Abstract
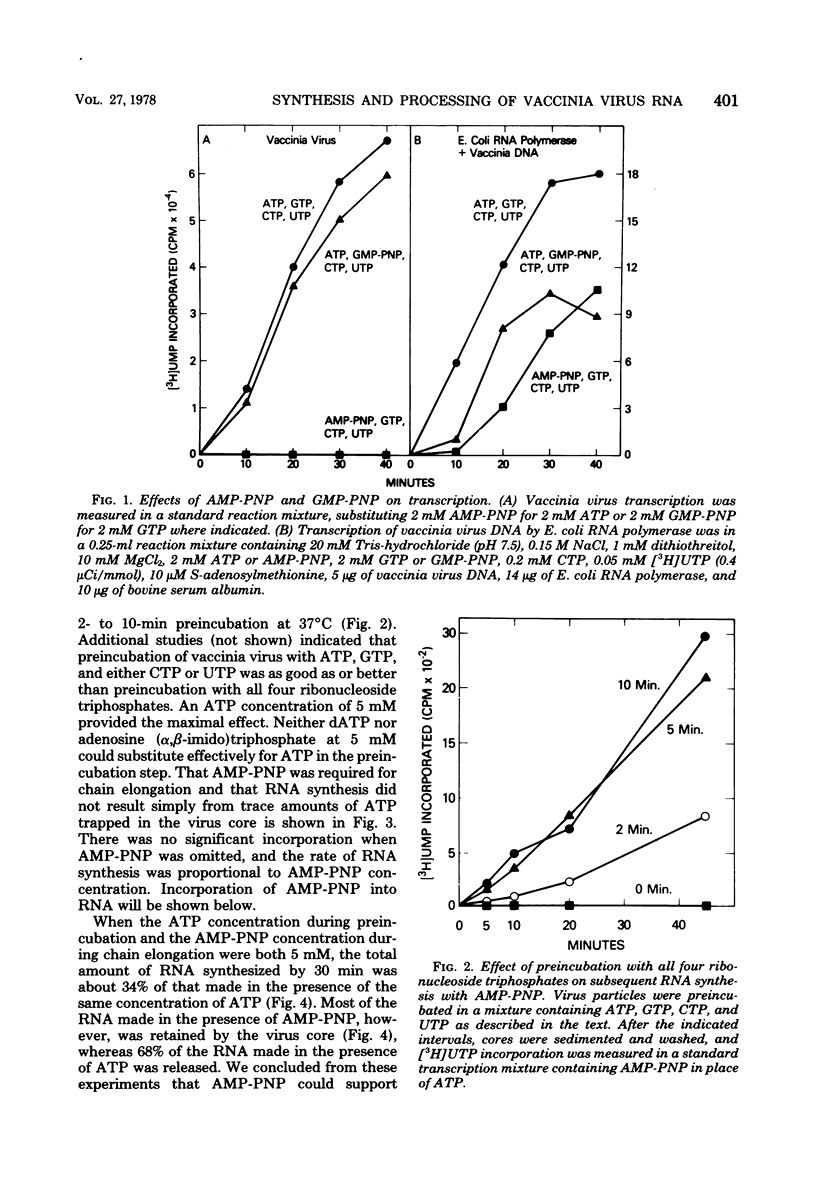
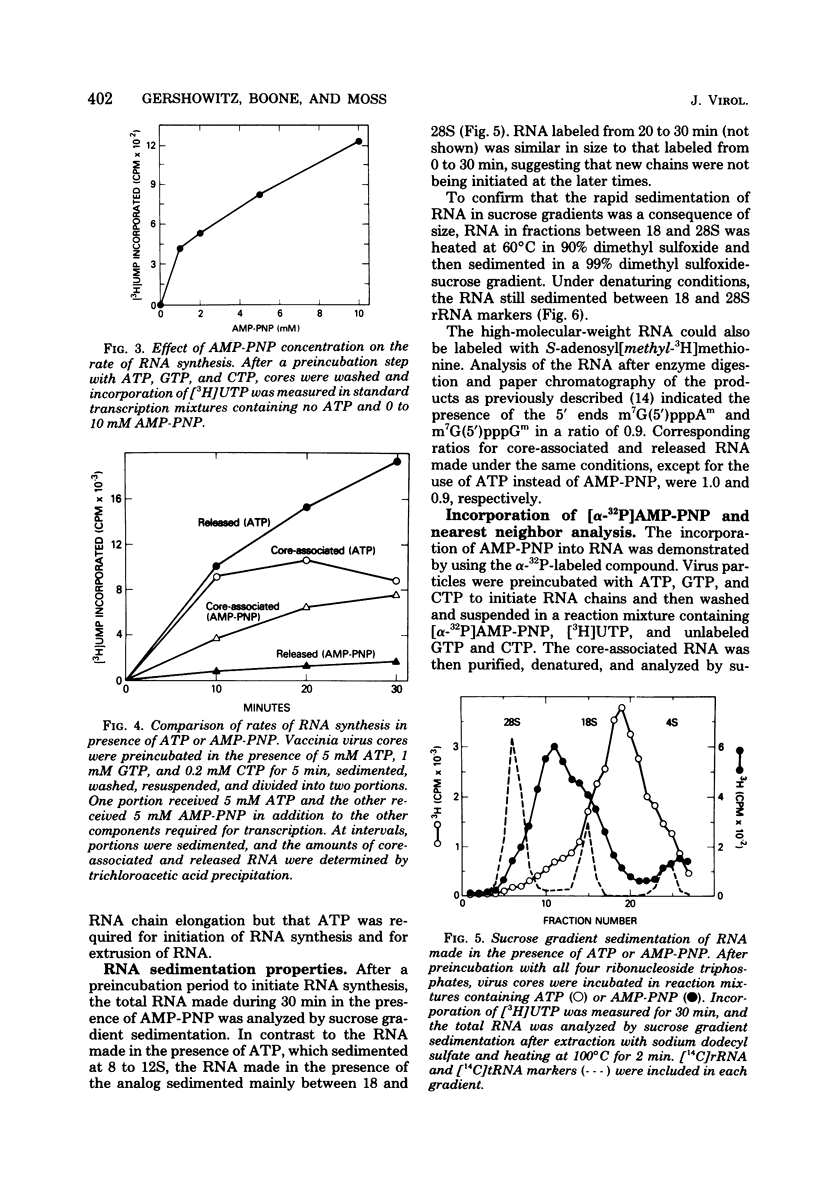
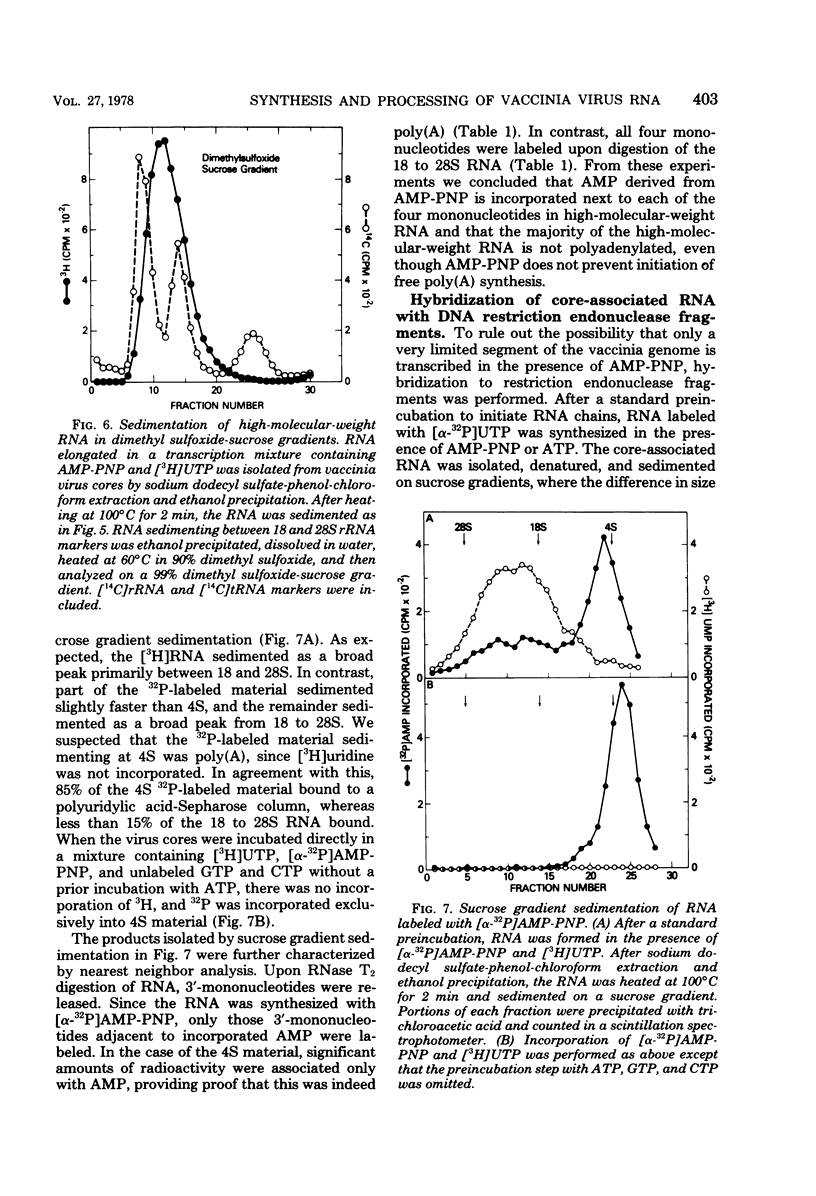
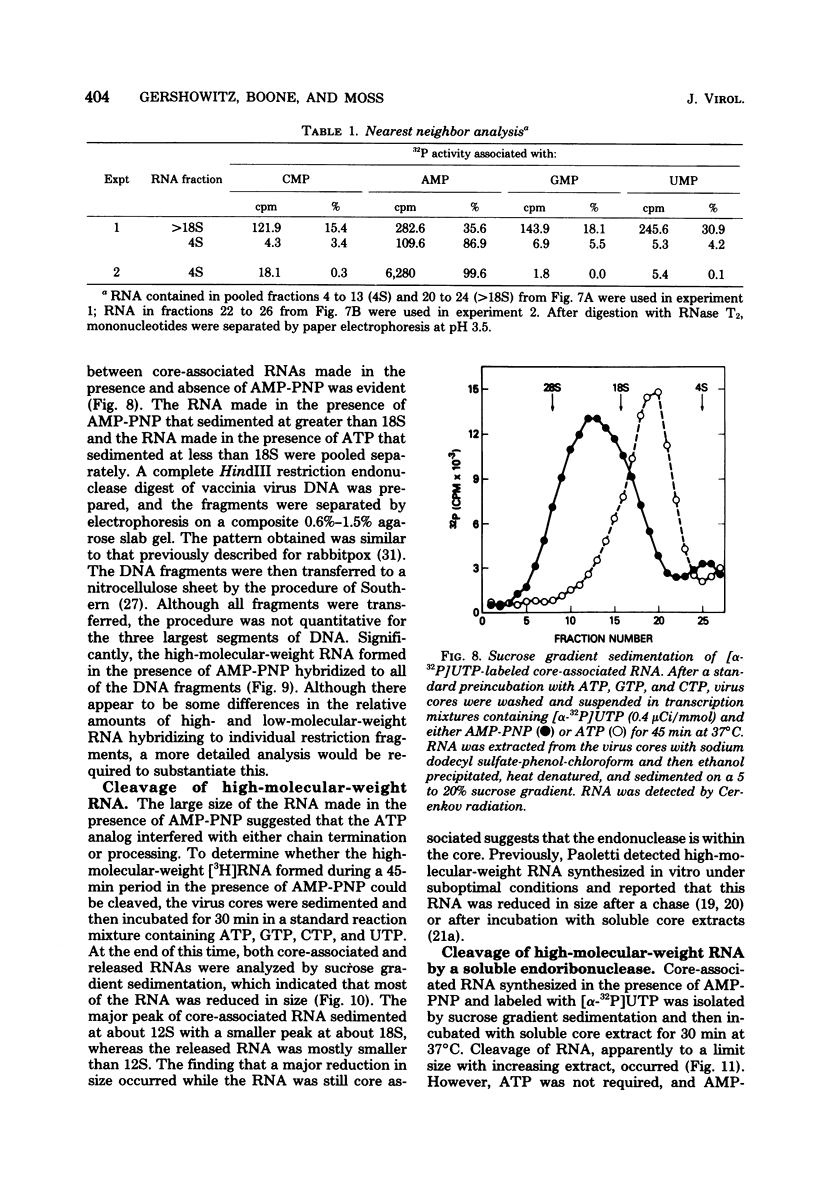
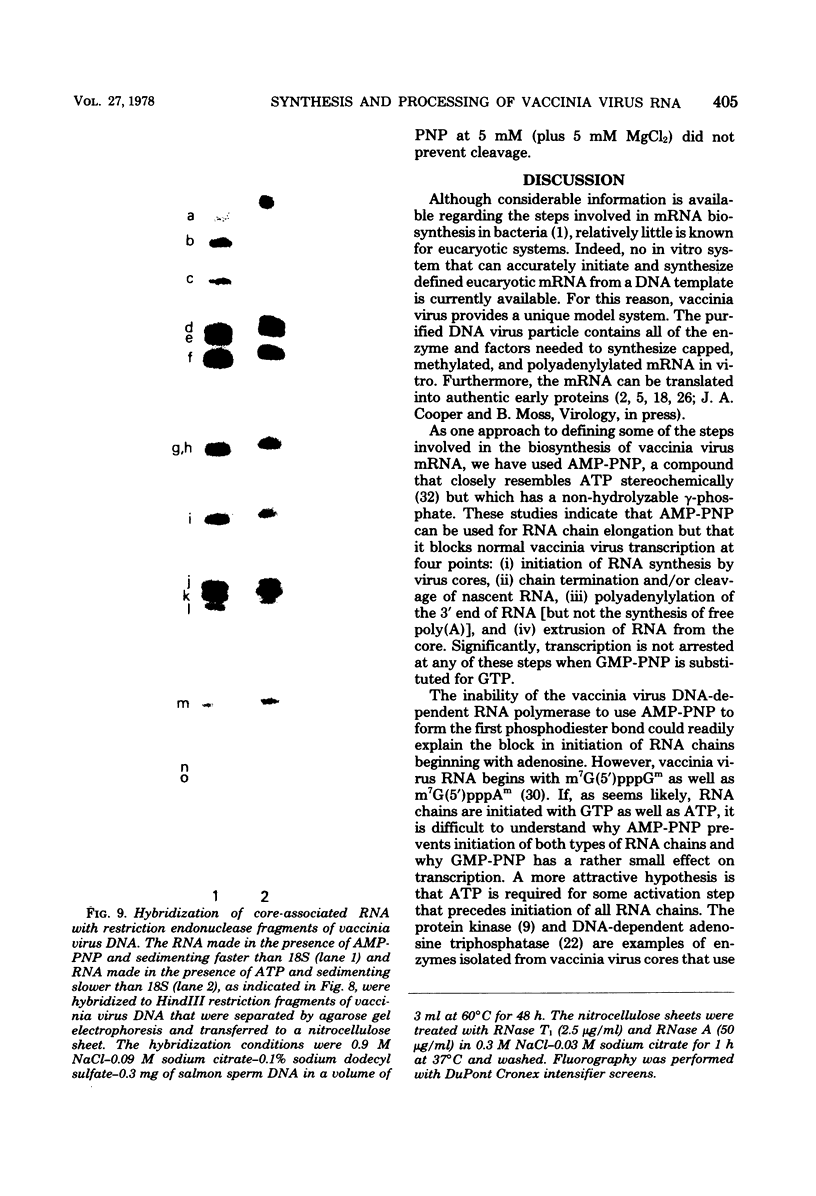
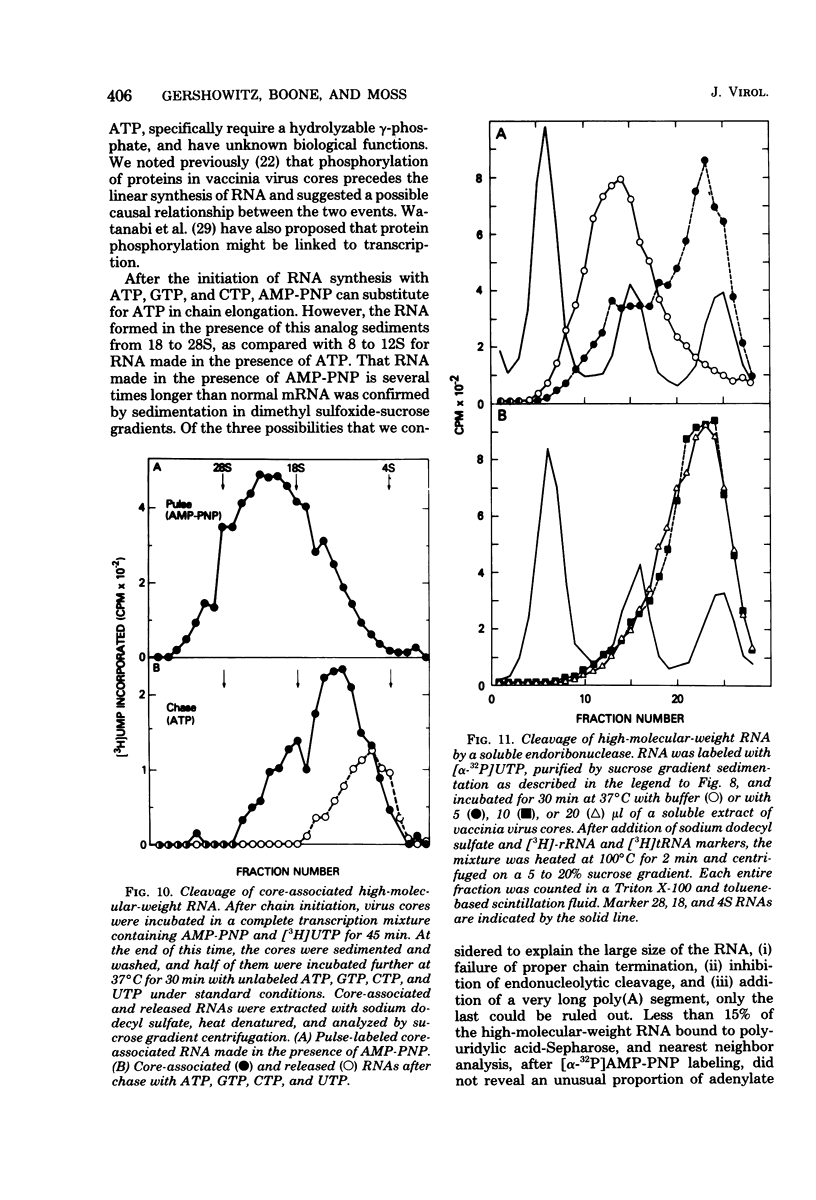
Adenosine (β,γ-imido)triphosphate (AMP-PNP) and guanosine (β,γ-imido)triphosphate (GMP-PNP) are analogs of ATP and GTP with non-hydrolyzable γ-phosphates. Although both AMP-PNP and GMP-PNP were used in place of ATP and GTP by Escherichia coli RNA polymerase to transcribe vaccinia virus DNA, only GMP-PNP was used by the transcriptase present within vaccinia virus cores. AMP-PNP specifically prevented initiation of transcription, since RNA initiated in the presence of ATP, GTP, and CTP was subsequently elongated by incubating the washed cores in the presence of AMP-PNP, GTP, CTP, and UTP. The RNA formed in this manner, however, was (i) several times longer than normal transcripts, indicating a defect in chain termination and/or cleavage of nascent RNA, (ii) was not polyadenylylated (although free polyadenylic acid formed), and (iii) was not extruded from the virus cores. Nearest neighbor analysis demonstrated that AMP-PNP was incorporated adjacent to all four nucleotides, and hybridization to restriction endonuclease fragments of vaccinia virus DNA indicated that the high-molecular-weight RNA was transcribed from representative fractions of the entire genome. The possibility of a block in processing rather than or in addition to a block in chain termination was suggested by the cleavage of the high-molecular-weight RNA within the core after replacement of AMP-PNP with ATP. Cleavage of purified high-molecular-weight RNA by a soluble endoribonuclease extracted from vaccinia virus cores, however, was not dependent upon ATP, nor was it inhibited by AMP-PNP. The latter results suggest that AMP-PNP blocks a step preceding cleavage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fournier F., Tovell D. R., Esteban M., Metz D. H., Ball L. A., Kerr I. M. The translation of vaccinia virus messenger RNA in animal cell-free systems. FEBS Lett. 1973 Mar 15;30(3):268–272. doi: 10.1016/0014-5793(73)80667-2. [DOI] [PubMed] [Google Scholar]
- Gold P. H., Dales S. Localization of nucleotide phosphohydrolase activity within vaccinia. Proc Natl Acad Sci U S A. 1968 Jul;60(3):845–852. doi: 10.1073/pnas.60.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. H., de Crombrugghe B. ATPase activity required for termination of transcription by the Escherichia coli protein factor rho. J Biol Chem. 1976 Apr 25;251(8):2520–2524. [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Jaureguiberry G., Ben-Hamida F., Chapeville F., Beaud G. Messenger activity of RNA transcribed in vitro by DNA-RNA polymerase associated to vaccinia virus cores. J Virol. 1975 Jun;15(6):1467–1474. doi: 10.1128/jvi.15.6.1467-1474.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. I. The mechanism of synthesis and release of RNA in vaccinia cores. J Mol Biol. 1970 May 28;50(1):1–18. doi: 10.1016/0022-2836(70)90100-2. [DOI] [PubMed] [Google Scholar]
- Kleiman J. H., Moss B. Characterization of a protein kinase and two phosphate acceptor proteins from vaccinia virions. J Biol Chem. 1975 Apr 10;250(7):2430–2437. [PubMed] [Google Scholar]
- Kleiman J. H., Moss B. Purification of a protein kinase and two phosphate acceptor proteins from vaccinia virions. J Biol Chem. 1975 Apr 10;250(7):2420–2429. [PubMed] [Google Scholar]
- Lowery-Goldhammer C., Richardson J. P. An RNA-dependent nucleoside triphosphate phosphohydrolase (ATPase) associated with rho termination factor. Proc Natl Acad Sci U S A. 1974 May;71(5):2003–2007. doi: 10.1073/pnas.71.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Moss B. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9330–9335. [PubMed] [Google Scholar]
- Martin S. A., Moss B. mRNA guanylyltransferase and mRNA (guanine-7-)-methyltransferase from vaccinia virions. Donor and acceptor substrate specificites. J Biol Chem. 1976 Dec 10;251(23):7313–7321. [PubMed] [Google Scholar]
- Moss B., Gershowitz A., Wei C. M., Boone R. Formation of the guanylylated and methylated 5'-terminus of vaccinia virus mRNA. Virology. 1976 Jul 15;72(2):341–351. doi: 10.1016/0042-6822(76)90163-x. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Gershowitz A. Characterization of a polyriboadenylate polymerase from vaccinia virions. J Biol Chem. 1975 Jun 25;250(12):4722–4729. [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Grace J. T., Jr RNA polymerase activity in purified infectious vaccinia virus. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2280–2287. doi: 10.1073/pnas.58.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Ospina J., Grace J. T., Jr Nucleotide phosphohydrolase in purified vaccinia virus. J Virol. 1968 Mar;2(3):167–172. doi: 10.1128/jvi.2.3.167-172.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Poly (A) sequences of vaccinia virus messenger RNA: nature, mode of addition and function during translation in vitra and in vivo. Virology. 1975 Jan;63(1):1–14. doi: 10.1016/0042-6822(75)90365-7. [DOI] [PubMed] [Google Scholar]
- Paolette E., Rosemond-Hornbeak H., Moss B. Two nucleid acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3273–3280. [PubMed] [Google Scholar]
- Paoletti E., Cooper N., Moss B. Regulation of synthesis of two immunologically distinct nucleic acid-dependent nucleoside triphosphate phosphohydrolases in vaccinia virus-infected HeLa cells. J Virol. 1974 Sep;14(3):578–586. doi: 10.1128/jvi.14.3.578-586.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E. High molecular weight virion-associated RNA of vaccinia. A possible precursor to 8 to 12 S mRNA. J Biol Chem. 1977 Feb 10;252(3):872–877. [PubMed] [Google Scholar]
- Paoletti E. In vitro synthesis of a high molecular weight virion-associated RNA by vaccinia. J Biol Chem. 1977 Feb 10;252(3):866–871. [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R. Soluble endoribonuclease activity from vaccinia virus: specific cleavage of virion-associated high-molecular-weight RNA. J Virol. 1978 Jun;26(3):822–824. doi: 10.1128/jvi.26.3.822-824.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Protein kinase and specific phosphate acceptor proteins associated with vaccinia virus cores. J Virol. 1972 Sep;10(3):417–424. doi: 10.1128/jvi.10.3.417-424.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Two nucleic acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Nucleotide substrate and polynucleotide cofactor specificities. J Biol Chem. 1974 May 25;249(10):3281–3286. [PubMed] [Google Scholar]
- Pelham H. R., Sykes J. M., Hunt T. Characteristics of a coupled cell-free transcription and translation system directed by vaccinia cores. Eur J Biochem. 1978 Jan 2;82(1):199–209. doi: 10.1111/j.1432-1033.1978.tb12012.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Use of coupled transcription and translation to study mRNA production by vaccinia cores. Nature. 1977 Oct 6;269(5628):532–534. doi: 10.1038/269532a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Sakuma S., Tanaka S. A possible biological function of the protein kinase associated with vaccinia and vesicular stomatitis virions. FEBS Lett. 1974 May 1;41(2):331–334. doi: 10.1016/0014-5793(74)81241-x. [DOI] [PubMed] [Google Scholar]
- Wei C. M., Moss B. Methylated nucleotides block 5'-terminus of vaccinia virus messenger RNA. Proc Natl Acad Sci U S A. 1975 Jan;72(1):318–322. doi: 10.1073/pnas.72.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Menna A., Schümperli D., Stoffel S., Müller H. K., Wyler R. HindIII and Sst I restriction sites mapped on rabbit poxvirus and vaccinia virus DNA. J Virol. 1977 Sep;23(3):669–678. doi: 10.1128/jvi.23.3.669-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]