Abstract
Background and aim
To investigate the possible modulating role of “Nigella sativa” (NS), a plant commonly used in Egyptian traditional medicine, on premalignant perturbations in three glycol-regulatory enzymes in an experimental rat model of hepatocellular carcinoma (HCC).
Methods
Thirty-six (36) male albino rats were divided into four groups (n = 9). Group 1 served as a normal control, group 2 was treated with methanolic extract of Nigella sativa (MENS) (1 g/kg/day, orally) for 14 weeks, group 3 received a single intraperitoneal dose of diethyl nitrosamine (DENA) (200 mg/kg), followed 2 weeks later by a subcutaneous injection of carbon tetrachloride (CCl4, 3 ml/kg/week/6 weeks) and group IV was treated with MENS for 2 weeks prior to administration of the carcinogenic combination (DENA + CCl4, as in group 3) until the end of the experiment. The total period of the experiment was 14 weeks.
Results
In the DENA + CCl4-treated group, there was a significant increase in the relative liver weight, serum alpha fetoprotein level and the activities of hexokinase, glyceraldehyde phosphate dehydrogenase and glucose 6 phosphate dehydrogenase in both the serum and liver homogenate; this was accompanied by a subsequent decrease in body weight. Pre-treatment with MENS significantly maintained these parameters close to the normal condition.
Conclusion
Based on these results, we conclude that MENS has a chemo-preventive effect against the progression into liver malignancy through its modulation of the energy metabolic pathways (i.e. glycolysis) that may be involved in hepatocarcinogenesis.
Keywords: Premalignant hepatocellular changes, Glyco-regulatory enzymes, Nigella sativa, Glucose metabolism, Chemoprevention
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide and the most common primary cancer of hepatocytes [1, 2]. It is responsible for approximately one million deaths each year. Variability in the incidence rates correspond closely to the prevalence and pattern of the primary etiologic factors. More than 80 % of HCC cases occur in developing countries. Areas of particularly high incidence are Eastern and South-eastern Asia and Sub-Saharan Africa [3]. According to recent reports, the incidence of HCC has increased sharply in the last decade, especially in Egypt, where there has been a doubling of the incidence rate during the last 10 years. This sharp rise has been attributed to several biological (e.g. hepatitis B and C virus infection) and environmental factors (e.g. aflatoxin), but many other factors, such as cigarette smoking, occupational exposure to chemicals (e.g. pesticides) and endemic infections in the community (e.g. Schistosomiasis) may also contribute to the etiology or progression of the disease [4]. Liver carcinogenesis may also develop through the progressive accumulation of different mutations (genetic) and/or gene products (protein), which eventually lead to malignant transformation [5, 6].
Due to the increasing awareness of the side effects of conventional medicine, the use of natural products as an alternative to conventional treatment in the healing and treatment of various diseases, including cancer, has been increasing in the last few decades [7]. Several herbal drugs have been evaluated for their potential to protect the liver against diethylnitrosamine (DENA)-induced hepatotoxicity in rats [8]. Nigella sativa (NS), commonly known as black seed or black cumin ‘Al-Habba Al-Sauda’ or ‘Habbet Al-Barakah’(in Arabic), is a seed of a capsulated plant that belongs to the Ranunculaceae family. NS has been employed for thousands of years as a spice and food preservative, as well as a protective and curative remedy for numerous disorders [9]. The seed contains 36–38 % fixed oils, proteins, alkaloids, saponin and 0.4–2.5 % essential oil as unsaturated fatty acids, including C20:2 arachidic and eicosadienoic acids [10]. The major active constituents are thymoquinone (TQ; 27.8–57.0 %), ρ-cymene (7.1–15.5 %), carvacrol (5.8–11.6 %), t-anethole (0.25–2.3 %), 4-terpineol (2.0–6.6 %) and longifoline (1.0–8.0 %) [11]. TQ readily dimerizes to form dithymoquinone (DTQ), which is believed to be nigellone [12, 13], and is the main bioactive constituent of the volatile oil of NS seeds. It has been shown to exert several pharmacological activities, including antioxidant, anti-inflammatory, chemotherapeutic and anti-tumor activities [14], as well as hepatoprotective activity [15].
Cancer cells are characterized by an abnormal pattern of energy metabolism that is manifested by an increase in glucose, fatty acid and amino acid metabolism and a decrease in oxidative phosphorylation [16]. Glucose is the primary energy source, and a high rate of glycolysis, which is one of the earliest discovered hallmarks of cancer cells, provides the tumor with metabolic and survival advantages [17, 18]. The early changes in carbohydrate metabolism are of particular interest, since anomalies of the glycolytic pathway are well-known biochemical disturbances in hepatomas [19, 20]. Elevated glucose catabolism is important for the production of the energy required by rapidly growing tumors. Early studies established the presence of abnormalities in the glucose-metabolizing enzymes with the transformation of normal liver cells into high glucose-utilizing hepatoma cell lines [21]. The high rate of aerobic glycolysis exhibited by some cancer cells is called the Warburg effect, in recognition of Otto Warburg’s discovery some 80 years ago, who determined that tumor cells produce lactate in the presence of oxygen. Based on this observation, Warburg championed the notion that aerobic glycolysis is necessary during carcinogenesis [22–24]. A definite correlation exists between tumor progression and the activities of glycolytic enzymes [19, 20]. Therefore, in the study reported here, we have focused on the assessment of alterations in the activity of hexokinase (HK), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and glucose-6-phosphate dehydrogenase (G6PD) enzymes in the serum and in the liver homogenate to investigate whether these enzymes are potential candidates as diagnostic and prognostic markers for HCC.
Materials and methods
Animals
Thirty-six adult male Wister Albino rats weighing 150–200 g were purchased from the Experimental Breeding House of Assiut University, Egypt. The animals were housed in plastic cage with wood chips for bedding and maintained at a temperature of 22 ± 2 °C, with 45 ± 4 % relative humidity under a 12/12-h (light/dark) cycle. The experiment commenced after acclimatization for 1 week to the animal house conditions. Both food and water were available for all animals ad libitum.
Chemicals
Diethylnitrosamine was purchased from Sigma Chemical Co. (St. Louis, MO). All other chemicals used were of analytical grade.
Preparation of Nigella sativa extract
Nigella sativa seeds, identified and harvested during the summer season, were purchased from the specialized Agricultural Institute in Dokki, Giza, Egypt. They were cleaned, dried, mechanically powdered, and extracted with 95 % aqueous methanol (1:10, w/v) for 24 h. The extract was filtered through a Buchner funnel, and the plant residue re-extracted with 50 % methanol for additional 2 h. The two extracts were combined and concentrated under reduced pressure on a rotatory evaporator at <40 °C until most of the methanol had been removed. The brownish-black crude extract was coded as methanolic extract of NS (MENS) and kept in a refrigerator at 4 °C. TQ is the main bioactive constituent of the aqueous alcoholic extract.
Experimental design
The research protocol, including treatment of the animals and the experimental design, was approved by the Research Ethics Committee of the College of Pharmacy, Minia University, Egypt and was performed in accordance to the Guidelines for Care and Use of Laboratory Animals of Minia University.
The rats were classified into four experimental groups of nine rats each:
Group 1: untreated control (designated IP); the rats were given saline only
Group 2: Nigella sativa control group (designated NS). The rats received orally 1 g/kg/day of methanolic extract of N. sativa (MENS) by gavage for 14 weeks [25].
Group 3: [DENA + carbon tetrachloride (CCl4) group]. The rats received a single intraperitoneal injection of DENA (200 mg/kg body weight), freshly dissolved in sterile 0.9 % saline [26]. Two weeks later, they received a subcutaneous injection of CCl4 (3 ml/kg/week) for 6 weeks to promote the carcinogenic effect of DENA [27–29].
Group 4. The rats were treated with MENS for 2 weeks prior to receiving the carcinogenic combination DENA + CCl4 (as in group 3) until the end of the experiment (14 weeks).
Preparation of serum and liver homogenate
At the end of the experimental period (14 weeks), the rats were fasted overnight, sacrificed and decapitated. Blood was collected and allowed to clot, then centrifuged; serum aliquots were kept frozen at −80 °C. The livers were immediately excised, rinsed with ice-cold saline, blotted dry and accurately weighed. The relative liver weight for each rat was calculated as the percentage ratio of absolute liver weight to the total body weight. A small portion of liver was kept in formalin for histopathological studies after hematoxylin and eosin (H&E) staining, while the remaining liver tissue was used for preparation of the homogenate. For the latter, 10 % of the homogenate was placed in 0.1 M Tris–HCl buffer (pH 7.4) using a Potter–Elvehjem homogenizer with a Teflon pestle. The liver homogenates were centrifuged at 4,000 rpm for 15 min and the supernatant stored in aliquots in Eppendorf tubes at −80 °C until enzyme activity was determined.
Biochemical analysis
The serum alpha fetoprotein (AFP) level was measured quantitatively by a solid phase enzyme-linked immunosorbent assay using a Calbiotech kit (Spring Valley, CA) following the instructions of the manufacturer. Enzyme activities of HK, GAPDH and G6PD were assayed spectrophotometrically in the serum and liver tissue homogenate. HK was measured according to Bergmeyer et al. [30], GAPDH was measured according to the procedures described by Velick [31] and G6PD was determined according to the method of Kornberg [32] using kits from Biodiagnostic (Dokki, Giza, Egypt). Total protein concentration in the supernatants of the tissue homogenates was determined according to the method adopted by Gornall et al. [33, 34] using a local kit (Spectrum).
Definition of units and specific activity
One unit of HK activity was taken to reduce 1 μmol of NADP+ per minute at pH 7.6 at 25 °C; 1 U of GAPDH activity was taken to reduce 1 μmol of NAD per minute at 25 °C and pH 8.5 and 1 U of G6PD activity was considered to reduce 1 μmol of NADP+ per minute at pH 7.6 at 25 °C. The specific activities of HK, GAPDH and G6PD were expressed as units per milligram of protein.
Statistical analysis
The results are presented as the mean ± standard deviation for nine rats in each group, and differences between mean values were determined by one-way analysis of variance, followed by Tukey’s test. For multiple comparisons, values of P > 0.05 were considered to be statistically significant. The GraphPad Prism ver. 5 software program (GraphPad, San Diego, CA)was used for this purpose.
Results
Body weight and relative liver weight
Table 1 shows the final body weight, liver weight and the relative liver weight of the four groups. There was significant decrease in the final body weight of rats subjected to DENA and CCl4, compared to control group. The body weights of the group 4 animals increased, indicating clearly that MENS had no toxic effects on the growth responses of the rats and was fairly well tolerated; in contrast, the change in relative liver weight of these animals was significant (P < 0.001) compared to the normal control (group I).
Table 1.
Effect of methanolic extract of Nigella sativa on body weight and relative liver weight after 2 weeks of treatment
| Groups | Final body weight (g) | Liver weight (g) | Relative liver weight (liver/100 g body weight) |
|---|---|---|---|
| 1 (normal control) | 228.5 ± 8.8 | 5.8 ± 0.4 | 2.51 ± 0.14 |
| 2 (MENS control) | 260 ± 21.3++ | 6.8 ± 0.41 | 2.63 ± 0.14 |
| 3 (premalignant group) | 203.1 ± 5.7# | 6.9 ± 0.46 | 3.40 ± 0.13### |
| 4 (premalignant treated group) | 243.4 ± 18.2*** | 6.7 ± 0.76 | 2.73 ± 0.15 *** |
MENS, Methanolic extract of Nigella sativa
Data are presented as the mean ± standard deviation (SD) (n = 9 in each group)
+MENS control group is compared to normal control, #premalignant group is compared to normal control, *premalignant treated group is compared to premalignant group (1 symbol = p < 0.05, 2 symbols = p < 0.01, 3 symbols = p < 0.001)
Biochemical investigations
Treatment with DENA + CCl4 caused a significant increase in the serum AFP level. However, the administration of MENS significantly decreased the serum AFP level in the cancer group in comparison to premalignant group (group 3) (Table 2). The activities of HK, GAPDH and G6PD in the serum and liver tissue homogenate were significantly increased among the premalignant group. However, relative to the premalignant group (group 3), the administration of MENS significantly decreased the activities of these three enzymes in both the serum and liver homogenate [serum: G6PD (P < 0.05), GAPDH (P < 0.01); liver homogenate: G6PD (P < 0.015), GAPDH (P < 0.001)] (Tables 2, 3 respectively).
Table 2.
Serum level of alpha fetoprotein and activities of serum HK, GAPDH and G6PD in the experimental rat groups
| Parameter | Groups | |||
|---|---|---|---|---|
| 1 (normal control) | 2 (MENS control) | 3 (premalignant group) | 4 (premalignant treated group) | |
| AFP (ng/ml) | 4.04 ± 0.61 | 5.13 ± 0.33 | 61.06 ± 2.71### | 17.9 ± 0.99*** |
| HK (mU/ml) | 5.7 ± 2.0 | 5.20 ± 2.6 | 9.84 ± 1. 65## | 6.56 ± 2.61* |
| GAPDH (mU/ml) | 25.3 ± 3.41 | 22.31 ± 5.12 | 33.16 ± 5.43## | 24.12 ± 3.7** |
| G6PD (mU/ml) | 0.50 ± 0.38 | 0.56 ± 0.32 | 2.4 ± 0.44### | 1.75 ± 0.46* |
AFP, Alpha fetoprotein; HK, hexokinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; G6PD, glucose-6-phosphate dehydrogenase
Data are presented as the mean ± SD (n = 9 in each group)
+MENS control group is compared to normal control, #premalignant group is compared to normal control, *premalignant treated group is compared to premalignant group (1 symbol = p < 0.05, 2 symbols = p < 0.01, 3 symbols = p < 0.001)
Table 3.
Activities of HK, GAPDH and G6PD in liver tissue homogenate of the experimental rat
| Parameters | Groups | |||
|---|---|---|---|---|
| 1 (normal control) | 2 (MENS control) | 3 (premalignant group) | 4 (premalignant treated group) | |
| HK (mU/mg protein) | 7.926 ± 2.319 | 8.473 ± 2.167 | 15.58 ± 2.725### | 10.66 ± 3.395** |
| GAPDH (mU/mg protein) | 28.34 ± 4.025 | 27.73 ± 4.275 | 46.42 ± 8.907### | 29.54 ± 4.025*** |
| G6PD (mU/mg protein) | 3.425 ± 1.316 | 4.438 ± 1.178 | 6.750 ± 0.707### | 4.625 ± 1.061** |
Data are presented as the mean ± SD (n = 9 in each group)
+MENS control group is compared to normal control, #premalignant group is compared to normal control, *premalignant treated group is compared to premalignant group (1 symbol = p < 0.05, 2 symbols = p < 0.01, 3 symbols = p < 0.001)
Histopathological examination
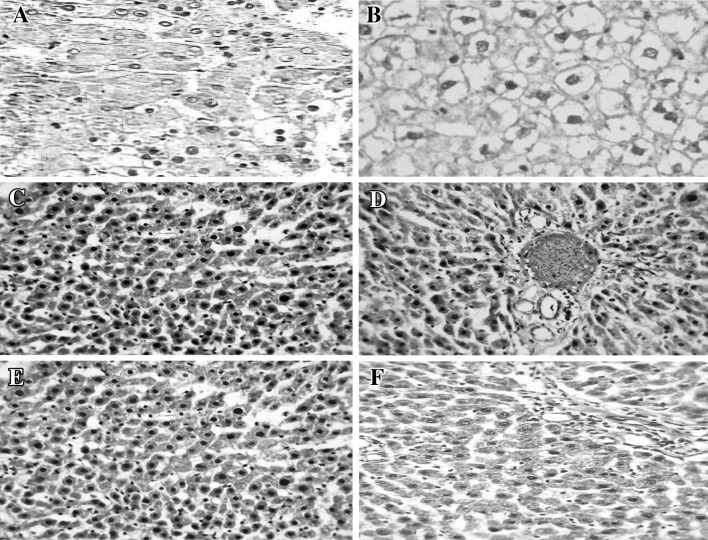
Representative samples were taken from the liver tissue of each animal and prepared for histopathological examination. Study of the liver tissue sections from rats in the normal and Nigella control groups revealed a normal hepatic lobular architecture and the presence of normal hepatocytes with granulated cytoplasm and small uniform nuclei and nucleolus, indicating the non-toxic nature of MENS (Fig. 1a, b). In contrast, the study of sections obtained from rats subjected to DENA + CCl4 treatment revealed hydropic degeneration, with most liver cells showing a cloudy swelling due to the presence of foamy cytoplasm that resulted from the intracellular accumulation of water. Changes in fat content, variable areas of necrosis, congestion of portal tracts, bile stasis and inflammatory infiltration were prominent (Fig. 1c–e). Animals pre-treated with MENS showed minimal changes in hepatocyte morphology and histology. Most of the histopathological changes seen in the cells of the premalignant HCC group were greatly reduced (Fig. 1f).
Fig. 1.
Representative photomicrographs of liver sections of the studied groups. a Normal control, b methanolic extract of Nigella sativa (MENS) control. Cells of both groups showed normal hepatic lobular architecture and hepatocytes with granulated cytoplasm and small uniform nucleus and nucleolus [hematoxylin and eosin (H&E) staining, ×200]. c–e Group 3 (premalignant group): c focal areas of dysplasia marked with large prominent hyperchromatic nuclei, atypia and eosinophilic cytoplasm, d markedly congested portal tract, proliferated bile ductules, bile stasis and infiltration with some inflammatory cells; adjacent liver cells show dysplasia, hydropic degeneration and focal areas of necrosis, e changes in fat (both micro- and macro-vesicular) (H&E, ×400). f Group 4 [animals pretreated with MENS, then DENA (diethyl nitrosamine) and CCl4 (carbon tetrachloride)]; cells showed more or less normal histology and no inflammation, as evidenced by lesser leukocyte infiltration. Collectively, most of the histological disturbances observed in the premalignant group were greatly reduced (H&E, ×400)
Discussion
The results of this study collectively and clearly demonstrate the efficacious effect of MENS on the activities of three glycol-regulatory enzymes (HK, GAPDH, G6PD) in experimentally induced hepatocarcinogenesis. Animals treated with DENA and CCl4 showed significant histological and biochemical variations, reflecting the instability of liver cell metabolism and leading to distinctive changes in serum enzyme activities and AFP (the relevant tumor marker). DENA is known to cause perturbations in the nuclear enzymes involved in DNA repair/replication [35] and is normally used to induce liver cancer in animal models [36, 37]; [38, 39]. Treatment with DENA and CCl4 has been shown to induce extensive necrosis and inflammatory infiltration, clusters of hepatocytes, necrosis, bile duct proliferation and marked atypia [38, 40].
In this study, the activities of three glyco-regulatory enzymes were up-regulated in group 3 rats (premalignant group). It is well-known that cancer cells generally need increased levels of aerobic glycolysis and therefore produce lactate even in the presence of oxygen (the Warburg effect) for ATP generation [22, 41]. Thus, increased glycolysis or pentose phosphate metabolism may confer adaptive advantages if excess pyruvate is synthesized for lipid synthesis, thereby providing essential anabolic substrates, such as ribose, for nucleic acid synthesis [42]. Glucose consumption through the pentose pathway may also provide essential reducing equivalents (NADPH) to reduce the toxicity of reactive oxygen species, conferring resistance to senescence [43, 44]. Consequently, cancer cells have a high glycolytic rate, which is advantageous in terms of tumor growth [45]. Rapidly growing, highly malignant tumor cells can obtain up to 60 % of their total ATP production from glycolysis. An elevated rate of glycolysis in tumor cells results in an increase in the intracellular concentration of glucose-6-phosphate, a key precursor in the de novo synthesis of nucleic acids, phospholipids and other macromolecules. This increased glucose-6-phosphate level is likely to be essential to keep pace with rapid cell division and membrane biosynthesis during tumor growth. Thus, rapidly proliferating tumors have an excess demand for nuclear energy, which is demonstrated by the nuclei having elevated activities of glycolytic enzymes [20].
The increased activity of rate-limiting glycolytic enzymes, such as HK, in tumor cells is one of the factors responsible for this increased aerobic glycolysis [46] in cancer cells and plays an important role in determining the glycolytic capacity of these cells [20]. We found that GAPDH, also a regulatory enzyme in the glycolytic pathway, was increased in our experimental hepatocarcinogenesis rat model. GAPDH has been reported to be overexpressed in cancers, including lung [47], colorectal-, prostate-, bladder-cancer [48] and several transformed tumor cell lines [49], and to be strongly up-regulated in advanced stages of HCC [50]. This enzyme is an also indicator of metastatic growth and increases specifically after metastasis. There have been reports of the increased activity of G6PD in hepatic carcinoma being a very useful diagnostic and prognostic tool to detect early pre-neoplastic lesions [51].
There is increasing evidence that G6PD activity is of major importance in NADPH production for defense against oxidative stress rather than for ribose production during proliferation [52]. Several key genetic alterations associated with tumor development have recently been shown to affect glycolysis directly, such as p53 mutation and the activation of hypoxia inducible factor [53, 54]. It is conceivable that the metabolic alterations in malignant cells may be exploited to develop therapeutic strategies to target this metabolic abnormality. One possibility is to inhibit glycolysis and preferentially kill the cancer cells that are dependent on glycolytic pathway for ATP generation [22, 41].
Our results indicate that the administration of MENS significantly curtailed liver tumor development and protected the cells against the histological and biochemical effects induced by the carcinogen. Earlier observations also support this proposal [55]. In an earlier study, a decoction of NS seed and some indigenous herbs was shown to have the potential to suppress hepatic tumor in rats induced by DENA [56]. It has also been shown to possess cytotoxic activity, even at low concentrations, against the human hepatoma HepG2 cell line through its inhibitory effects on DNA synthesis [57–60]. In this respect, the possible modes of action of thymoquinon (TQ) include antioxidant activity, interference with DNA synthesis and enhancement of the detoxification processes. Moreover, the aqueous extract of NS seeds was found to have a strong inhibitory effect on NO production by murine macrophages [61].
It can be concluded that the administration of MENS was highly successful in revert DENA-induced alterations in glyco-regulatory enzyme activities in liver tissue and the circulation back to the normal condition. Collectively, these data highlight one of the mechanisms by which N. sativa may exert its chemo-preventive potential, i.e. by modulation of energy metabolism in both the target organ and circulation, possibly through its potency in normalizing abnormal cell behavior. The lack of toxicity associated with this natural agent combined with its cost effectiveness are additional advantages for its use as a chemo-preventive agent. Based on our results, we recommend the use of NS either as a decoction or its dried methanolic extract for individuals at risk for HCC in order to prevent hepatocarcinogenesis or to break the link between the risk and carcinogenesis.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Somi MH. Hepatocellular-carcinoma: a review article. Hepatitis Monthly. 2005;11:65–76. [Google Scholar]
- 2.Davis GL, Dempster J, Meler JD, Orr DW, Walberg MW, Brown B, Berger BD, O’Connor JK, Goldstein RM. Hepatocellular carcinoma: management of an increasingly common problem. Proc Bayl Univ Med Center. 2008;21:266–280. doi: 10.1080/08998280.2008.11928410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Anwar WA, Khaled HM, Amra HA, El-Nezami H, Loffredo CA. Changing pattern of hepatocellular carcinoma (HCC) and its risk factors in Egypt: possibilities for prevention. Mutat Res. 2008;659:176–184. doi: 10.1016/j.mrrev.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 5.MacPhee DG. Time-dependent mutagenesis and cancer: a new role for antimutagenesis in cancer prevention. Mutat Res. 1998;402:29–39. doi: 10.1016/S0027-5107(97)00279-0. [DOI] [PubMed] [Google Scholar]
- 6.Seufi AM, Ibrahim SS, Elmaghraby TK, Hafez EE. Preventive effect of the flavonoid, quercetin, on hepatic cancer in rats via oxidant/antioxidant activity: molecular and histological evidences. J Exp Clin Cancer Res. 2009;28:80. doi: 10.1186/1756-9966-28-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaefer CM, Milner JA. The role of herbs and spices in cancer prevention. J Nutr Biochem. 2008;19:347–361. doi: 10.1016/j.jnutbio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramakrishnan G, Raghavendran HR, Vinodhkumar R, Devaki T. Suppression of N-nitrosodiethylamine induced hepatocarcinogenesis by silymarin in rats. Chem Biol Interact. 2006;161:104–114. doi: 10.1016/j.cbi.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Sayed MD. Traditional medicine in health care. J Ethnopharm. 1980;2:19–22. doi: 10.1016/0378-8741(80)90023-9. [DOI] [PubMed] [Google Scholar]
- 10.Houghton PJ, Zarka R, de las Heras B, Hoult JR. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61:33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 11.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::AID-PTR621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 12.Omar A, Ghosheh S, Abdulghani A, Houdi A, Crookscor PA. High performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa L) J Pharm Biomed Anal. 1999;19:757–762. doi: 10.1016/S0731-7085(98)00300-8. [DOI] [PubMed] [Google Scholar]
- 13.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharm. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Padhye S, Banerjee S, Ahmad A, Mohammad R, Sarkar FH. From here to eternity—the secret of Pharaohs: therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008;6:495–510. [PMC free article] [PubMed] [Google Scholar]
- 15.Salama AF, Hegazi MAM, Hozifa HM. Natural products and treatment of liver fibrosis in female albino rats. J Med Res Inst. 2006;27:94–101. [Google Scholar]
- 16.Haussinger D, Schliess F. Glutamine metabolism and signaling in the liver. Front Biosci. 2007;12:371–391. doi: 10.2741/2070. [DOI] [PubMed] [Google Scholar]
- 17.Beckner ME, Stracke ML, Liotta LA, Schiffmann E. Glycolysis as primary energy source in tumor cell chemotaxis. J Natl Cancer Inst. 1990;82:1836–1840. doi: 10.1093/jnci/82.23.1836. [DOI] [PubMed] [Google Scholar]
- 18.Greiner EF, Guppy M, Brand K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J Biol Chem. 1994;269:31484–31490. [PubMed] [Google Scholar]
- 19.Permalatha B, Sujatha V, Sachdanandam P. Modulating effect of Semecarpus anacardium L. nut extract on glucose metabolizing enzymes in aflatoxin B1-induced experimental hepatocellular carcinoma. Pharm Res. 1997;36:187–192. doi: 10.1006/phrs.1997.0214. [DOI] [PubMed] [Google Scholar]
- 20.Sivanesan D, Begum VH. Modulating effect of Gynandropsis gynandra L on glucose metabolizing enzymes in aflatoxin B1-induced experimental hepatocellular carcinoma in rats. Indian J Biochem Biophys. 2007;44:477–480. [PubMed] [Google Scholar]
- 21.Parimala R, Sachdanandam P. Effect of Plumbagin on some glucose metabolising enzymes studied in rats in experimental hepatoma. Mol Cell Biochem. 1993;125:59–63. doi: 10.1007/BF00926835. [DOI] [PubMed] [Google Scholar]
- 22.Zhivotovsky B, Orrenius S. The Warburg effect returns to the cancer stage. Semin Cancer Biol. 2009;19:1–3. doi: 10.1016/j.semcancer.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Harris RA. Glycolysis, overview. Encycl Biol Chem. 2004;2:266–271. doi: 10.1016/B0-12-443710-9/00280-5. [DOI] [Google Scholar]
- 24.Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65:613–621. doi: 10.1158/0008-5472.CAN-04-4313. [DOI] [PubMed] [Google Scholar]
- 25.Musa D, Dilsiz N, Hatice Gumushan, Ulakoglu G, Bitiren M. Antitumor activity of an ethanol extract of Nigella sativa seeds. Biol Brat. 2004;59: 735–40.
- 26.Sarkar A, Basak R, Bishayee A, Basak J, Chatterjee M. β-Carotene inhibits rat liver chromosomal aberrations and DNA chain break after a single injection of diethylnitrosamine. Br J Cancer. 1997;76:855–861. doi: 10.1038/bjc.1997.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian P, Mirunalini S, Dakshayani KB, Pandi-Perumal SR, Trakht I, Cardinali DP. Prevention by melatonin of hepatocarcinogenesis in rats injected with N-nitrosodiethylamine. J Pineal Res. 2007;43:305–312. doi: 10.1111/j.1600-079X.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 28.Dakshayani KB, Subramanian P, Manivasagam T, Essa MM, Manoharan S. Melatonin modulates the oxidant–antioxidant imbalance during N-nitrosodiethylamine induced hepatocarcinogenesis in rats. J Pharm Sci. 2005;8:316–321. [PubMed] [Google Scholar]
- 29.Singha BN, Braj R, Singh BR, Sarma BK, Singha HB. Potential chemoprevention of N-nitrosodiethylamine-induced hepatocarcinogenesis by polyphenolics from Acacia nilotica bark. Chem Biol Interact. 2009;181:20–28. doi: 10.1016/j.cbi.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Bergmeyer HU, Grassl M, Walter HE. In: Bergmeyer HU (ed) Methods of enzymatic analysis, 3rd edn. Deerf: Verlag Chem; 1983.
- 31.Velick SF. Glyceraldehyde-3-phosphate dehydrogenase from muscle. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 1. New York: Academic Press 1955, p.401–6.
- 32.Kornberg A. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 1. New York: Academic Press 1955, p. 232.
- 33.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the Biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 34.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 35.Bhosale P, Motiwale L, Ingle AD, Gadre RB, Rao KVK. Protective effect of Rhodotorula glutinis NCIM3353 on the development of hepatic preneoplastic lesions. Curr Sci. 2002;83:303–308. [Google Scholar]
- 36.Loeppky RN. The mechanism of bioactivation of N-nitrosodiethanolamine. Drug Metab Rev. 1999;31:175–193. doi: 10.1081/DMR-100101913. [DOI] [PubMed] [Google Scholar]
- 37.Lijinsky W. N-nitroso compounds in the diet. Mutat Res. 1999;443:129–138. doi: 10.1016/S1383-5742(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 38.Sundaresan S, Subramanian P. S-allycysteine inhibits circulatory lipid peroxidation and promotes antioxidants in N-nitrosodiethylamine-induced carcinogenesis. Pol J Pharmacol. 2003;55:37–42. [PubMed] [Google Scholar]
- 39.Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther. 1996;71:57–81. doi: 10.1016/0163-7258(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 40.Al-Rejaie SS, Aleisa AM, Al-Yahya AA, Bakheet SA, Alsheikh A, Fatani AG, Al-Shabanah OA, Sayed-Ahmed MM. Progression of diethylnitrosamine-induced hepatic carcinogenesis in carnitine-depleted rats. World J Gastroenterol. 2009;15:1373–1380. doi: 10.3748/wjg.15.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gatenby RA, Gillies RJ. Glycolysis in cancer: a potential target for therapy. Int J Biochem Cell Biol. 2007;39:1358–1366. doi: 10.1016/j.biocel.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 42.Homem de Bittencourt PI, Peres CM, Yano MM, Hiratea MH, Curi R. Pyruvate is a lipid precursor for rat lymphocytes in culture: evidence for a lipid exporting capacity. Biochem Mol Biol Int. 1993;30:631–641. [PubMed] [Google Scholar]
- 43.Kondoh H, Lieonart ME, Bernard D, Gil J. Protection from oxidative stress by enhanced glycolysis; a possible mechanism of cellular immortalization. Histol Histopathol. 2007;22:85–90. doi: 10.14670/HH-22.85. [DOI] [PubMed] [Google Scholar]
- 44.Kondoh H, Lieonart ME, Gil J, Beach D, Peters G. Glycolysis and cellular immortalization. Drug Discov Today. 2005;2:263–267. doi: 10.1016/j.ddmec.2005.05.001. [DOI] [Google Scholar]
- 45.Peng S, Lai P, Pan H, Hsiao L, Hsu H. Aberrant expression of the glycolytic enzymes aldolase B and type II hexokinase in hepatocellular carcinoma are predictive markers for advanced stage, early recurrence and poor prognosis. Oncol Rep. 2008;19:1045–1053. [PubMed] [Google Scholar]
- 46.King CP. Molecular imaging. Hepatocellular carcinoma screening, diagnosis, and management. National Institutes of Health: Bethesda; 2004. p. 109–112.
- 47.Tokunaga K, Nakamura Y, Sakata K, Fujimori K, et al. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987;47:5616–5619. [PubMed] [Google Scholar]
- 48.Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–1020. doi: 10.1016/j.ygeno.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Bhatia P, Taylor WR, Greenberg AH, Wright JA. Comparison of glyceraldehyde-3-phosphate dehydrogenase and 28S-ribosomal RNA gene expression as RNA loading controls for northern blot analysis of cell lines of varying malignant potential. Anal Biochem. 1994;216:223–226. doi: 10.1006/abio.1994.1028. [DOI] [PubMed] [Google Scholar]
- 50.Waxman S, Wurmbach E. De-regulation of common housekeeping genes in hepatocellular carcinoma. BMC Genomics. 2007;8:243. doi: 10.1186/1471-2164-8-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiihler A, Van Noorden C. Initial velocities in situ of G6PDH and PGDH and expression of proliferating cell nuclear antigen (PCNA): sensitive diagnostic markers of environmentally induced hepatocellular carcinogenesis in a marine flatfish (Platichthys flesus L.) Aquat Toxicol. 1998;40:233–252. doi: 10.1016/S0166-445X(97)00050-7. [DOI] [Google Scholar]
- 52.Winzer K, Becker W, Van Noorden CJ, Köhler A. Quantitative cytochemical analysis of glucose-6-phosphate dehydrogenase activity in living isolated hepatocytes of European flounder for rapid analysis of xenobiotic effects. J Histochem Cytochem. 2001;49:1025–1032. doi: 10.1177/002215540104900810. [DOI] [PubMed] [Google Scholar]
- 53.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. doi: 10.1016/S0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 54.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 55.Zaher KS, Ahmed WM, Zerize SN. Observations on the biological effects of black cumin seed (Nigella sativa) and green tea (Camellia sinensis) Glob Veterin. 2008;2:198–204. [Google Scholar]
- 56.Ali B, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 57.Thabrewa MI, Mitryb RR, Morsy MA, Hughes RD. Cytotoxic effects of a decoction of Nigella sativa, Hemidesmus indicus and Smilax glabra on human hepatoma HepG2 cells. Life Sci. 2005;77:1319–1330. doi: 10.1016/j.lfs.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 58.Abdallah NM, Noaman ENA, Hala M, Mohamed HE. Freshly crushed black seeds good protective effect during tumor induction and radiotherapy in rats. JASMR. 2008;3:1–9. [Google Scholar]
- 59.Gali-Muhtasib H, Diab-Assaf M, Boltze C, Al-Hmaira J, Hartig R, Roessner A, et al. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004;25:857–866. [PubMed] [Google Scholar]
- 60.Ahmed WA, Hassan SA, Galeb FM, El-Taweel MA, Abu-Bedair FA. The in vitro promising therapeutic activity of thymoquinone on hepatocellular carcinoma (HepG2) cell line. Glob Veterin. 2008;2:233–241. [Google Scholar]
- 61.Mohamed A, Shoker A, Bendjelloul F, Mare A, Alzrigh M, Benghuzzi H, Desin T. Improvement of experimental allergic encephalomyelitis (EAE) by thymoquinone; an oxidative stress inhibitor. Biomed Sci Instrum. 2003;39:440–445. [PubMed] [Google Scholar]