parental strain NZ131. The number of bacteria in an OK171 colony was found to be approximately 2.5×106, while the number of cells in large colonies (NZ131) was found to be approximately 1.3×107. The obtained ratio of about 5.2 roughly corresponds to the difference in size of OK171 and NZ131 colonies on both BP and THY plates (Fig. 7a). Since the survival kinetics in unbuffered TH or THY media showed that OK171 did differ from the parental strain NZ131, allowing these cells to persist for a longer period before culture acidification occurred, it was of interest to see whether this strain reverted to the original form by a mutational event or by the proposed epigenetic event (Leonard et al., 1998). To answer that question a modified Luria–Delbruck fluctuation test was carried out on strain OK171 as described in Methods. Fig. 7(c) shows the reversion frequency from the small-colony phenotype of OK171 to a large form in four independent experiments. The reversion pattern presented clearly shows the presence of individual cultures with ‘jackpots’, a strong indication that the small-colony phenotype reverts to a large-colony form by a mutational event. The calculated mean mutation rate for the four separate experiments was 2.03×10−9 mutations per generation, a value very close to the one observed for the appearance of ciprofloxacin resistance (3.2×10−9) (Scott et al., 2008). Since ciprofloxacin resistance results from single-hit mutations (i.e. a single-base change in parC) (Pletz et al., 2006), the reversion rate to the WT large-colony morphology by OK171 suggests that similar single hits are responsible here, too.
Fig. 7.
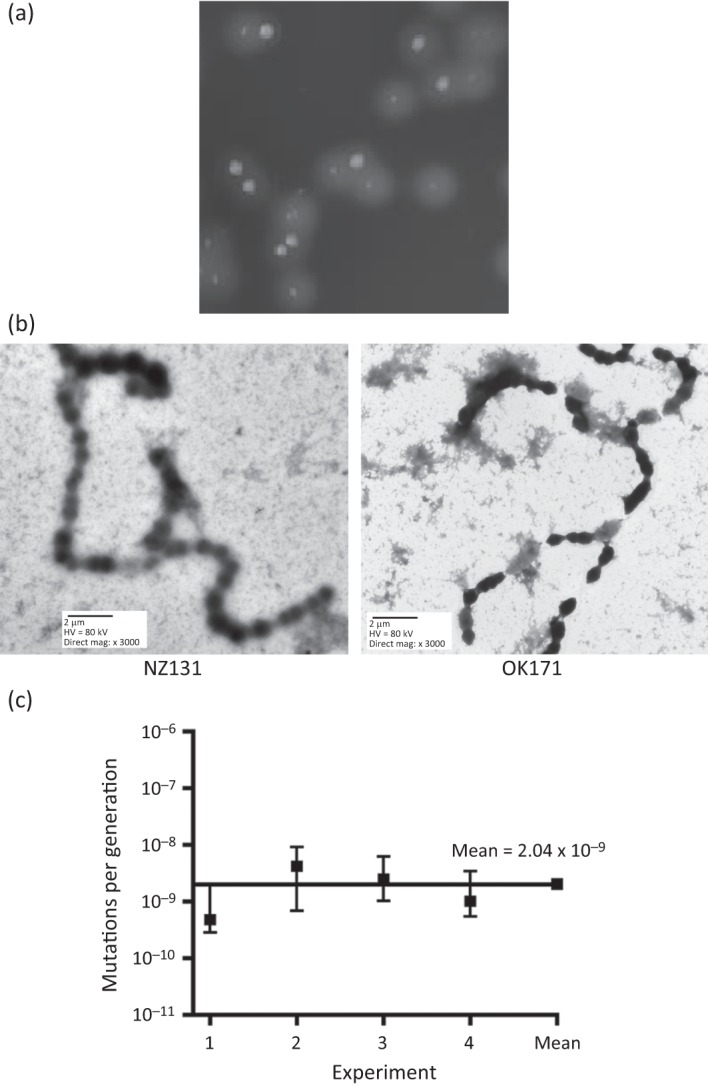
Rate of reversion by OK171 to WT colonial morphology. (a) The large- and small-colony morphologies associated with NZ131 and OK171, respectively, are shown. OK171 was a spontaneous small-colony variant of NZ131 and occasionally generates large-colony revertants. (b) Electron micrographs of NZ131 and OK171. Microscopy showed that OK171 cells have an elongated, ellipsoidal shape, which contrasts with the spherical shape of NZ131. This difference is reflected in the size of the cells, with NZ131 having cells with a mean diameter of 0.9 µm, while OK171 cells have dimensions of 1.0×0.7 µm (length×width). A minimum of five measurements were made for each cell type. (c) Using a modified Luria–Delbruck fluctuation test, the reversion of strain OK171 from small colonial morphology to the large WT morphology was determined in four independent experiments. The software package ft (Shaver & Sniegowski, 2003) calculated the mutations per generation (μ) for each strain; the mean rate of the four trials was μ = 2.04×10−9, a value consistent with our previously published rate for a single-hit mutation (Scott et al., 2008).
Electron microscopy
Electron microscopy of NZ131 and OK171 showed that a morphological change had occurred in strain OK171, which was no longer spherical like NZ131 but now had an ellipsoidal shape (Fig. 7b).
Discussion
We here report the survival of M49 strain NZ131 and its small-colony variant OK171 in long-term culture in rich media. In standard media for the culture of S. pyogenes, both strains survived for a short period (1 and 2 weeks for NZ131 and OK171, respectively); however, both cultures eventually became totally unviable. The loss of viability in both cases was accompanied by a drop in the pH of the culture to below 5.5. Strain NZ131 acidified the media to pH <5.5 by the end of exponential growth on day 1 (Fig. 1), and the kinetics of culture death appeared to be linear over time, with no living cells present after 1 week. By contrast, strain OK171 delayed acidification of the culture to pH <5.5 until about day 5, and then loss of culture viability followed, although death of the culture may follow a brief period of stabilization from days 6 to 9 before final loss of living cells (Figs 1 and 3). S. pyogenes strains SF370 and CS101, the strains employed in earlier long-term culture studies (Wood et al., 2005, 2009), both had survival patterns similar to that of NZ131 (results not shown). Doubling the concentration of glucose in THY medium (final concentration 0.4 %) accelerated the death of the NZ131 cultures, but had little effect on the survival of the OK171 culture (Fig. 5). In contrast, a substantial increase in the glucose concentration to 1.0 % was not reflected in the survival of NZ131 (results not shown) but did decrease to some extent the survival of OK171 (death usually occurring on day 9 or 10; results not shown) as compared with their survival in medium containing 0.4 % glucose (Fig. 5). A possible explanation could be that the capacity of S. pyogenes to transport and catabolize glucose is saturated somewhere between 0.4 and 1 % glucose. Consequently, a higher amount of glucose would not affect the final amount of lactic acid produced by a fixed population of S. pyogenes. The same rationale may not apply to OK171, whose partial deficiency in glucose uptake or catabolism was not affected by the small increment in glucose concentration from 0.2 to 0.4 % (Figs 3 and 5). However, a higher glucose concentration (1 %) might overcome the partial inability of OK171 to use this sugar, resulting in higher levels of lactic acid and a decreased survival time.
These results suggest that the metabolism of glucose to lactic acid with the accompanying lowering of the pH is related directly to the death of the tested cultures. This model is also supported by experiments with buffered medium. When the pH of the medium was controlled by the addition of a buffer, strains NZ131 and OK171 both stabilized their populations at ~105 cells ml−1, and culture viability continued for at least 70 days (Fig. 6).
The experiment with additional glucose argues against the possibility that H2O2 might be the killing agent. Namely, it has been found that induction of H2O2 coincides with the depletion of glucose in the medium (Seki et al., 2004). Similarly, experiments with external acidification of cultures in the early phase of growth when glucose was still abundant in the medium (Fig. 4) also suggest that H2O2 is not the lethal agent. H2O2 might co-participate in killing in the late stationary phase, although the experiment with buffered media, where survival of the bacteria was extended by up to at least 70 days (Fig. 6), when all the glucose was used up, again argues against that possibility.
With the exception of the small-colony derivative of NZ131 (OK171) that survives for up to 11 days, the results presented show that S. pyogenes types M1 and M49 lose the ability to form colonies or to establish growth in fresh medium after 6–7 days of incubation at 37 °C. The data also point to a strong correlation between the acidification of the medium and death of the bacterial culture. Even though the slow-growing mutant demonstrates overall a longer life expectancy than NZ131, when the pH value of the culture drops below about 5.6–5.5, its lifespan beyond that point becomes more or less the same (5–6 days; Fig. 3).
These data are in sharp contrast to the finding elsewhere that pH values in TH medium vary between 5.6 and 6.2 and rarely drop below pH 5.6 (Wood et al., 2005). Trainor and colleagues also studied survival of S. pyogenes in stationary cultures, but their analysis was mostly limited to chemically defined medium. In the single experiment with TH medium, the titre of a stationary culture was reduced by five orders of magnitude after 9 days, with the survival curve tending to a zero value when the experiment was interrupted for reasons that were not explained. Any change in pH values in that study was not reported (Trainor et al., 1999).
The discrepancy between the two studies, both of which employed a simple and straightforward experimental approach and used the same media for bacterial growth [TH broth and yeast extract (Difco)], is surprising. In our studies, we could detect no difference in survival between any of the strains examined, including CS101, the strain employed in the earlier studies of long-term culture of S. pyogenes (Wood et al., 2005, 2009).
The same workers reported in a more recent paper that most cultures of CS101 stabilize at a pH above 5.5, allowing long-term survival of the cells, although many of the cultures that they initiated dropped below pH 5.5, with a loss of culture viability (Wood et al., 2009). They suggested that lot-to-lot variations in commercial media might be responsible for the unexpectedly low pH and loss of survival in these cultures. Nothing similar happened in more than 20 of our experiments in a time span of about a year and half. To explain the unusual inconsistency of the survival pattern (Wood et al., 2009), an alternative explanation is that an isolate of CS101 underwent an uncharacterized mutation that allowed an initial stabilization of cultures that do not acidify the medium to the same extent as WT strains. However, occasional revertants to WT may occur during the early exponential phase, and generate descendants that acidify the media to <pH 5.5 and thereby lead to cell death.
Previous to these studies, we had serendipitously discovered that the spontaneous small-colony variant of NZ131, OK171, was acid-resistant (D. J. Savic & W. M. McShan, unpublished results). Thanks to this strain characteristic, we employed OK171 as an internal control in the experiments with standard S. pyogenes strains. Given that the unusual features of the strain might be of interest for the broader picture of S. pyogenes long-term survival, we also performed several analyses not directly related to the central theme of the study. A fluctuation test (Fig. 7) clearly demonstrated that reversion of the small colony-producing mutation in OK171 is a mutation event and not an epigenetic change, as suggested by Leonard and co-workers for their colonial variants (Leonard et al., 1998). The reversion to the large-colony phenotype by OK171 occurred at the basal mutation rate of ~10−9 mutations per generation (Fig. 7c), suggesting that only one gene is involved in the observed phenotype switches (the reversion frequency of a double mutation would certainly have a double-digit negative exponent). At present, the gene involved is unknown; however, electron microscopy of NZ131 and OK171 showed a change from spherical NZ131 cells to ellipsoidal OK171 cells (Fig. 7b), which may be the result of a mutation in a gene involved in cell wall synthesis or in segregation. The answer should be obtained in future studies, perhaps including sequencing of the genome of OK171 and its comparison with the known genome sequence of NZ131.
The ability of a small-colony variant strain of S. pyogenes OK171 to survive by delaying the acidification of the surrounding environment may provide a clue to one potential mechanism for persistence in natural streptococcal infections. Such variants may be able to survive for an extended period when host defences lower the pH as compared with large-morphology cells, increasing the chance of escape and survival when conditions change. Additionally, while it is currently unclear whether the large and small variants have equal fitness in a natural infection, the ability of each type to generate the other phenotype by random mutation raises the possibility that these alterations may provide a mechanism of survival or enhanced pathogenesis, depending upon the environmental conditions. Small-colony variants that produce persistent infections have been found in many bacterial genera, but they have been most studied for staphylococci (Proctor et al., 2006). In addition to their small colony size, their characteristics include slower growth in liquid medium, decreased concentrations of glycolytic pathway enzymes, decreased oxidative metabolism of sugars, and decreased production of ATP. These phenotypes are reminiscent of some of the features of OK171, and further study of the long-term survival and variability of the small-colony mutant strain OK171 could well shed light on an important mechanism of S. pyogenes persistence and survival in natural infections.
Acknowledgements
This publication was made possible by NIH grant number P20 RR016478 from the INBRE Program of the National Center for Research Resources and by NIH grant number 1R15A1072718 and Oklahoma Center for the Advancement of Science & Technology grant HR11-133 to W. M. M. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations:
- GAS
group A streptococcus
- WT
wild-type
References
- Atlas R. M. (2004). Handbook of Microbiological Media, 3rd edn Boca Raton, FL: CRC Press; 10.1201/9781420039726 [DOI] [Google Scholar]
- Cleary P. P., Cue D. (2000). High frequency invasion of mammalian cells by beta hemolytic streptococci. Subcell Biochem 33, 137–166. [DOI] [PubMed] [Google Scholar]
- Cleary P. P., McLandsborough L., Ikeda L., Cue D., Krawczak J., Lam H. (1998). High-frequency intracellular infection and erythrogenic toxin A expression undergo phase variation in M1 group A streptococci. Mol Microbiol 28, 157–167. 10.1046/j.1365-2958.1998.00786.x [DOI] [PubMed] [Google Scholar]
- Courtney H. S., Hasty D. L., Dale J. B. (2002). Molecular mechanisms of adhesion, colonization, and invasion of group A streptococci. Ann Med 34, 77–87. 10.1080/07853890252953464 [DOI] [PubMed] [Google Scholar]
- Cunningham M. W. (2000). Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 13, 470–511. 10.1128/CMR.13.3.470-511.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev A. V., McDowell E. J., Chaussee M. S. (2008). Inter- and intraserotypic variation in the Streptococcus pyogenes Rgg regulon. FEMS Microbiol Lett 284, 43–51. 10.1111/j.1574-6968.2008.01171.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard T. H., Sledjeski D. D., Boyle M. D. (2001). Mouse skin passage of a Streptococcus pyogenes Tn917 mutant of sagA/pel restores virulence, beta-hemolysis and sagA/pel expression without altering the position or sequence of the transposon. BMC Microbiol 1, 33. 10.1186/1471-2180-1-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facinelli B., Spinaci C., Magi G., Giovanetti E., Varaldo P. E. (2001). Association between erythromycin resistance and ability to enter human respiratory cells in group A streptococci. Lancet 358, 30–33. 10.1016/S0140-6736(00)05253-3 [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., McShan W. M., Ajdic D., Savic D. J., Savic G., Lyon K., Primeaux C., Sezate S., Suvorov A. N., et al. (2001). Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc Natl Acad Sci U S A 98, 4658–4663. 10.1073/pnas.071559398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluckiger U., Jones K. F., Fischetti V. A. (1998). Immunoglobulins to group A streptococcal surface molecules decrease adherence to and invasion of human pharyngeal cells. Infect Immun 66, 974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haanes E. J., Heath D. G., Cleary P. P. (1992). Architecture of the vir regulons of group A streptococci parallels opacity factor phenotype and M protein class. J Bacteriol 174, 4967–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes W. (2004). Virulence factors of the group A streptococci and genes that regulate their expression. Front Biosci 9, 3399–3433. 10.2741/1491 [DOI] [PubMed] [Google Scholar]
- Kaplan E. L., Oakes J. M., Johnson D. R. (2007). Unexpected individual clinical site variation in eradication rates of group A streptococci by penicillin in multisite clinical trials. Pediatr Infect Dis J 26, 1110–1116. 10.1097/INF.0b013e31814615ac [DOI] [PubMed] [Google Scholar]
- Leonard B. A., Woischnik M., Podbielski A. (1998). Production of stabilized virulence factor-negative variants by group A streptococci during stationary phase. Infect Immun 66, 3841–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty M. (1966). The nature of the opaque colony variation in group A streptococci. J Hyg (Lond) 64, 185–190. 10.1017/S0022172400040444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShan W. M., Ferretti J. J., Karasawa T., Suvorov A. N., Lin S., Qin B., Jia H., Kenton S., Najar F., et al. (2008). Genome sequence of a nephritogenic and highly transformable M49 strain of Streptococcus pyogenes. J Bacteriol 190, 7773–7785. 10.1128/JB.00672-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari G., Chhatwal G. S. (1999). Streptococcal invasion. Curr Opin Microbiol 2, 56–61. 10.1016/S1369-5274(99)80010-1 [DOI] [PubMed] [Google Scholar]
- Österlund A., Engstrand L. (1997). An intracellular sanctuary for Streptococcus pyogenes in human tonsillar epithelium–studies of asymptomatic carriers and in vitro cultured biopsies. Acta Otolaryngol 117, 883–888. 10.3109/00016489709114219 [DOI] [PubMed] [Google Scholar]
- Österlund A., Popa R., Nikkilä T., Scheynius A., Engstrand L. (1997). Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope 107, 640–647. 10.1097/00005537-199705000-00016 [DOI] [PubMed] [Google Scholar]
- Pletz M. W., McGee L., Van Beneden C. A., Petit S., Bardsley M., Barlow M., Klugman K. P. (2006). Fluoroquinolone resistance in invasive Streptococcus pyogenes isolates due to spontaneous mutation and horizontal gene transfer. Antimicrob Agents Chemother 50, 943–948. 10.1128/AAC.50.3.943-948.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., von Eiff C., Kahl B. C., Becker K., McNamara P., Herrmann M., Peters G. (2006). Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4, 295–305. 10.1038/nrmicro1384 [DOI] [PubMed] [Google Scholar]
- Rosche W. A., Foster P. L. (2000). Determining mutation rates in bacterial populations. Methods 20, 4–17. 10.1006/meth.1999.0901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Slomska J., Boué A., Caravano R. (1972). Induction of L-variants in human diploid cells infected by group A streptococci. Infect Immun 5, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J., Thompson-Mayberry P., Lahmamsi S., King C. J., McShan W. M. (2008). Phage-associated mutator phenotype in group A streptococcus. J Bacteriol 190, 6290–6301. 10.1128/JB.01569-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M., Iida K.-I., Saito M., Nakayama H., Yoshida S.-I. (2004). Hydrogen peroxide production in Streptococcus pyogenes: involvement of lactate oxidase in coupling with aerobic utilization of lactate. J Bacteriol 186, 2046–2051. 10.1128/JB.186.7.2046-2051.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver A. C., Sniegowski P. D. (2003). Spontaneously arising mutL mutators in evolving Escherichia coli populations are the result of changes in repeat length. J Bacteriol 185, 6076–6082. 10.1128/JB.185.20.6076-6082.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor V. C., Udy R. K., Bremer P. J., Cook G. M. (1999). Survival of Streptococcus pyogenes under stress and starvation. FEMS Microbiol Lett 176, 421–428. 10.1111/j.1574-6968.1999.tb13692.x [DOI] [PubMed] [Google Scholar]
- Wood D. N., Chaussee M. A., Chaussee M. S., Buttaro B. A. (2005). Persistence of Streptococcus pyogenes in stationary-phase cultures. J Bacteriol 187, 3319–3328. 10.1128/JB.187.10.3319-3328.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. N., Weinstein K. E., Podbielski A., Kreikemeyer B., Gaughan J. P., Valentine S., Buttaro B. A. (2009). Generation of metabolically diverse strains of Streptococcus pyogenes during survival in stationary phase. J Bacteriol 191, 6242–6252. 10.1128/JB.00440-09 [DOI] [PMC free article] [PubMed] [Google Scholar]