Abstract
Dynamin2 is a large GTPase that regulates vesicle trafficking, and the GTPase activity of dynamin2 is required for the multistep process of adenovirus infection. Activity of dynamin2 may be regulated by post-translational phosphorylation and S-nitrosylation modifications. In this study, we demonstrate a role for dynamin2 S-nitrosylation in adenovirus infection of epithelial cells. We show that adenovirus serotype 5 (Ad5) infection augments production of nitric oxide (NO) in epithelial cells and causes the S-nitrosylation of dynamin2, mainly on cysteine 86 (C86) and 607 (C607) residues. Forced overexpression of dynamin2 bearing C86A and/or C607A mutations decreases Ad5 infection. Diminishing NO synthesis by RNAi-induced knockdown of endogenous endothelial NO synthase (eNOS) expression attenuates virus infection of target cells. Ad5 infection promotes the kinetically dynamic S-nitrosylation of dynamin2 and eNOS: there is a rapid decrease in eNOS S-nitrosylation and a concomitant increase in the dynamin2 S-nitrosylation. These results support the hypothesis that dynamin2 S-nitrosylation following eNOS activation facilitates adenovirus infection of host epithelial cells.
Introduction
Adenoviruses are non-enveloped, icosahedral DNA viruses about 90 nm in diameter that spread widely in the human population. In fact, the majority of the human population will have experienced at least one adenoviral infection by the age of 10 years (Echavarría, 2008). Currently, there are 56 known immunologically distinct types of adenovirus that can infect humans (Kennedy & Parks, 2009). Depending on the organ in the body that is affected, the outcomes of adenoviral infections may be haemorrhagic cystitis, haemorrhagic colitis, pancreatitis, nephritis or encephalitis (Lynch et al., 2011).
The life cycle of adenovirus consists of early and late phases that correspond with viral DNA replication and assembly, respectively (McConnell & Imperiale, 2004). In the early phase, E1A is the first expressed adenovirus transcript and functions to transactivate other early viral transcription units, which include E1B, E2, E3 and E4 (Berk, 1986). The early transcription units exert additional functions during adenovirus replication. For example, E1B-encoded protein can inhibit infected cell apoptosis, products of E2 are essential to initiate viral genome replication, E3-encoded protein functions to destabilize host immune reaction, and the proteins of the E4 region are responsible for cell cycle control and transformation (Ben-Israel & Kleinberger, 2002; de Jong et al., 2003). In the late phase, the major late promoter facilitates transcription of five regions, L1–L5 that lead to the production of sufficient structural proteins to ensure viral assembly (McConnell & Imperiale, 2004; Vellinga et al., 2004; Vigne et al., 1999).
Entry involves two sets of interactions between the virus and the host cell. First, binding of virus fibre protein through the knob domains to particular host cell receptors initiates the infection. Two such receptors are currently known to function in adenovirus infection of human cells, namely coxsackievirus adenovirus receptor and group B adenovirus serotype-specific CD46 (Bergelson et al., 1997). Several studies have reported that major histocompatibility complex molecules and sialic acid residues may contribute to virus adherence. Second, a specialized motif in the virus penton base protein interacts with cellular αvβ3 and αvβ5 integrins (Wickham et al., 1993), resulting in endocytosis of the virus particle via clathrin-coated pits. Virus attachment to αvβ3 and αvβ5 integrins can activate phosphoinositide 3-kinase (PI3K) that phosphorylates PIP2 to generate PIP3, which, in turn, regulates vesicle trafficking (Nemerow & Stewart, 1999).
The most commonly used portal of adenovirus entry involves clathrin-dependent endocytosis, where dynamin plays a critical role by ‘pinching off’ invaginated virus-containing vesicles from the plasma membrane (Conner & Schmid, 2003). The primary structure of dynamin comprises five domains: N-terminal GTPase, middle, pleckstrin homology (PH), GTPase effector and C-terminal proline-rich (Conner & Schmid, 2003; Praefcke & McMahon, 2004). Ubiquitously expressed dynamin2 has been implicated in many cellular functions, including receptor-mediated endocytosis, intracellular membrane trafficking, actin reorganization and apoptosis (Fish et al., 2000; Mooren et al., 2009; Tanabe & Takei, 2009). The activity of dynamin2 is regulated by self-assembly into higher-order aggregates, thereby leading to increased GTPase activity (Conner & Schmid, 2003; Durieux et al., 2010). In addition to self-assembly, dynamin2 activity may be regulated by post-translational modifications such as phosphorylation and S-nitrosylation (Ahn et al., 1999, 2002; Kang-Decker et al., 2007; Wang et al., 2006, 2011) that play important roles in the enzyme’s subcellular localization and GTPase activity.
Among the adenovirus types, entry of species C adenovirus-2 (Ad2) and adenovirus-5 (Ad5) is best characterized. Ad2/Ad5 internalization is clathrin-dependent and involves dynamin (Gastaldelli et al., 2008; Meier & Greber, 2004; Wang et al., 1998; Wickham et al., 1993). In this study, we examined mechanisms involved in the dynamin2-dependent Ad5 infection of host epithelial cells with the emphasis on dynamin2 post-translational modification by S-nitrosylation. Our results suggest that dynamin2 S-nitrosylation plays an important role in Ad5 infection of epithelial cells and that Ad5 infection induces the dynamic, but reciprocal, endothelial nitric oxide synthase (eNOS) and dynamin2 S-nitrosylation. These findings provide a mechanistic understanding of adenovirus infection and present rationale to target dynamin2 S-nitrosylation to control this common viral infection.
Results
Nitric oxide (NO) regulates Ad5–DsRed infection
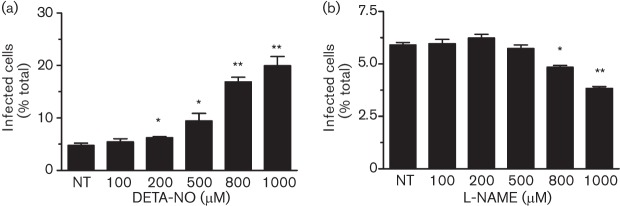
Ad5–DsRed virus encodes a Discosoma sp. red fluorescent protein (DsRed) and is thus a practical tool for quantifying the number of infected cells by flow cytometry. Further, Ad5–DsRed lacks the E1 gene, rendering it capable of infecting but not replicating in the host cells. We used Ad5–DsRed to quantify the initial virus entry into host human bladder epithelial cancer (BEC) cells. Cells were pretreated with NO donor NONOate diethylenetriamine (DETA-NO), which elicited a dose-dependent increase in the virus infection (Fig. 1a). BEC cells endogenously express NOSs (Heeringa et al., 1998) and the treatment with NOS inhibitor L-NAME resulted in a significant reduction of virus infection (Fig. 1b). Admittedly, infectivity under basal conditions was modest; only about 5 % of cells became infected. These results show that NO may regulate Ad5–DsRed infection of host human BEC cells.
Fig. 1.
Effect of NO on Ad5–DsRed infection of epithelial cells. (a) BEC cells were treated with escalating doses of DETA-NO for 8 h before mixing with Ad5–DsRed. (b) BEC cells were treated with increasing concentrations of L-NAME for 8 h before mixing with Ad5–DsRed. In both cases, virus internalization was analysed 36 h after infection. Experiments were repeated three times and data are expressed as mean±sem. *, P<0.05 and **, P<0.01 versus non-treated (NT) samples.
Dynamin2 is S-nitrosylated on residues C86 and C607 in response to Ad5–DsRed infection
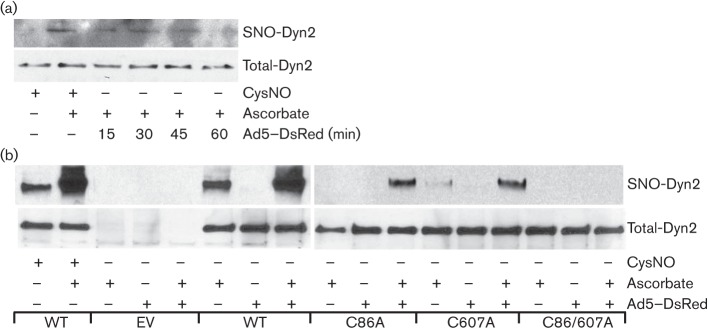
It is well established that Ad5 endocytosis is a clathrin-dependent event that involves dynamin GTPase activity (Gastaldelli et al., 2008; Greber et al., 1993; Wickham et al., 1993). Given that dynamin2 activity is controlled by post-translational modifications (Ahn et al., 1999, 2002; Kang-Decker et al., 2007; Wang et al., 2006) and our recent finding that dynamin2 regulates uropathogenic Escherichia coli invasion (Wang et al., 2011), we tested if virus infection regulates dynamin2 S-nitrosylation. The results show that Ad5–DsRed infection promoted the time-regulated S-nitrosylation of endogenous dynamin2 (Fig. 2a and b). We and others have previously reported that dynamin2 is S-nitrosylated primarily on the C86 and C607 residues and that S-nitrosylation of these residues controls GTPase activity (Kang-Decker et al., 2007; Wang et al., 2011). To determine the functional relevance of these two sites with regard to the Ad5–DsRed infection, BEC cells were transfected with wild-type (WT), C86A, C607A or C86/607A mutated forms of dynamin2, and S-nitrosylation of overexpressed dynamin2 was analysed post-Ad5–DsRed infection. The results indicate that overexpressed C86A or C607A mutated forms of dynamin2 had significantly reduced S-nitrosylation signal both at the basal level and in response to Ad5–DsRed infection (Fig. 2b). Furthermore, the C86/607A mutant dynamin2 had completely lost its S-nitrosylation signal (Fig. 2b), suggesting that Ad5–DsRed infection induces S-nitrosylation of dynamin2 principally on two critical cysteine residues, C86 and C607.
Fig. 2.
Ad5–DsRed infection causes dynamin2 S-nitrosylation. (a) Ad5–DsRed infection promotes endogenous dynamin2 S-nitrosylation. BEC cells were infected with Ad5–DsRed for the indicated time and equal amounts of cell lysate were subjected to the biotin switch assay (top). (b) Ad5–DsRed infection promotes dynamin2 S-nitrosylation on C86 and C607 residues. BEC cells were transfected with cDNAs encoding HA-tagged dynamin2 [wild-type (WT), C86A, C607A, or C86/607A]. After 48 h, cells were mixed, or not, with Ad5–DsRed for 30 min. Cell lysates were harvested and subjected to the biotin switch method (top). For both panels, total cell lysates were immunoblotted with anti-dynamin2 antibody to show the equal protein loading (bottom), and lysate from cells treated with CysNO (100 µM) was used as a positive control. SNO-Dyn2, S-nitrosylated dynamin2.
S-nitrosylation of dynamin2 is vital for Ad5–DsRed infection
To test if the S-nitrosylation of dynamin2 on C86 and C607 is regulatory for the adenovirus infection, BEC cells transfected with WT or mutant forms of dynamin2 (Fig. 3a) were infected with Ad5–DsRed and the efficiency of infection was measured by flow cytometry. Dynamin2 K44A mutant is a GTPase-deficient form that inhibits vesicle trafficking and was previously shown to significantly reduce adenovirus infection (Wang et al., 1998). Overexpression of WT dynamin2 increased, while dominant-negative dynamin2 K44A significantly reduced the adenovirus infection (Fig. 3b). Concordantly, forced overexpression of individual dynamin2 C86A, C607A or C86/607A caused a significant decrease in Ad5–DsRed infection, compared with empty vector-transfected BEC cells (Fig. 3b). These results suggest a correlation between dynamin2 S-nitrosylation and Ad5–DsRed infection.
Fig. 3.
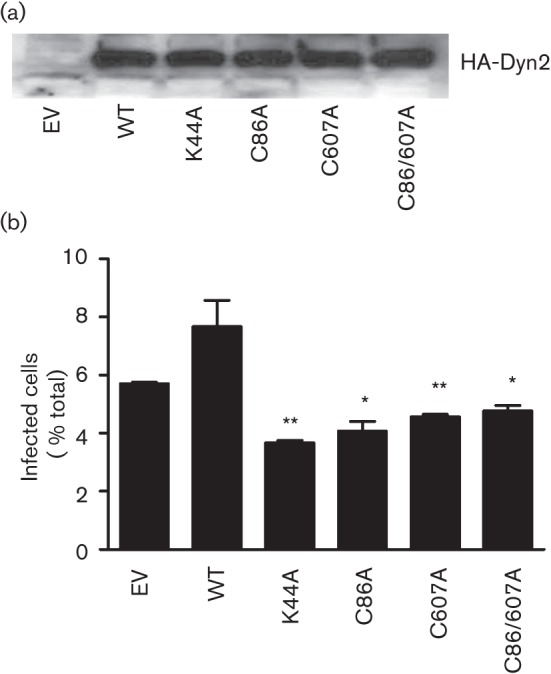
Requirement of dynamin2 S-nitrosylation for Ad5–DsRed entry into cells. BEC cells were transfected with cDNAs encoding HA-tagged dynamin2 [wild-type (WT), K44A, C86A, C607A and C86/607A] for 48 h, and then infected with Ad5–DsRed for 24 h. (a) Cells were harvested and subjected to immunoblot analysis with an anti-HA antibody. (b) Virus infection efficiency was analysed by flow cytometry, exactly as described for Fig. 1. Experiments were repeated three times and data are expressed as mean±sem. *, P<0.05, **, P<0.01 versus empty vector (EV)-transfected cells.
Ad5–DsRed infection of target cells increases NO concentration
Diaminofluoresceins (DAFs) are fluorescence indicators that may be used to detect the release of NO from cells (Nakatsubo et al., 1998). We used DAFs to measure NO in cells following Ad5–DsRed infection. First, to validate specificity and sensitivity of DAFs to detect NO, cells were loaded with 4,5-diaminofluorescein diacetate (DAF-2DA) and treated with the endogenous NO donor S-nitrosoglutathione (GSNO). Time lapse images of fluorescent DAF-2T evidenced increased NO signals following GSNO treatment (data not shown). Remarkably, Ad5–DsRed infection also promoted the time-dependent NO production, which was detected at 15 min, peaked at 30 min and significantly decreased at 60 min (Fig. 4a). The time frame of NO production parallels that of virus endocytosis (Sanlioglu et al., 2000), suggesting a relationship between NO production and Ad5 infection.
Fig. 4.
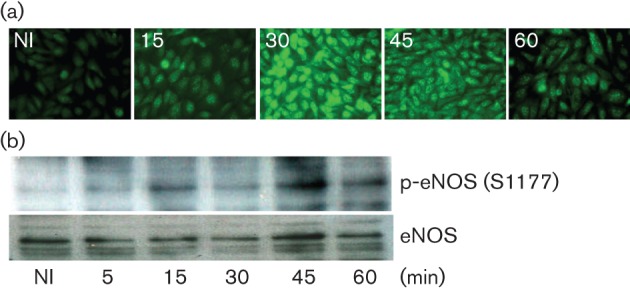
Effect of Ad5–DsRed on NOS activation. (a) Ad5–DsRed infection promotes NO production. BEC cells were infected with Ad5–DsRed for the indicated time and then loaded with DAF-2DA for 2 min. The representative images were acquired using a Leica DM 6000 microscope at the indicated time (in min). (b) eNOS is phosphorylated at S1177 upon Ad5–DsRed infection. BEC cells were mixed with Ad5–DsRed for the indicated time and cell lysates were analysed by immunoblotting with an antibody against phospho-S1177-eNOS. The nitrocellulose membrane was stripped and immunoblotted with an antibody against eNOS to establish equal protein loading.
There are three isoforms of NOS, namely nNOS (NOS I), iNOS (NOS II) and eNOS (NOSIII). We focused our attention on eNOS based on the findings that in model mouse lungs, iNOS expression is significantly induced by adenovirus only days after infection (Zsengellér et al., 2001), nNOS expression is generally low in epithelial cells, and our previous studies linking dynamin2 S-nitrosylation to eNOS. The eNOS is phosphorylated at multiple serine and threonine residues that dictate its activation. Among the potential phosphorylation sites, S1177 is crucial for enzymic activity (Dudzinski et al., 2006). Phosphorylation of eNOS at S1177 significantly increased within 5 min of virus infection, and was detectable up to 60 min (Figs 4b and 7). These results provide evidence that Ad5–DsRed infection activated eNOS, thereby promoting NO production in vivo.
eNOS activation can directly mediate dynamin2 S-nitrosylation
Based on the observations that dynamin2 forms a complex with eNOS and that dynamin2 can be S-nitrosylated, we investigated if eNOS mediates dynamin2 S-nitrosylation. COS-7 cells do not express detectable eNOS (Erwin et al., 2005) and were used in the next set of experiments. WT eNOS and HA-tagged dynamin2 were co-transfected into COS-7 cells and both NO concentrations and dynamin2 S-nitrosylation were determined. Cells expressing eNOS, alone or together with dynamin2, exhibited significant increases in NO levels compared with control cells (Fig. 5a). Remarkably, dynamin2 was S-nitrosylated when co-expressed with eNOS (Fig. 5b). These results imply a direct relationship between eNOS activation and dynamin2 S-nitrosylation.
Fig. 5.
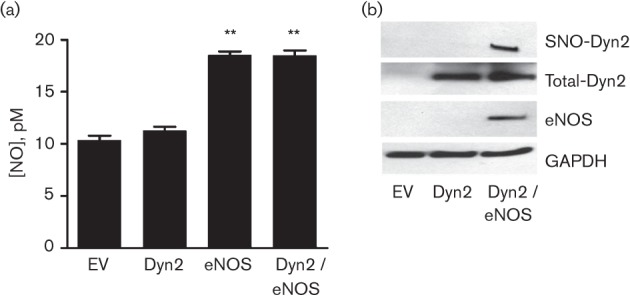
eNOS promotes dynamin2 S-nitrosylation. (a) COS-7 cells were transfected with cDNAs encoding HA-tagged dynamin2, WT bovine eNOS or both. After 24 h, the culture medium from the different transfected groups was mixed with an equal volume of ethanol for 20 min, followed by centrifugation. NO levels in the supernatant were quantified by a chemiluminescence detector after reaction with ozone using an NO analyser (Sievers). Experiments were repeated three times and data are expressed as mean±sem. **, P<0.01 versus empty vector (EV) pcDNA3.1-transfected cells. (b) Transfected COS-7 cells were harvested and analysed for dynamin2 S-nitrosylation (SNO-Dyn2) using the biotin switch assay. The cell lysates were immunoblotted with antibodies against dynamin2 (Total-Dyn2), eNOS or GAPDH.
eNOS is key in Ad5 infection
Two opposing approaches were used to further study the role of eNOS in virus infection, by either increasing (Fig. 6a, upper panels) or decreasing (Fig. 6b, upper panels) the expression of eNOS in human BEC cells prior to virus infection. Forced overexpression of eNOS caused a modest but significant increase in Ad5–DsRed infection (Fig. 6a). Concordantly, knockdown of endogenous eNOS expression with small hairpin RNA caused a significant decrease in Ad5–DsRed infection (Fig. 6b). These results give support to the hypothesis that eNOS functions as a critical regulator of Ad5–DsRed infection by way of dynamin2 S-nitrosylation.
Fig. 6.
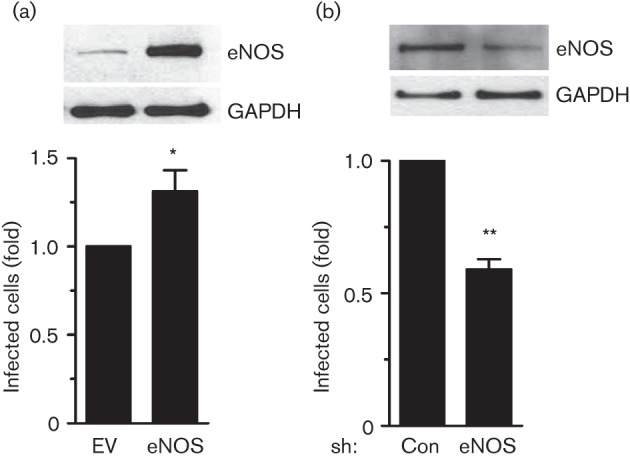
eNOS regulates Ad5–DsRed infection. (a) Ad5–DsRed infection is increased as a result of forced overexpression of eNOS. BEC cells were transfected with pcDNA3.1 (EV) or pcDNA3.1-eNOS (eNOS) for 24 h. Cells were then infected with Ad5–DsRed for an additional 24 h and cell lysates were harvested and immunoblotted with antibodies against eNOS or GAPDH. (b) Ad5–DsRed infection is decreased in response to the knockdown of endogenous eNOS expression. Cells were infected with adenovirus encoding control shRNA that targets green fluorescent protein (GFP) (Con) or shRNA that targets eNOS (eNOS) for 36 h. Next, cells were infected with Ad5–DsRed for 24 h and cell lysates were immunoblotted with antibodies against eNOS or GAPDH. For both panels, the cells were also analysed for Ad5–DsRed internalization using flow cytometry, as described. Infection ratio of the control group was arbitrarily assigned a value of 1, to which the infection ratio of cells with overexpression of eNOS or knockdown of endogenous eNOS was normalized. Experiments were repeated three times and data are expressed as mean±sem. *, P<0.05, **, P<0.01 versus empty vector (EV)-transfected, or shRNA-GFP (Con)-infected cells.
Akt mediates eNOS activation upon Ad5–DsRed infection
Our results suggest that activated eNOS mediates the Ad5–DsRed entry into host cells by means of dynamin2 S-nitrosylation. The eNOS may be activated by multiple mechanisms, including phosphorylation. Among all the phosphorylation sites of eNOS, S1177 phosphorylation by the kinases Akt (protein kinase B), cyclic AMP-dependent protein kinase (PKA), AMP-activated protein kinase (AMPK), protein kinase G (PKG) or calcium/calmodulin-dependent protein kinase II (CaM kinase II) is indicative of activation (Chen et al., 1999; Dudzinski et al., 2006; Fulton et al., 2001; Michell et al., 2001). Previous studies have shown that adenovirus infection can induce Akt and PKA activation in different cell types (Rajala et al., 2005; Suomalainen et al., 2001). Ad5–DsRed infection induced the phosphorylation of both Akt and eNOS (Fig. 7, left panels). To directly implicate Akt in the Ad5–DsRed-mediated eNOS phosphorylation, cells were treated with the PI3K inhibitor LY294002 prior to Ad5–DsRed infection. LY294002 effectively blocked the Ad5–DsRed-induced Akt and eNOS phosphorylation (Fig. 7, right panels). Treatment of BEC cells with the PKA inhibitor H89 prior to Ad5–DsRed infection did not impact the eNOS phosphorylation (data not shown). These results suggest that activation of eNOS in response to Ad5–DsRed infection proceeds, at least in part, through Akt.
Fig. 7.
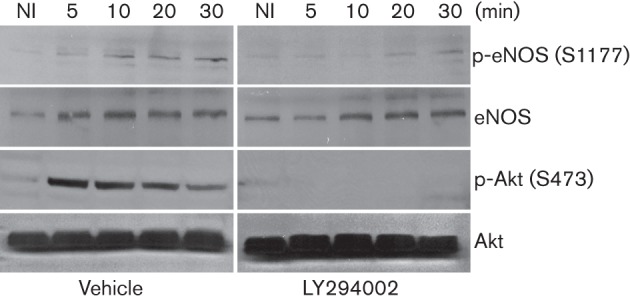
Impact of Akt on Ad5–DsRed-induced eNOS phosphorylation. BEC cells were treated, or not, with PI3K inhibitor LY294002 (50 µM) for 2 h and then infected with Ad5–DsRed. Cells were harvested and lysates were fractionated on SDS-PAGE. Antibodies against phospho-S1177-eNOS and phosho-S473-Akt were used to detect phosphorylation of eNOS and Akt, respectively. The nitrocellulose membrane was stripped and immunoblotted with antibodies against total eNOS and Akt to show the equal protein loading. NI, Not-infected.
eNOS and dynamin2 are dynamically S-nitrosylated during Ad5–DsRed infection
eNOS undergoes receptor-mediated S-nitrosylation (Dudzinski et al., 2006) and we tested if it, like dynamin2, undergoes S-nitrosylation modification upon Ad5–DsRed infection. Cells expressing eNOS and dynamin2 were infected with Ad5–DsRed and analysed for protein S-nitrosylation. Results show that virus infection had an inverse effect on the S-nitrosylation signal of eNOS and dynamin2: we observed a time-dependent decrease in eNOS S-nitrosylation (Fig. 8a), but an increase in dynamin2 S-nitrosylation (Fig. 8b).
Fig. 8.
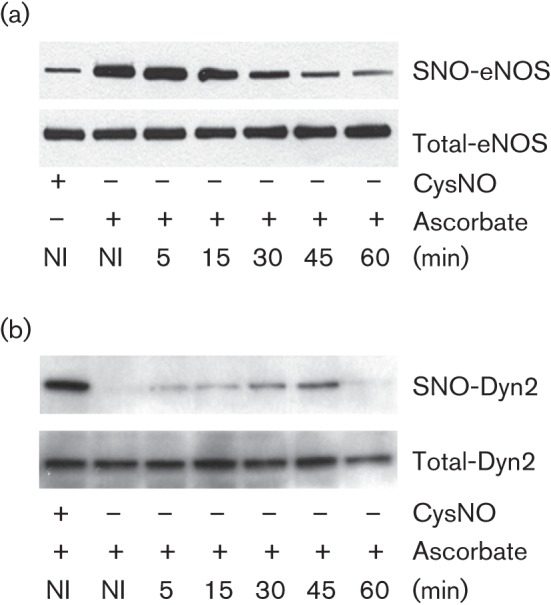
eNOS and dynamin2 are dynamically S-nitrosylated in response to Ad5–DsRed infection. BEC cells were transfected with cDNAs encoding HA-dynamin2 and bovine eNOS for 48 h and then infected with Ad5–DsRed for the indicated time. BEC cell lysates were subjected to a biotin switch assay to detect S-nitrosylated (a) eNOS (SNO-eNOS) or (b) dynamin2 (SNO-Dyn2). Treatment with CysNO served as a positive control and total dynamin2 or eNOS levels are shown to evidence equal protein loading among the different samples.
Discussion
Adenoviruses are non-enveloped DNA viruses that cause a significant number of human respiratory and gastrointestinal diseases. Adenovirus infections are common worldwide and occur throughout the year. Structure, life cycle and cell tropism of adenoviruses have all been well studied, but mechanisms underlying the initial step of infection, or entry into target cells, remain incompletely understood. Here, we show that Ad5–DsRed infection of BEC cells in culture activates eNOS leading to the S-nitrosylation of dynamin2 that may facilitate the adenovirus internalization, as measured by the increased DsRed signal in host BEC cells.
Previous work has shown that adenovirus internalization and infection of HeLa cells require dynamin2 (Wang et al., 1998). Our results are consistent with this finding: BEC cells overexpressing WT dynamin2 take up more adenovirus, whereas cells overexpressing dominant-negative dynamin2 K44A suggest limited virus entry, compared with non-transfected cells (Fig. 3). We have previously shown that C86 is a key S-nitrosylation site for dynamin1 (Wang et al., 2006). For dynamin2, we find that both C86 and C607 are key S-nitrosylation sites in response to Ad5–DsRed infection (Figs 2b, 3). Dynamin2 has six cysteine residues and what distinguishes these two S-nitrosylation sites from the other cysteine residues remains poorly understood, although it is reasonable to speculate that conformation and site availability may be determining factors. The results show that eNOS is an endogenous NO source, which can directly interact with (Cao et al., 2001) and S-nitrosylate dynamin2 (Fig. 5). This interaction may elicit changes in dynamin2 conformation that favour S-nitrosylation of the C607 and C86 residues. Likewise, eNOS also has two known nitrosylation sites, C94 and C99, unique amongst 30 cysteine residues (Erwin et al., 2005; Ravi et al., 2004). Our results show that eNOS undergoes denitrosylation in response to Ad5–DsRed infection (Fig. 8). Hence, the interaction between dynamin2 and eNOS during Ad5–DsRed infection may induce not only dynamin2 S-nitrosylation, but also eNOS denitrosylation. It is tempting to speculate that dynamin2 S-nitrosylation may proceed through eNOS-mediated NO production and/or transfer of NO group from the S-nitrosylated eNOS to dynamin2 through a recently appreciated process termed trans-nitrosylation (Kornberg et al., 2010; Nakamura et al., 2010).
eNOS enzymic activity and subcellular localization are well controlled by post-translational modifications, including phosphorylation, S-nitrosylation and acylation (Dudzinski et al., 2006). There are three activating serine residues in eNOS that can be phosphorylated: S1177, S617 and S635. Among these sites, phosphorylation of S1177 is a key indicator of its activation (Bauer et al., 2003; Dudzinski et al., 2006). Besides phosphorylation, reversible S-nitrosylation controls eNOS activity in endothelial cells; S-nitrosylation of eNOS attenuates its activity (Dudzinski et al., 2006). Our results are consistent with these observations: eNOS is rapidly activated through phosphorylation on S1177 and denitrosylation consequent upon Ad5–DsRed infection.
In mouse models, adenovirus infection typically induces strong, but delayed, inflammatory responses that are likely mediated by iNOS (Zsengellér et al., 2001). We saw in our studies that eNOS phosphorylation and dynamin2 S-nitrosylation occurred within minutes after infection, a time frame that is insufficient for iNOS-mediated NO production. Also, endogenous eNOS resides in the plasma membrane (Dudzinski et al., 2006) and forms a complex with dynamin2 (Cao et al., 2001), thereby making eNOS, and not iNOS, the likely candidate to mediate dynamin2 S-nitrosylation. This conclusion is confirmed by the result that iNOS expression is not detected 1 h post-Ad5–DsRed infection (data not shown). Our hypothesis is in accord with phosphorylation studies documenting maximal eNOS phosphorylation of S1177 within 5 min, accompanied by rapid eNOS denitrosylation. In addition, iNOS produces high levels of NO that contribute to the later pathophysiological responses to adenoviral infection. Our results show that eNOS-derived NO is at low concentrations, which may facilitate adenovirus infection.
Taken together, the results show that dynamin2 and eNOS are critical partners in the process of Ad5 infection. Ad5 infection promotes eNOS activation that, in turn, facilitates dynamin2 S-nitrosylation at conserved C86 and C607 residues. Just how Ad5 infection promotes eNOS activation remains an open question, although our results suggest involvement of PI3K and Akt. Interference of eNOS activation and dynamin2 S-nitrosylation may provide an opportunity to control adenovirus infection that is globally widespread.
Methods
Reagents.
Adeno–DsRed2 (Ad5–DsRed), a serotype 5 recombinant adenovirus with deletion of the E1/E3 genes and expression of Discosoma sp. red fluorescent protein, was obtained from Clontech. This virus is incapable of replicating after infection of host cells, and infected cells can be detected by flow cytometry. The BEC cell line 5637 was obtained from ATCC (HBT-9) and was cultured in RPMI 1640 medium supplemented with 10 % FBS without antibiotics at 37 °C in a humidified atmosphere of 5 % CO2. The African green monkey kidney fibroblast-like cell line COS-7 was also obtained from ATCC (CRL-1651) and was cultured in Dulbecco’s modified Eagle’s medium supplemented with 10 % FBS with penicillin–streptomycin (1 %) at 37 °C in a humidified atmosphere of 5 % CO2. NOS inhibitor Nω-nitro-l-arginine methyl ester hydrochloride (L-NAME) and NO donor DETA-NO were purchased from Sigma-Aldrich. The endogenous NO donor GSNO was purchased from Cayman Chemical Company, and S-nitrosocysteine (CysNO) was freshly prepared by mixing l-cysteine-HCl with sodium nitrite, as described previously (Wang et al., 2011). LY294002 was purchased from Cell Signaling Technology. cDNAs encoding WT and K44A dynamin2 were described previously (Ahn et al., 1999, 2002). Mutations of dynamin2 were created with a site-directed mutagenesis kit (QuikChange; Stratagene) and were verified by sequencing.
Measurement of viral infection.
To analyse adenovirus infection, BEC cells were mixed with Ad5–DsRed for 1 h, followed by washing with culture medium to remove free virus. BEC cells were cultured for 36 h post-infection, trypsinized and washed three times with PBS. Efficiency of virus infection was measured by flow cytometry (FACSContor; Becton Dickinson) and analysed with Cell Quest software.
Fluorescence imaging for real-time NO production.
The membrane permeable fluorescent indicator DAF-2DA was used to measure intracellular NO concentrations (Kojima et al., 1998). Briefly, BEC cells either treated with NO donor (used as positive control) or infected with virus were washed twice with phenol red-free RPMI 1640 medium and incubated at 37 °C for 10 min with DAF-2DA (1 µM) in phenol red-free RPMI 1640 medium. Dye-loaded cells were analysed using the Leica DM 6000 Imaging System to capture the fluorescence signal.
NO release.
Prior to cell harvest, the culture medium of appropriately treated cells was collected and analysed for NO release. Briefly, cell culture medium (100 µl) was mixed with ethanol (to precipitate proteins) and refluxed in sodium iodide/glacial acetic acid for measurement of the basal NO. Net NO release was calculated by NO-specific chemiluminescence after subtracting basal release from non-transfected cells as described previously (Fulton et al., 1999).
Western blot analysis.
Total and phosphorylated protein levels were detected by immunoblotting. Cells were harvested with lysis buffer (25 mM HEPES, pH 7.4, 150 mM NaCl, 0.5 mM EDTA, 5 mM MgCl2, 1 % Triton X-100, 1 mM DTT, 1 mM PMSF and protease inhibitor cocktail). Cleared cell lysates were subjected to protein quantification using the Bradford method. Equal amounts of protein were resolved by SDS-PAGE, transferred to nitrocellulose membranes and immunoblotted with antibodies against dynamin2 (1 : 1000 dilution; Cell Signaling Technology), phospho-S1177-eNOS (1 : 500 dilution; BD Biosciences Pharmingen), eNOS (1 : 1000 dilution; BD Biosciences Pharmingen), haemagglutinin (HA; 1 : 2000 dilution; Abcam), glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1 : 5000; Millipore), Akt (1 : 1000 dilution; Cell Signaling Technology), or phospho-S473-Akt (1 : 1000 dilution; Cell Signaling Technology). Filters were incubated with appropriate HRP-conjugated secondary antibody and visualized with an enhanced chemiluminescence detection system (ECL; Amersham).
Protein S-nitrosylation.
Appropriately treated cells from a 10 cm dish were lysed (250 mM HEPES, pH 7.7, 1 mM EDTA, 0.1 mM neocuproine, 1 % Nonidet P-40 and protease inhibitor cocktail) and cell extracts were diluted with HEN buffer (250 mM HEPES, pH 7.7, 1 mM EDTA and 0.1 mM neocuproine) to 1 ml. Detection of dynamin2 and eNOS S-nitrosylation was performed using the biotin switch method (Wang et al., 2006, 2011). Briefly, cell lysates were treated with the thiol-specific methylthiolating agent methyl methanethiosulfonate to block free thiols. Ascorbate was then used to reduce nitrosothiol to thiol that reacts with the thiol-specific reagent N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio) propionamide (biotin-HPDP). Proteins labelled with biotin were purified using immobilized streptavidin agarose beads (Sigma) and detected by immunoblotting.
Knockdown of eNOS.
Adenovirus-encoding shRNA targeting human eNOS (gift from D. Fulton, Georgia Health Sciences University, Augusta, GA) was used to silence endogenous eNOS gene expression, as described previously (Wang et al., 2011). Briefly, cells were infected with shRNA adenovirus at an m.o.i. of 100. After 36 h, infected BEC cells were harvested and cell lysates were used to confirm the decreased expression of the eNOS proteins.
Statistical analysis.
Experiments were repeated at least three times and data were expressed as mean±sem. Statistical analysis was performed by one way ANOVA with Tukey post-test using Prism 5.0 software (GraphPad Software, Inc.).
Acknowledgements
We thank Dr D. Fulton for providing the Ad-sh-eNOS and Dr L. Huang for technical assistance. We also thank Dr Z. Nie and Ms E. Grigson for helpful comments and suggestions. This work was supported by the Public Health Service from the National Institutes of Health grant AI079014 (to Y. D.).
References
- Ahn S., Maudsley S., Luttrell L. M., Lefkowitz R. J., Daaka Y. (1999). Src-mediated tyrosine phosphorylation of dynamin is required for β2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem 274, 1185–1188 10.1074/jbc.274.3.1185 [DOI] [PubMed] [Google Scholar]
- Ahn S., Kim J., Lucaveche C. L., Reedy M. C., Luttrell L. M., Lefkowitz R. J., Daaka Y. (2002). Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the epidermal growth factor receptor. J Biol Chem 277, 26642–26651 10.1074/jbc.M201499200 [DOI] [PubMed] [Google Scholar]
- Bauer P. M., Fulton D., Boo Y. C., Sorescu G. P., Kemp B. E., Jo H., Sessa W. C. (2003). Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem 278, 14841–14849 10.1074/jbc.M211926200 [DOI] [PubMed] [Google Scholar]
- Ben-Israel H., Kleinberger T. (2002). Adenovirus and cell cycle control. Front Biosci 7, d1369–d1395 10.2741/ben [DOI] [PubMed] [Google Scholar]
- Bergelson J. M., Cunningham J. A., Droguett G., Kurt-Jones E. A., Krithivas A., Hong J. S., Horwitz M. S., Crowell R. L., Finberg R. W. (1997). Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275, 1320–1323 10.1126/science.275.5304.1320 [DOI] [PubMed] [Google Scholar]
- Berk A. J. (1986). Adenovirus promoters and E1A transactivation. Annu Rev Genet 20, 45–77 10.1146/annurev.ge.20.120186.000401 [DOI] [PubMed] [Google Scholar]
- Cao S., Yao J., McCabe T. J., Yao Q., Katusic Z. S., Sessa W. C., Shah V. (2001). Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J Biol Chem 276, 14249–14256 [DOI] [PubMed] [Google Scholar]
- Chen Z. P., Mitchelhill K. I., Michell B. J., Stapleton D., Rodriguez-Crespo I., Witters L. A., Power D. A., Ortiz de Montellano P. R., Kemp B. E. (1999). AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443, 285–289 10.1016/S0014-5793(98)01705-0 [DOI] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. (2003). Regulated portals of entry into the cell. Nature 422, 37–44 10.1038/nature01451 [DOI] [PubMed] [Google Scholar]
- de Jong R. N., van der Vliet P. C., Brenkman A. B. (2003). Adenovirus DNA replication: protein priming, jumping back and the role of the DNA binding protein DBP. Curr Top Microbiol Immunol 272, 187–211 [DOI] [PubMed] [Google Scholar]
- Dudzinski D. M., Igarashi J., Greif D., Michel T. (2006). The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol 46, 235–276 10.1146/annurev.pharmtox.44.101802.121844 [DOI] [PubMed] [Google Scholar]
- Durieux A. C., Prudhon B., Guicheney P., Bitoun M. (2010). Dynamin 2 and human diseases. J Mol Med (Berl) 88, 339–350 10.1007/s00109-009-0587-4 [DOI] [PubMed] [Google Scholar]
- Echavarría M. (2008). Adenoviruses in immunocompromised hosts. Clin Microbiol Rev 21, 704–715 10.1128/CMR.00052-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin P. A., Lin A. J., Golan D. E., Michel T. (2005). Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem 280, 19888–19894 10.1074/jbc.M413058200 [DOI] [PubMed] [Google Scholar]
- Fish K. N., Schmid S. L., Damke H. (2000). Evidence that dynamin-2 functions as a signal-transducing GTPase. Mol Biol Cell 11, 1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D., Gratton J. P., McCabe T. J., Fontana J., Fujio Y., Walsh K., Franke T. F., Papapetropoulos A., Sessa W. C. (1999). Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399, 597–601 10.1038/21218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D., Gratton J. P., Sessa W. C. (2001). Post-translational control of endothelial nitric oxide synthase: why isn’t calcium/calmodulin enough? J Pharmacol Exp Ther 299, 818–824 [PubMed] [Google Scholar]
- Gastaldelli M., Imelli N., Boucke K., Amstutz B., Meier O., Greber U. F. (2008). Infectious adenovirus type 2 transport through early but not late endosomes. Traffic 9, 2265–2278 10.1111/j.1600-0854.2008.00835.x [DOI] [PubMed] [Google Scholar]
- Greber U. F., Willetts M., Webster P., Helenius A. (1993). Stepwise dismantling of adenovirus 2 during entry into cells. Cell 75, 477–486 10.1016/0092-8674(93)90382-Z [DOI] [PubMed] [Google Scholar]
- Heeringa P., van Goor H., Moshage H., Klok P. A., Huitema M. G., de Jager A., Schep A. J., Kallenberg C. G. M. (1998). Expression of iNOS, eNOS, and peroxynitrite-modified proteins in experimental anti-myeloperoxidase associated crescentic glomerulonephritis. Kidney Int 53, 382–393 10.1046/j.1523-1755.1998.00780.x [DOI] [PubMed] [Google Scholar]
- Kang-Decker N., Cao S., Chatterjee S., Yao J., Egan L. J., Semela D., Mukhopadhyay D., Shah V. (2007). Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2. J Cell Sci 120, 492–501 10.1242/jcs.03361 [DOI] [PubMed] [Google Scholar]
- Kennedy M. A., Parks R. J. (2009). Adenovirus virion stability and the viral genome: size matters. Mol Ther 17, 1664–1666 10.1038/mt.2009.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H., Nakatsubo N., Kikuchi K., Kawahara S., Kirino Y., Nagoshi H., Hirata Y., Nagano T. (1998). Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 70, 2446–2453 10.1021/ac9801723 [DOI] [PubMed] [Google Scholar]
- Kornberg M. D., Sen N., Hara M. R., Juluri K. R., Nguyen J. V. K., Snowman A. M., Law L., Hester L. D., Snyder S. H. (2010). GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol 12, 1094–1100 10.1038/ncb2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. P., III, Fishbein M., Echavarria M. (2011). Adenovirus. Semin Respir Crit Care Med 32, 494–511 10.1055/s-0031-1283287 [DOI] [PubMed] [Google Scholar]
- McConnell M. J., Imperiale M. J. (2004). Biology of adenovirus and its use as a vector for gene therapy. Hum Gene Ther 15, 1022–1033 10.1089/hum.2004.15.1022 [DOI] [PubMed] [Google Scholar]
- Meier O., Greber U. F. (2004). Adenovirus endocytosis. J Gene Med 6 (Suppl 1), S152–S163 10.1002/jgm.553 [DOI] [PubMed] [Google Scholar]
- Michell B. J., Chen Zp, Tiganis T., Stapleton D., Katsis F., Power D. A., Sim A. T., Kemp B. E. (2001). Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem 276, 17625–17628 10.1074/jbc.C100122200 [DOI] [PubMed] [Google Scholar]
- Mooren O. L., Kotova T. I., Moore A. J., Schafer D. A. (2009). Dynamin2 GTPase and cortactin remodel actin filaments. J Biol Chem 284, 23995–24005 10.1074/jbc.M109.024398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Wang L., Wong C. C. L., Scott F. L., Eckelman B. P., Han X. M., Tzitzilonis C., Meng F. J., Gu Z. Z. & other authors (2010). Transnitrosylation of XIAP regulates caspase-dependent neuronal cell death. Mol Cell 39, 184–195 10.1016/j.molcel.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsubo N., Kojima H., Kikuchi K., Nagoshi H., Hirata Y., Maeda D., Imai Y., Irimura T., Nagano T. (1998). Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett 427, 263–266 10.1016/S0014-5793(98)00440-2 [DOI] [PubMed] [Google Scholar]
- Nemerow G. R., Stewart P. L. (1999). Role of α(v) integrins in adenovirus cell entry and gene delivery. Microbiol Mol Biol Rev 63, 725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praefcke G. J. K., McMahon H. T. (2004). The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol 5, 133–147 10.1038/nrm1313 [DOI] [PubMed] [Google Scholar]
- Rajala M. S., Rajala R. V. S., Astley R. A., Butt A. L., Chodosh J. (2005). Corneal cell survival in adenovirus type 19 infection requires phosphoinositide 3-kinase/Akt activation. J Virol 79, 12332–12341 10.1128/JVI.79.19.12332-12341.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi K., Brennan L. A., Levic S., Ross P. A., Black S. M. (2004). S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci U S A 101, 2619–2624 10.1073/pnas.0300464101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlioglu S., Benson P. K., Yang J. S., Atkinson E. M., Reynolds T., Engelhardt J. F. (2000). Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation. J Virol 74, 9184–9196 10.1128/JVI.74.19.9184-9196.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M., Nakano M. Y., Boucke K., Keller S., Greber U. F. (2001). Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. EMBO J 20, 1310–1319 10.1093/emboj/20.6.1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K., Takei K. (2009). Dynamic instability of microtubules requires dynamin 2 and is impaired in a Charcot-Marie-Tooth mutant. J Cell Biol 185, 939–948 10.1083/jcb.200803153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellinga J., Rabelink M. J., Cramer S. J., van den Wollenberg D. J., Van der Meulen H., Leppard K. N., Fallaux F. J., Hoeben R. C. (2004). Spacers increase the accessibility of peptide ligands linked to the carboxyl terminus of adenovirus minor capsid protein IX. J Virol 78, 3470–3479 10.1128/JVI.78.7.3470-3479.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne E., Mahfouz I., Dedieu J. F., Brie A., Perricaudet M., Yeh P. (1999). RGD inclusion in the hexon monomer provides adenovirus type 5-based vectors with a fiber knob-independent pathway for infection. J Virol 73, 5156–5161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. N., Huang S., Kapoor-Munshi A., Nemerow G. (1998). Adenovirus internalization and infection require dynamin. J Virol 72, 3455–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. F., Moniri N. H., Ozawa K., Stamler J. S., Daaka Y. (2006). Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc Natl Acad Sci U S A 103, 1295–1300 10.1073/pnas.0508354103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. M., Humphrey C., Frilot N., Wang G. F., Nie Z. Z., Moniri N. H., Daaka Y. (2011). Dynamin2- and endothelial nitric oxide synthase-regulated invasion of bladder epithelial cells by uropathogenic Escherichia coli. J Cell Biol 192, 101–110 10.1083/jcb.201003027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham T. J., Mathias P., Cheresh D. A., Nemerow G. R. (1993). Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73, 309–319 10.1016/0092-8674(93)90231-E [DOI] [PubMed] [Google Scholar]
- Zsengellér Z. K., Ross G. F., Trapnell B. C., Szabó C., Whitsett J. A. (2001). Adenovirus infection increases iNOS and peroxynitrite production in the lung. Am J Physiol Lung Cell Mol Physiol 280, L503–L511 [DOI] [PubMed] [Google Scholar]