Abstract
Two novel obligately anaerobic, Gram-stain-positive, saccharolytic and non-proteolytic spore-forming bacilli (strains CD3 : 22T and N1T) are described. Strain CD3 : 22T was isolated from a biopsy of the small intestine of a child with coeliac disease, and strain N1T from the saliva of a healthy young man. The cells of both strains were observed to be filamentous, approximately 5 to >20 µm long, some of them curving and with swellings. The novel organisms produced H2S, NH3, butyric acid and acetic acid as major metabolic end products. Phylogenetic analyses, based on comparative 16S rRNA gene sequencing, revealed close relationships (98 % sequence similarity) between the two isolates, as well as the type strain of Eubacterium saburreum and four other Lachnospiraceae bacterium-/E. saburreum-like organisms. This group of bacteria were clearly different from any of the 19 known genera in the family Lachnospiraceae. While Eubacterium species are reported to be non-spore-forming, reanalysis of E. saburreum CCUG 28089T confirmed that the bacterium is indeed able to form spores. Based on 16S rRNA gene sequencing, phenotypic and biochemical properties, strains CD3 : 22T and N1T represent novel species of a new and distinct genus, named Lachnoanaerobaculum gen. nov., in the family Lachnospiraceae [within the order Clostridiales, class Clostridia, phylum Firmicutes]. Strain CD3 : 22T ( = CCUG 58757T = DSM 23576T) is the type strain of the type species, Lachnoanaerobaculum umeaense gen. nov., sp. nov., of the proposed new genus. Strain N1T ( = CCUG 60305T = DSM 24553T) is the type strain of Lachnoanaerobaculum orale sp. nov. Moreover, Eubacterium saburreum is reclassified as Lachnoanaerobaculum saburreum comb. nov. (type strain CCUG 28089T = ATCC 33271T = CIP 105341T = DSM 3986T = JCM 11021T = VPI 11763T).
Coeliac disease (CD) in children has features analogous to those of an infectious disease. Between 1985 and 1996, the incidence of childhood CD in Sweden among children younger than 2 years was four times higher than it was before or after (Ivarsson et al., 2000). We have studied the microbial flora in biopsies from the jejunal mucosa of children born during the so-called ‘Swedish epidemic’ period and compared the microflora with those of control patients and CD children born after the epidemic period using scanning electron microscopy (SEM), cultivation-based analyses and 16S rRNA gene sequencing (Forsberg et al., 2004; Ou et al., 2009). We found that the microbiota of children with CD born during the Swedish epidemic had different compositions from those of children with CD born after the epidemic or those of controls, with a significant enrichment of rod-shaped bacteria of the Clostridiales, Prevotella and Actinomyces. Any or all of these bacteria may contribute to the aetiology or pathogenesis of CD in children. One of these rod-shaped bacteria was a spore-forming bacterium initially believed to belong to the clostridia (Ou et al., 2009). The phylogenetic relationship of this Clostridiales sp. strain CD3 : 22 revealed that it was more closely related (98 % 16S rRNA gene sequence similarity) to the type strain of Eubacterium saburreum (CCUG 28089T = JCM 11021T = ATCC 33271T) and to several isolates and clones of the Lachnospiraceae than to the so-called ‘true’ clostridia belonging to cluster I of the subphylum Clostridium (Collins et al., 1994). However, eubacteria have been described to be non-spore-forming (Holdeman et al., 1977). The known habitat of E. saburreum is the oral cavity, where it has been isolated from dental plaque (L. V. Holdeman; type strain ATCC 33271T) and from the tongue dorsum (Tyrrell et al., 2003).
This study describes the phenotypic and genotypic characterization of strain CD3 : 22T, strain N1T, a previously unreported, anaerobic oral strain, and the type strain of E. saburreum, CCUG 28089T. Additionally, we describe the phylogenetic relationships between the three isolates and other members of the family Lachnospiraceae based upon comparative 16S rRNA gene sequence analyses.
Strain CD3 : 22T was isolated from a biopsy of the proximal small intestine of a girl with CD born in 1995, i.e. during the Swedish CD epidemic. She was on a gluten-free diet when the biopsy was taken at the Department of Paediatrics, Umeå University Hospital, Umeå, in 2007. The biopsy was weighed, homogenized and serially tenfold diluted in fastidious anaerobe broth medium (Lab M) and immediately plated onto selective and non-selective agar media. Strain N1T was isolated from the saliva of a healthy young man in 1998 at the Karolinska Institute, Karolinska University Hospital, Huddinge, Stockholm, Sweden. E. saburreum CCUG 28089T, initially isolated from human dental plaque, was obtained from the Culture Collection University of Gothenburg (CCUG), Sweden. Pure cultures of the three strains grew well on blood agar plates [Columbia or Brucella agar base (BBL) supplemented with 5 % horse blood], on chocolate agar plates and in Brucella broth, under an anaerobic atmosphere (10 % H2, 5 % CO2 in N2) at 37 °C. Vitamin K and haemin were not required for growth and were not added to the media.
Colony morphology and presumptive identification tests by diagnostic discs (Jousimies-Somer et al., 2002) were examined on blood agar plates after incubation for 2–3 days. Strains CD3 : 22T and N1T and E. saburreum CCUG 28089T are all strictly anaerobic; they did not grow in the presence of oxygen. However, all three strains survived exposure to air for more than 12 h.
When the bacterial strains were cultured on a blood agar medium for 3 days, individual colonies of strain CD3 : 22T were 1–3 mm in diameter, flat, spreading and circular with erose edges. The colonies were speckled and the centre of the colonies had a more compact appearance than the outer parts and, when viewed by stereomicroscope, they were sometimes multi-coloured, with pink, green, white and grey areas (Fig. S1, available in IJSEM Online). Colonies of strain N1T had a similar size and speckled structure to those of CD3 : 22T but with edges more rhizoid and with sometimes pyramidal centres, making the colony morphology clostridium-like. The colony morphology of E. saburreum CCUG 28089T was also similar to that of CD3 : 22T, although the colonies lacked the compact appearance at the centre observed for CD3 : 22T (Fig. S1).
The morphology of bacterial cells was investigated by light microscopy after Gram-staining, dark-field microscopy, SEM and transmission electron microscopy (TEM). Cells of strains CD3 : 22T and N1T were filamentous, 5 to >20 µm long. Some of the cells were curved and with swellings; chain forms were occasionally observed (Fig. S2a). The cells of strain N1T were frequently observed in aggregates. Cells of E. saburreum CCUG 28089T were longer, more slender and did not display the swellings or spore-like structures observed in CD3 : 22T and N1T. All three strains were easily decolorized during Gram-staining.
The presence of spores was studied by Gram-staining, a specific spore-staining test, using malachite green (Shaeffer and Fulton spore stain kit; Sigma), and TEM. Spore formation was enhanced by growing the bacteria in Brucella broth at 37 °C for more than 4 days. Gram-staining detected spore-like structures in strains CD3 : 22T and N1T (Fig. S2b) but not in E. saburreum CCUG 28089T. However, spores were detected in all three strains by spore-staining (Fig. S2c, d). To confirm the presence of spores, TEM was performed on cultures of CD3 : 22T and E. saburreum CCUG 28089T, identifying spores inside the cells and extracellular spores in both cultures (Fig. S2e, f). Treatment of bacterial cells with heat (70 °C for 10 min) killed the spores of the novel strains as well as spores from spore-forming positive-control strains of Clostridium perfringens and C. innocuum. Spores of the novel strains were killed by 75 % ethanol treatment for 30 min, but not by 50 % ethanol, whereas the clostridial spores were resistant to 75 % ethanol treatment but not 85 %.
All three strains exhibited a temperature optimum for growth at 37 °C. The pH optima were 6.5–7.0 for CD3 : 22T and N1T and 7.0–7.5 for E. saburreum CCUG 28089T. Motility was not observed. None of the strains was haemolytic. All three strains produced H2S and NH3. Growth on glucose as the sole carbon source yielded the following volatile fatty acids: for strain CD3 : 22T, butyric acid and small amounts of acetic acid; for N1T and E. saburreum CCUG 28089T, butyric acid, acetic acid, lactic acid and small amounts of succinic acid.
Strains CD3 : 22T and N1T and E. saburreum CCUG 28089T were resistant to 10 µg colistin and susceptible to 5 µg vancomycin, 1 mg kanamycin, 5 µg metronidazole and 20 % bile. Susceptibilities to penicillin G (M.I.C. Evaluator Strips; Oxoid) were determined to be 0.06 mg l−1 for strain CD3 : 22T, 0.3 mg l−1 for N1T and 0.004 mg l−1 for E. saburreum CCUG 28089T.
The nucleotide sequences of the 16S rRNA genes of strains CD3 : 22T and N1T were determined by primer walking, covering the gene, and by cloning and sequencing of PCR amplification fragments, also covering the gene (Sambrook et al., 1989). Other 16S rRNA gene sequences for comparative analyses were retrieved from the NCBI sequence database (Sayers et al., 2010). The 16S rRNA gene sequence of CD3 : 22T was unusual in that it was longer (1594 bp) than most bacterial 16S rRNA genes. It contained an insert of 110 nt at position 1436 compared with the sequence of E. saburreum CCUG 28089T. The sequence outside the insert exhibited 98 % similarity to that of E. saburreum CCUG 28089T. The sequence of strain N1T did not contain this insert and was 98 % identical to the sequences of the other two strains (for CD3 : 22T outside the insert). Fig. 1 shows the phylogenetic tree reconstructed by the maximum composite likelihood model, using 16S rRNA gene sequences, to display the relationships between strains CD3 : 22T and N1T, E. saburreum JCM 11021T ( = CCUG 28089T), E. saburreum-like isolate C27KA (Dewhirst et al., 2010), Lachnospiraceae bacterium isolate F0167 and two sequences from related uncharacterized, uncultivated bacteria, one member each of the 19 genera of the family Lachnospiraceae, three species belonging to Clostridium cluster XIVa and a member of Clostridium cluster I (Clostridium butyricum VPI 3266T). Strains CD3 : 22T and N1T and E. saburreum CCUG 28089T and related bacteria form a separate group distinct from the 19 known genera in the family Lachnospiraceae. This phylogenetic group is also distinct from members of Clostridium clusters XIVa and I. Based on cluster analyses and 16S rRNA gene sequence similarities between CD3 : 22T, N1T and E. saburreum CCUG 28089T, these bacteria can be proposed to represent a novel genus, for which the name Lachnoanaerobaculum gen. nov. is proposed.
Fig. 1.
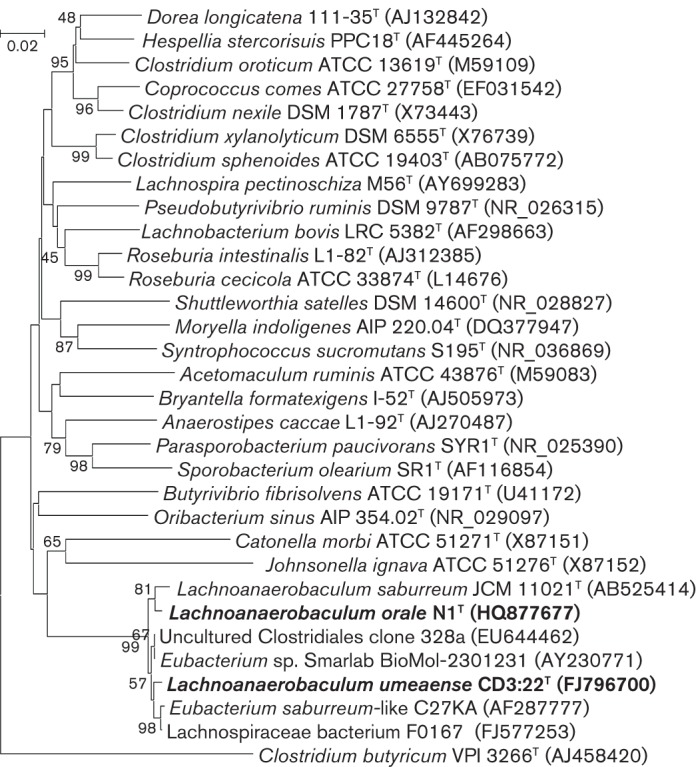
Phylogenetic tree based on 16S rRNA gene sequencing of members of Lachnoanaerobaculum gen. nov. and one species each of the other 19 recognized genera within the family Lachnospiraceae together with three remaining species of the genus Clostridium that are members of cluster XIVa. The 16S rRNA gene sequence of Clostridium butyricum VPI 3266T served as an outgroup. Bar, 0.02 substitutions per nucleotide position.
The DNA G+C contents of the three strains were determined by HPLC as 35 mol% for strain CD3 : 22T, 38 mol% for strain N1T and 37 mol% for E. saburreum CCUG 28089T.
Analyses of biochemical characteristics (Rapid ID 32A and API 20A; bioMérieux) showed that the three strains were saccharolytic and non-proteolytic (Table S1). However, they differed from each other in several respects. CD3 : 22T and N1T exhibited β-glucuronidase activity, while E. saburreum CCUG 28089T did not. E. saburreum CCUG 28089T and N1T displayed indole production and pyroglutamic acid arylamidase activity, in contrast to CD3 : 22T. Acid production from carbohydrates was not detected in strain N1T by the API 20A test, whereas strains CD3 : 22T and E. saburreum CCUG 28089T were more active. However, when grown in peptone yeast glucose broth, strain N1T also formed volatile fatty acids. Strain N1T was the only urease-positive strain. Another difference was that only CD3 : 22T produced acid from mannose and raffinose. Finally, E. saburreum CCUG 28089T displayed α-fucosidase activity, in contrast to the other two strains. Differentiating characteristics between the three strains are summarized in Table 1.
Table 1. Discriminating characteristics of strains CD3 : 22T and N1T and E. saburreum CCUG 28089T.
Strains: 1, CD3 : 22T; 2, N1T; 3, E. saburreum CCUG 28089T. Data were obtained in this study.
| Characteristic | 1 | 2 | 3 |
| DNA G+C content (mol%) | 35.0 | 37.8 | 37.0 |
| Enzyme activities | |||
| Urease | − | + | − |
| α-Fucosidase | − | − | + |
| β-Glucuronidase | + | + | − |
| Pyroglutamic acid arylamidase | − | + | + |
| Indole production | − | + | + |
| Utilization of (API 20A): | |||
| Mannose | + | − | − |
| Arabinose | + | − | + |
| Raffinose | + | − | − |
Cell fatty acid methyl ester analyses were performed using a standardized protocol, similar to that of the MIDI Sherlock MIS system (described at http://www.ccug.se/pages/CFA_method_2008 and in File S1) (Sasser, 2001). Strains were grown anaerobically (85 % N2, 10 % H2, 5 % CO2) under the same conditions, using chocolate agar as a cultivation medium at 37 °C, and harvested after 40±2 h. Cellular fatty acids (CFAs) were extracted and saponified by mild alkaline methanolysis and the released fatty acids were methylated. CFAs were identified and quantified by GC (Hewlett Packard HP 5890). Retention times of CFA peaks were converted to equivalent chain-length (ECL) values and the relative amount (w/w) of each fatty acid was expressed as a percentage of the total fatty acids in the profile of the respective strain (Table S2). The major CFAs detected in strains CD3 : 22T, N1T and E. saburreum CCUG 28089T were C14 : 0, C16 : 0 and C18 : 1ω7c dimethylacetal (DMA). CFAs occurred in different relative amounts in the three strains. Thus, C18 : 1ω7c DMA constituted 16, 12 and 8 %, respectively, of the total CFAs for CD3 : 22T, N1T and E. saburreum CCUG 28089T. There were also distinct differences in minor fatty acids (Table S2). The CFA profiles of the three strains differed, primarily, only in the relative amounts of any given CFA. The overall profiles of the three strains were distinctly similar. The isolation and characterization of more strains will enable more evidence to be presented as to whether the quantitative differences noted between the strains are characteristic of the individual strains or whether they represent species-level differences. Moreover, the fatty acid profiles of strains CD3 : 22T and N1T and E. saburreum CCUG 28089T were distinct from the CFA compositions of type strains from the genera Eubacterium and Clostridium (Table S2). Overall, these data support the conclusion from the phylogenetic analyses that they indeed represent different bacterial species in a distinct and new genus.
Reciprocal DNA–DNA hybridization experiments were performed with strains CD3 : 22T and N1T and E. saburreum CCUG 28089T at 60 °C for 16 h (Urdiain et al., 2008). Low levels of genomic DNA–DNA relatedness (16.2–52.2 %) were observed between the three strains. The values were 52.1 and 38.7 % for CD3 : 22T versus N1T, 16.2 and 40.2 % for N1T versus E. saburreum CCUG 28089T and 37.5 and 24.1 % for E. saburreum CCUG 28089T versus CD3 : 22T. As the genomic DNA hybridization values were well below 70 %, the three strains can be confirmed to represent different species (Stackebrandt & Goebel, 1994).
We therefore conclude that strains CD3 : 22T and N1T represent novel species named Lachnoanaerobaculum umeaense gen. nov. and Lachnoanaerobaculum orale sp. nov., respectively. Finally, we propose that Eubacterium saburreum be reclassified as Lachnoanaerobaculum saburreum comb. nov.
Description of Lachnoanaerobaculum gen. nov.
Lachnoanaerobaculum (Lach.no.an.ae.ro.ba′cu.lum. Gr. n. lachnos wool; Gr. pref. an- negating prefix; Gr. n. aer air; L. neut. n. baculum rod; N.L. neut. n. Lachnoanaerobaculum anaerobic rod forming woolly colonies).
Obligately anaerobic, Gram-stain-positive (easily decolorized and may appear as Gram-negative), spore-forming rods. Spores may be difficult to detect by Gram-staining. The cells are filamentous (5 to >20 µm long), sometimes curving and with swellings. Colony morphology on blood agar appears variably speckled and spreading; the edges are erose or rhizoid. Some colonies appear flat and others pyramidal. Temperature and pH optima for growth are 37 °C and pH 6.5–7.5. Saccharolytic and non-proteolytic, with butyric acid and acetic acid as major end products from glucose metabolism. Predominant CFAs are C14 : 0, C16 : 0 and C18 : 1ω7c DMA, accounting for 56–58 % of the total CFA profile. The G+C content of the DNA is 35–38 mol%. Strains have been isolated from the oral cavity, small intestine, blood and amniotic fluid (Han et al., 2009) of humans. The type species is Lachnoanaerobaculum umeaense.
Description of Lachnoanaerobaculum umeaense sp. nov.
Lachnoanaerobaculum umeaense (u.me.a.en′se. N.L. neut. n. umeaense of or pertaining to Umeå, referring to the discovery of the type strain at Umeå University).
Has the following properties in addition to those described for the genus. On blood agar, colonies are 1–3 mm in diameter, flat, spreading and circular with erose edges after 72 h at 37 °C. Colonies are non-haemolytic. Optimum growth at pH 6.5–7.0. Produces butyric acid, acetic acid, H2S and NH3. Acid is produced from glucose, mannose, xylose, arabinose, lactose, sucrose, maltose, trehalose, raffinose and melezitose. Activities for α-galactosidase, β-galactosidase, α-glucosidase, β-glucosidase and β-glucuronidase are observed but indole production and urease activity are not observed.
The type strain is CD3 : 22T ( = CCUG 58757T = DSM 23576T), isolated from a biopsy of the small intestine of a child with CD. The DNA G+C content of the type strain is 35 mol%.
Description of Lachnoanaerobaculum orale sp. nov.
Lachnoanaerobaculum orale (o.ra′le. L. neut. adj. orale belonging to the mouth, referring to the isolation of the type strain from the oral cavity).
Has the following properties in addition to those described for the genus. Cells frequently occur in aggregates. On blood agar, colonies are 1.5–3 mm in diameter, spreading and circular with rhizoid edges and pyramidal centres after 72 h at 37 °C. Colonies are non-haemolytic. Optimum growth at pH 6.5–7.0. Produces butyric acid, acetic acid, lactic acid, indole, H2S and NH3. Acid production is not detected from any of 17 common mono- or oligosaccharides or sugar alcohols. Activity for α-galactosidase, β-galactosidase, α-glucosidase, β-glucosidase, β-glucuronidase and pyroglutamic acid arylamidase is observed. Urease activity is observed.
The type strain is N1T ( = CCUG 60305T = DSM 24553T), isolated from the saliva of a healthy young man. The DNA G+C content of the type strain is 38 mol%.
Description of Lachnoanaerobaculum saburreum comb. nov.
Laehnoanaerobaculum saburreum (sa.bur′re.um. L. n. saburra sand; L.neut. stuff. -eum suffix used with the sense of belonging to; N.L. neut. adj. saburreum sandy). Basonym: Eubacterium saburreum (Prévot 1966) Holdeman and Moore 1970 (Approved Lists 1980).
Has the following properties in addition to those described for the genus. On blood agar, colonies are 1–2 mm in diameter, flat, spreading and circular with erose edges after 72 h at 37 °C. Colonies are non-haemolytic. Optimum growth at pH 7.0–7.5. Produces butyric acid, acetic acid, lactic acid, indole, H2S and NH3. Acid is produced from glucose, xylose, arabinose, lactose, sucrose, maltose, and trehalose. Activity for α-galactosidase, β-galactosidase, α-glucosidase, β-glucosidase, α-fucosidase and pyroglutamic acid arylamidase is observed.
The type strain is CCUG 28089T ( = ATCC 33271T = CIP 105341T = DSM 3986T = JCM 11021T = VPI 11763T), originally isolated from dental plaque. The DNA G+C content of the type strain is 37 mol%.
Acknowledgements
We thank Ms Anne Israelsson and Dr Lena Sundberg at Umeå University and Elisabeth Inganäs, Maria Ohlén and Kent Molin at the CCUG for skilful technical assistance. The research leading to these results has received funding from the European Union’s Seventh Framework Programme under grant agreement no. 222720 (to M.-L. H. and O. H.), the Swedish Research Council-Natural and Engineering Sciences (to M.-L. H., S. H. and S. N. W.) and the Swedish Research Council-Medicine and Health (O. H.). E. R. B. M. and L. S. were supported, in part, by ALF-medel ALFGBG-11574 and FOU projects VGFOUREG 30781 and VGFOUREG-72241.
Abbreviations:
- CD
coeliac disease
- CFA
cellular fatty acid
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
Footnotes
Two supplementary figures, two supplementary tables and a supplementary method are available with the online version of this paper.
References
- Collins M. D., Lawson P. A., Willems A., Cordoba J. J., Fernandez-Garayzabal J., Garcia P., Cai J., Hippe H., Farrow J. A. E. (1994). The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol 44, 812–826 10.1099/00207713-44-4-812 [DOI] [PubMed] [Google Scholar]
- Dewhirst F. E., Chen T., Izard J., Paster B. J., Tanner A. C., Yu W. H., Lakshmanan A., Wade W. G. (2010). The human oral microbiome. J Bacteriol 192, 5002–5017 10.1128/JB.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg G., Fahlgren A., Hörstedt P., Hammarström S., Hernell O., Hammarström M.-L. (2004). Presence of bacteria and innate immunity of intestinal epithelium in childhood celiac disease. Am J Gastroenterol 99, 894–904 10.1111/j.1572-0241.2004.04157.x [DOI] [PubMed] [Google Scholar]
- Han Y. W., Shen T., Chung P., Buhimschi I. A., Buhimschi C. S. (2009). Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol 47, 38–47 10.1128/JCM.01206-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdeman L. V., Moore W. E. C. (1970). Eubacterium. In Outline of Clinical Methods in Anaerobic Bacteriology, 2nd revision, pp. 23–30 Edited by Cato E. P., Cummings C. S., Holdeman L. V., Johnsson J. L., Moore W. E. C., Smibert R. M., Smith L. D. S. Blacksburg, VA: Virginia Polytechnic Institute Anaerobe Laboratory [Google Scholar]
- Holdeman L. V., Cato E. P., Moore W. E. C. (editors) (1977). Anaerobe Laboratory Manual, 4th edn Blacksburg, VA: Virginia Polytechnic Institute and State University [Google Scholar]
- Ivarsson A., Persson L. Å., Nyström L., Ascher H., Cavell B., Danielsson L., Dannaeus A., Lindberg T., Lindquist B. & other authors (2000). Epidemic of coeliac disease in Swedish children. Acta Paediatr 89, 165–171 10.1111/j.1651-2227.2000.tb01210.x [DOI] [PubMed] [Google Scholar]
- Jousimies-Somer H. R., Summanen P., Citron D. M., Baron E. J., Wexler H. M., Finegold S. M. (2002). Wadsworth-KTL Anaerobic Bacteriology Manual, 6th edn Belmont, CA: Star [Google Scholar]
- Ou G., Hedberg M., Hörstedt P., Baranov V., Forsberg G., Drobni M., Sandström O., Wai S. N., Johansson I. & other authors (2009). Proximal small intestinal microbiota and identification of rod-shaped bacteria associated with childhood celiac disease. Am J Gastroenterol 104, 3058–3067 10.1038/ajg.2009.524 [DOI] [PubMed] [Google Scholar]
- Prévot A. R. (1966). Manual for the Classification and Determination of the Anaerobic Bacteria. Philadelphia: Lea and Febiger [Google Scholar]
- Sambrook J., Fritsch E. F., Maniatis T. (1989). Molecular Cloning: a Laboratory Manual, 2nd edn Cold Spring Harbor, NY: Cold Spring Harbor Laboratory [Google Scholar]
- Sasser M. (2001) Identification of bacteria by gas chromatography of cellular fatty acids, MIDI Technical Note 101. Newark, DE: MIDI Inc [Google Scholar]
- Sayers E. W., Barrett T., Benson D. A., Bolton E., Bryant S. H., Canese K., Chetvernin V., Church D. M., Dicuccio M. & other authors (2010). Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 38, D5–D16 10.1093/nar/gkp967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackebrandt E., Goebel B. M. (1994). Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol 44, 846–849 10.1099/00207713-44-4-846 [DOI] [Google Scholar]
- Tyrrell K. L., Citron D. M., Warren Y. A., Nachnani S., Goldstein E. J. C. (2003). Anaerobic bacteria cultured from the tongue dorsum of subjects with oral malodor. Anaerobe 9, 243–246 10.1016/S1075-9964(03)00109-4 [DOI] [PubMed] [Google Scholar]
- Urdiain M., López-López A., Gonzalo C., Busse H. J., Langer S., Kämpfer P., Rosselló-Móra R. (2008). Reclassification of Rhodobium marinum and Rhodobium pfennigii as Afifella marina gen. nov. comb. nov. and Afifella pfennigii comb. nov., a new genus of photoheterotrophic Alphaproteobacteria and emended descriptions of Rhodobium, Rhodobium orientis and Rhodobium gokarnense. Syst Appl Microbiol 31, 339–351 10.1016/j.syapm.2008.07.002 [DOI] [PubMed] [Google Scholar]


