Abstract
Objective
Folate (vitamin B9) plays key roles in cell growth and proliferation through regulating the synthesis and stabilization of DNA and RNA, and its deficiency leads to lymphocytopenia and granulocytopenia. However, precisely how folate deficiency affects the distribution of a variety of white blood cell subsets, including the minor population of basophils, and the cell specificity of the effects remain unclear. Therefore, we examined the effects of a folate-deficient diet on the circulating number of lymphocyte subsets [T-lymphocytes, B-lymphocytes, and natural killer (NK) cells] and granulocyte subsets (neutrophils, eosinophils, and basophils) in rats.
Methods
Rats were divided into two groups, with one receiving the folate-deficient diet (FAD group) and the other a control diet (CON group). All rats were pair-fed for 8 weeks.
Results
Plasma folate level was dramatically lower in the FAD group than in the CON group, and the level of homocysteine in the plasma, a predictor of folate deficiency was significantly higher in the FAD group than in the CON group. The number of T-lymphocytes, B-lymphocytes, and NK cells was significantly lower in the FAD group than in the CON group by 0.73-, 0.49-, and 0.70-fold, respectively, indicating that B-lymphocytes are more sensitive to folate deficiency than the other lymphocyte subsets. As expected, the number of neutrophils and eosinophils was significantly lower in the FAD group than in the CON group. However, the number of basophils, the least common type of granulocyte, showed transiently an increasing tendency in the FAD group as compared with the CON group.
Conclusion
These results suggest that folate deficiency induces lymphocytopenia and granulocytopenia in a cell-specific manner.
Keywords: Dietary folate-deficiency, Lymphocyte subsets, Granulocyte subsets
Introduction
Folate (water-soluble vitamin B9) is a critical co-enzyme that mediates single carbon transfer reactions which lead to the biosynthesis of purine nucleotides and deoxythymidylic acid, which in turn are essential for the synthesis of DNA and RNA [1–3]. Human cells are not able to synthesize folate and since it is an essential nutrient for cell growth and proliferation, humans must obtain folate in their diet, predominantly in legumes, green leafy vegetables, and liver [1, 2, 4]. Folate deficiency is still seen in many developing countries [5, 6]. Furthermore, folate deficiency can be induced by an increased folate requirement, such as during pregnancy because cell growth and proliferation actively occurs in a fetus [1–6].
Folate deficiency is closely associated with anemia, lymphocytopenia, and granulocytopenia [1–9] since blood cells, such as red blood cells (RBCs), lymphocytes, and granulocytes have rapid turnover rates [10]. On the other hand, recent epidemiological data indicate that low serum folate levels and the TT genotype of the MTHFR-C677T polymorphism are associated with increased self-reported doctor-diagnosed asthma and attacks of shortness of breath [11]. The distribution of each lymphocyte and granulocyte subset is essential for the immune responses because the continuous migration of immune cells ensures the detection of antigens and neoplasms and promotes cellular interactions that enable the immune system to execute rapid and effective responses [12–14].
However, precisely how folate deficiency affects the distribution of a variety of white blood cell subsets, including the minor population of basophils, and the cell specificity of the effects remain unclear. In the study reported here, we systematically studied the effects of a folate-deficient diet on the number of lymphocyte subsets [T-lymphocytes, B-lymphocytes, and natural killer (NK) cells] and granulocyte subsets (neutrophils, eosinophils, and basophils) in rats.
Materials and methods
Animal care and experimental procedure
Five-week-old male Sprague–Dawley rats (n = 22) were purchased from CLEA Japan (Tokyo, Japan). The rats were individually housed in stainless steel wire mesh cages at 23–25 °C and a relative humidity of 50–60 %, and light was automatically provided from 0700 to 1900 hours [15]. The animals had free access to food and water throughout the study. After a 5-day acclimation to their new environment, the rats were divided into two groups, with one group (n = 12; initial body weight 175 ± 2 g) receiving the folate-deficient diet (FAD; 7 μg folate/100 g diet; Oriental Yeast Co., Tokyo, Japan) and one group (n = 10; initial body weight 175 ± 2 g) receiving the control diet [CON; normal requirement folate (20 mg folate/100 g diet); Oriental Yeast Co.] (Fig. 1). The FAD rats were fed their respective diets for 8 weeks (Fig. 1). One-to-one pair feeding was conducted in the experiment [16]. The nutritional composition of both diets is presented in Table 1. Eight weeks after the initiation of the FAD diet, body weight, body weight gain, total food intake, food efficiency and total water intake of all rats were determined. Food efficiency was calculated from the ratio of body weight gain to total food intake [16].
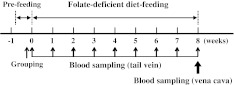
Fig. 1.
Experimental protocol. Pre-feedings were carried out for 5 days. After the pre-feeding, rats were divided into two groups, with one group receiving the folate-deficient diet (FAD) and the other the control diet (CON). During the experimental period, the number of total white blood cells, total lymphocytes, neutrophils, eosinophils, and basophils was analyzed using blood obtained from the tail vein in a repetitive manner. At the end of the experiment, the parameters of red blood cells (the number of red blood cells, hemoglobin concentration, and hematocrit) and the number of lymphocyte subsets [T-lymphocytes, B-lymphocytes, and natural killer (NK) cells] were analyzed using blood obtained from tail vein, and the plasma folate and homocysteine concentrations were assessed using plasma prepared from blood obtained from vena cava
Table 1.
Composition of the experimental diet used in the study
| Ingredient (g/kg diet) | Group | |
|---|---|---|
| CON | FAD | |
| Vitamin free casein | 200 | 200 |
| l-Cystine | 3 | 3 |
| Cornstarch | 397.5 | 397.5 |
| α-Cornstarch | 132 | 132 |
| Sucrose | 90 | 90 |
| Soybean oil | 70 | 70 |
| Cellulose | 50 | 50 |
| s93g | 35 | 35 |
| Vitamin mix (AIN-93G-VX + folate)a | 10 | – |
| Vitamin mix (AIN-93G-VX − folate)b | – | 10 |
| Choline bitartrate | 2.5 | 2.5 |
| tert-Butylhydroquinone | 0.014 | 0.014 |
| Succinyl sulfathiazole | 10 | 10 |
CON Control group, FAD folate-deficient group
aVitamin mix (AIN-93G-VX + folate), with folate at a normal dietary level (20 mg/100 g diet)
bVitamin mix (AIN-93G-VX + folate), with folate at a deficient dietary level (7 μg/100 g diet)
All experimental procedures and animal care were approved by the Animal Ethics Committee, Waseda University (No. 2011-A001). This study was carried out in accordance with the “Guiding Principle for the Care and Use of Animals in the Field of Physiological Science” of the Physiological Society of Japan. The experiment was performed with the least possible pain or discomfort to the rats.
Assays of plasma folate and homocysteine concentrations
On the final day of the experiment, the rats were anesthetized by the intraperitoneal administration of sodium pentobarbital (50 mg/kg body weight), and blood samples were collected in the heparinized condition from the inferior vena cava and subsequently centrifuged at 3,000 g for 10 min at 4 °C to obtain plasma. Plasma samples were stored at −80 °C until each analysis. The assay of plasma folate concentration was carried out using the reagent kit of Access Folic Acid (FOL2) (Beckman Coulter, Brea, CA) with UniCell Dxl 800 (Beckman Coulter). Plasma homocysteine concentration was analyzed by high-performance liquid chromatography (Shimadzu Co., Kyoto, Japan) [17].
Analyses of RBCs, total white blood cells, total lymphocytes, and granulocyte subsets
Whole blood samples were collected from the tail vein into heparinized microcapillary tubes, and 50-μl samples were immediately diluted by 20 % with Cellpack reagent (Sysmex Co., Hyogo, Japan) [13, 14, 16]. All analyses pertaining to the number of RBCs, hemoglobin concentration, and hematocrit, as well as the number of total white blood cells (WBCs), total lymphocytes, neutrophils, eosinophils, and basophils were performed using hematology analyzers (models SF-3000 and SFVU-1; Sysmex Co.) [13, 14, 16]. The number of basophils was independently determined in separate chamber of the analzyer. In the chamber, the blood sample is treated with stromatolyser-FB (lysing reagent for the analysis of basophils), which leads to nuclear membrane dissolution and cell shrinkage of WBCs other than basophils. In our study, the circulating number of basophils from rat tail vein was estimated as 170 ± 26 cells/μl (coefficient of variance 48 %).
Count analyses of lymphocyte subsets
On the final day of the experiment, the number of T-lymphocytes, B-lymphocytes, and NK cells were determined by direct immunofluorescent staining with a flow-cytometric analysis according to routine methods [16]. We used the Beckman Coulter flow cytometry reagent IOTest Anti-Rat CD3-FITC/CD45RA-PC7/CD161a-APC. Briefly, 25-μl whole blood samples collected from the tail vain were incubated with 25 μl of the primary antibodies for 20 min at room temperature in the dark, followed by the addition of 1 ml of BD FACS Lysing Solution (Becton–Dickinson, Franklin Lakes, NJ) and a further 10-min incubation and then by centrifugation at 1,500 g for 3 min at 4 °C. The supernatant was then discarded and replaced with 0.5 ml of phosphate-buffered saline (PBS, pH 7.4), the cell mixture was filtered through Cell Strainer (strong nylon mesh, pore size 40 μm; Becton–Dickinson), and the cells were analyzed using FACS Calibur (Becton–Dickinson).
Statistical analyses
Experimental data are presented as the mean ± standard error of the mean (SEM). The effects of folate deficiency on body weight, food and water intake parameters, plasma folate and homocysteine concentrations, RBC parameters (Table 2), the number of various lymphocyte subsets (Fig. 3), and the cell ratios of lymphocyte subsets (Table 3) and granulocyte subsets (Table 4) were tested by Student’s t test. Two-way analysis of variance [one-way between (group) and one-way within (time) factors] with repeated measures was used to examine the effects of folate deficiency on the number of total WBCs, total lymphocytes (Fig. 2), and various granulocyte subsets (Fig. 4). When the factors were significant, subsequent post hoc analyses to determine differences between the FAD and CON groups were performed by Fisher’s PLSD test. The differences were considered significant at p was <0.05.
Table 2.
The induction of folate deficiency and anemic phenotype
| Parameters | Group | FAD/CONa | |
|---|---|---|---|
| CON | FAD | ||
| Body weight (g) | 508 ± 8 | 494 ± 6 | 0.97 |
| Body weight gain (g) | 323 ± 5 | 307 ± 6 | 0.95 |
| Total food intake (kg) | 1.22 ± 0.01 | 1.21 ± 0.01 | 0.99 |
| Food efficiencya | 0.26 ± 0.004 | 0.25 ± 0.005 | 0.96 |
| Total water intake (kg) | 1.75 ± 0.08 | 1.73 ± 0.06 | 1.00 |
| Plasma folic acid (ng/ml) | 44.0 ± 2.5 | 1.6 ± 0.1*** | 0.04 |
| Plasma homocysteine (nmol/ml) | 4.7 ± 2.0 | 24.3 ± 2.1*** | 5.13 |
| Red blood cells (×104/μl) | 920 ± 7.8 | 862 ± 12.1*** | 0.94 |
| Hemoglobin (g/dl) | 17.0 ± 0.2 | 16.3 ± 0.2** | 0.96 |
| Hematocrit (%) | 46.2 ± 0.4 | 43.7 ± 0.5*** | 0.94 |
**p < 0.01 and ***p < 0.001 (vs. CON groups)
Data are presented as the mean ± standard error of the mean (SEM) (n = 10–12)
aData are given as the relative ratios of the FAD groups to the CON groups
Fig. 3.
Effects of FAD-feeding on the number of total lymphocytes (a), T-lymphocytes (b), B-lymphocytes (c), and NK cells (d). Values are shown as the mean ± SEM. Open bars CON groups (n = 10), closed bars FAD groups (n = 12). Values in parentheses Relative ratios of the FAD groups to the CON groups. *p < 0.05, **p < 0.01, ***p < 0.001 (vs. CON groups)
Table 3.
Effects of folate-deficient diet on cell ratioa of lymphocyte subsets
| Parameters | Group | FAD/CONb | |
|---|---|---|---|
| CON | FAD | ||
| T-lymphocytes (%) | 59.3 ± 2.7 | 67.2 ± 1.6* | 1.13 |
| B-lymphocytes (%) | 35.2 ± 2.8 | 26.6 ± 1.7* | 0.76 |
| NK cells (%) | 5.5 ± 0.6 | 6.2 ± 0.8 | 1.11 |
*p < 0.05 (vs. CON groups)
Values are shown as the mean ± SEM (n = 10–12)
aCell ratio of lymphocyte subsets was calculated by the ratio of each lymphocyte subset number to total lymphocyte numbers at the end of the experiment
bData are given as the relative ratios of the FAD groups to the CON groups
Table 4.
Effects of the folate-deficient diet on the cell ratioa of granulocyte subsets
| Parameters | Group | FAD/CONb | |
|---|---|---|---|
| CON | FAD | ||
| Neutrophils (%) | 91 ± 0.01 | 92 ± 0.01 | 1.02 |
| Eosinophils (%) | 9 ± 0.01 | 5.6 ± 0.01** | 0.68 |
| Basophils (%) | 0.5 ± 0.03 | 1.8 ± 0.11* | 3.29 |
*p < 0.05, **p < 0.01 (vs. CON groups)
Values are shown as mean ± SEM (n = 10–12)
aCell ratio of granulocyte subsets was calculated by the ratio of each granulocyte subset number to total granulocyte numbers at the end of the experiment
bData are given as the relative ratios of the FAD groups to the CON groups
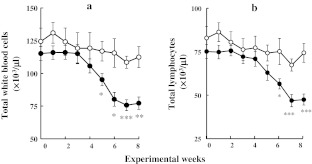
Fig. 2.
Effects of FAD-feeding on the number of total white blood cells (a) and total lymphocytes (b). Values are shown as the mean ± standard error of the mean (SEM). Open circles CON groups (n = 10), closed circles FAD groups (n = 12). *p < 0.05, **p < 0.01, ***p < 0.001 (vs. CON groups)
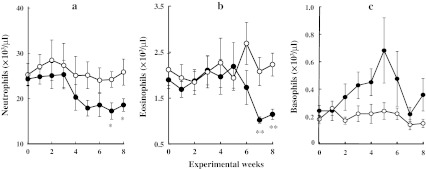
Fig. 4.
Effects of FAD-feeding on the number of neutrophils (a), eosinophils (b), and basophils (c). Values are shown as the mean ± SEM. Open circles CON groups (n = 10), closed circles FAD groups (n = 12). *p < 0.05, **p < 0.01 (vs. CON groups)
Results
Induction of folate deficiency
Body weight, body weight gain, total food intake, food efficiency, and total water intake were not different between the two groups of rats (FAD and CON) after 8 weeks of dietary treatment (Table 2). Compared to the CON group, the FAD group had a dramatically lower plasma folate level and a significantly higher level of plasma homocysteine, which is a predictor of folate deficiency [18, 19] (Table 2). Anemic phenotypes were also clearly observed among the FAD rats (Table 2).
Effects of folate deficiency on the number and cell ratio of lymphocyte subsets
The number of total WBCs and total lymphocytes in the CON group did not change during the experimental period (Fig. 2a, b). However, these parameters in the FAD group began show a significant reduction after 5 and 6 weeks of FAD-feeding, as compared with the CON group (Fig. 2a, b). After 8 weeks of FAD-feeding, the number of total lymphocytes was significantly lower in the FAD group than in the CON group (Fig. 3a). Among the lymphocyte subsets, the number of T-lymphocytes, B-lymphocytes, and NK cells were significantly lower in the FAD group than in the CON group, respectively (Fig. 3b, c, d).
In addition, the changes in the cell ratio of each lymphocyte subset to total lymphocytes after 8 weeks of FAD-feeding were also analyzed. Among the lymphocyte subsets, the cell ratio of T-lymphocytes was significantly higher in the FAD group than in the CON group (Table 3), although that of B-lymphocytes was significantly lower in the FAD group than in the CON group (Table 3).
Effects of folate-deficiency on the number and cell ratio of granulocyte subsets
After 7 and 8 weeks of FAD-feeding, the FAD rats had a significantly lower number of granulocyte subsets, including neutrophils and eosinophils, than the CON group (Figs. 4a, b). In contrast, the number of basophils showed transiently a tendency to increase in the FAD group, as compared with the CON group, during the experimental period, although the differences in the values between the two groups were not significant (Fig. 4c).
The cell ratio of each granulocyte subset to total granulocytes after 8 weeks from the onset of the FAD diet were analyzed (Table 4). The cell ratio of neutrophils was similar between the FAD and CON groups, although that of eosinophils was significantly lower in the FAD group than in the CON group. In addition, the cell ratio of basophils was markedly higher in the FAD group than in the CON group.
Discussion
It is generally accepted that folate deficiency leads to lymphocytopenia [9]. Indeed, Dhur et al. [20] demonstrated that the number of CD4- and CD8-double positive immature T-lymphocytes in the thymus and Thy-1-positive mature T-lymphocytes in the spleen was decreased in rats fed a FAD. However, these authors did not systematically elucidate the effects of a FAD on the circulating number of various lymphocyte subsets. In this study, we demonstrated that the circulating number of T-lymphocytes, B-lymphocytes, and NK cells were generally reduced after 8 weeks from the onset of FAD in rats. The reduction ratio of B-lymphocytes was greater than that of other lymphocyte subsets, such as T-lymphocytes and NK cells, suggesting that B-lymphocytes are more sensitive to folate-deficiency than other lymphocyte subsets. It has been reported that turnover rates of B-lymphocytes, T-lymphocytes, and NK cells are approximately 1, 2, and 2 % per day, respectively [10]. These results indicate that the turnover rates of lymphocyte subsets are not associated with folate deficiency-induced reduction in the respective cell numbers.
One in vitro study revealed that the proliferative activity of primary human CD8-positive cytotoxic T-lymphocytes was inhibited by folate deficiency [21]. It has also been demonstrated in vitro that folate deficiency induces apoptotic cell death of phytohemagglutinin-stimulated human primary lymphocytes from the blood through triggering mitochondrial membrane depolarization [22].
In contrast, little is known about the effects of folate deficiency on the number of B-lymphocytes. In our study, the number of B-lymphocytes in the FAD rats fell at a faster rate than the number of T-lymphocytes. It is conceivable that the sensitivity of B-lymphocytes to folate and/or other metabolites is higher than that of other subsets. The extensive reduction in the number of circulating B-lymphocytes due to folate deficiency may cause antibody production against pathogenic microoganisms to be depressed. Further study is necessary to clarify the mechanism(s) of these phenomena in more detail, as well as the effects of FAD on antibody production and susceptibility to infection. We also demonstrated that the circulating number of NK cells was also clearly reduced by the FAD, although the cell ratio was not significantly changed. Kim et al. [23] reported that the cytotoxicity of NK cells was impaired by a FAD. Based on these findings, we suggest that the FAD both reduces the number and suppresses the function of NK cells, and that this process may result in the attenuation of immune responses against viral infection and tumorigenesis.
Folate deficiency also leads to granulocytopenia [7, 8, 24]. In our study, within granulocyte subsets, the number of neutrophils and eosinophils gradually declined with increasing length of time that the rats were on the FAD, although the number of basophils in FAD rats showed only transiently an increasing tendency during this same period. These results suggest that the production of basophils is not suppressed by folate deficiency. In addition, the cell ratio analysis indicates that the reduction in the ratio of eosinophils is greater than that of neutrophils and that the cell ratio of basophils is increased after feeding on the FAD for 8 weeks.
We previously demonstrated that the circulating number of basophils was transiently and dramatically increased when rats were suspended (whole-body suspension in which rats were held in a denim suspension harness, resulting in no hindlimb skeletal muscle loading and reduced movement) for 10 days, but that it did not change during immobilization (rats were kept in small cages, resulting in restricted physical activity and reduced skeletal muscle movement) for the same length of time; in contrast that of neutrophils and eosinophils did increase significantly under both conditions throughout the experimental period [13]. These findings indicate that regulatory mechanism of basophil production and/or recruitment is different from that of other granulocytes, such as neutrophils and eosinophils [13].
Based on our results, it is conceivable that the transiently increased (tendency, not significant) circulating number of basophils reflects decreasing plasma folate level due to feeding on a FAD. However, since folate is an essential nutrient for cell growth and proliferation, activation of the production and/or recruitment of basophils may be gradually attenuated with folate deficiency. To elucidate whether the activity is actually elevated by decreasing plasma folate level, it is necessary to analyze the time-course changes in the plasma folate level after the onset of FAD-feeding, as well as the number and cell ratio of basophils in bone marrow cells, particularly after 5 weeks from the onset of the FAD-feeding.
In conclusion, as expected, FAD-feed resulted in a reduction in the total number of circulating lymphocytes and granulocytes. However, within the lymphocyte subsets, the number of B-lymphocytes was relatively more susceptible to the effects of a FAD than the T-lymphocytes and NK cells—at least after 8 weeks of FAD-feeding. In addition, the number of basophils tended to increase during the period of FAD-feeding, although that of neutrophils and eosinophils significantly reduced during the same period. These results suggest that folate deficiency is able to induce lymphocytopenia and granulocytopenia in a cell-specific manner.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Young Scientists (B) (2010–2011, 22790545 for K. Shirato) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and a Grant-in-Aid from Global Center of Excellence (COE) program, Graduate School of Sport Sciences, Waseda University (2009-2013, for K. Imaizumi) of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of interest
None.
References
- 1.Krishnaswamy K, Madhavan Nair K. Importance of folate in human nutrition. Br J Nutr. 2001;85:S115–S124. doi: 10.1079/BJN2000303. [DOI] [PubMed] [Google Scholar]
- 2.Sethuraman U. Vitamins. Pediatr Rev. 2006;27:44–55. doi: 10.1542/pir.27-2-44. [DOI] [PubMed] [Google Scholar]
- 3.Iyer R, Tomar SK. Folate: a functional food constituent. J Food Sci. 2009;74:R114–R122. doi: 10.1111/j.1750-3841.2009.01359.x. [DOI] [PubMed] [Google Scholar]
- 4.Allen LH. Causes of vitamin B12 and folate deficiency. Food Nutr Bull. 2008;29:S20–S37. doi: 10.1177/15648265080292S105. [DOI] [PubMed] [Google Scholar]
- 5.Antony AC. In utero physiology: role of folic acid in nutrient delivery and fetal development. Am J Clin Nutr. 2007;85:598S–603S. doi: 10.1093/ajcn/85.2.598S. [DOI] [PubMed] [Google Scholar]
- 6.Metz J. A high prevalence of biochemical evidence of vitamin B12 or folate deficiency does not translate into a comparable prevalence of anemia. Food Nutr Bull. 2008;29:S74–S85. doi: 10.1177/15648265080292S111. [DOI] [PubMed] [Google Scholar]
- 7.Liu YK. Effects of alcohol on granulocytes and lymphocytes. Semin Hematol. 1980;17:130–136. [PubMed] [Google Scholar]
- 8.Koury MJ, Horne DW. Apoptosis mediates and thymidine prevents erythroblast destruction in folate deficiency anemia. Proc Natl Acad Sci USA. 1994;91:4067–4071. doi: 10.1073/pnas.91.9.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duthie SJ, Hawdon A. DNA instability (strand breakage, uracil misincorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. FASEB J. 1998;12:1491–1497. [PubMed] [Google Scholar]
- 10.De Boer RJ, Mohri H, Ho DD, Perelson AS. Turnover rates of B cells, T cells, and NK cells in simian immunodeficiency virus-infected and uninfected rhesus macaques. J Immunol. 2003;170:2479–2487. doi: 10.4049/jimmunol.170.5.2479. [DOI] [PubMed] [Google Scholar]
- 11.Thuesen BH, Husemoen LL, Ovesen L, Jørgensen T, Fenger M, Gilderson G, et al. Atopy, asthma, and lung function in relation to folate and vitamin B12 in adults. Allergy. 2010;65:1446–1454. doi: 10.1111/j.1398-9995.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 12.Engler H, Dawils L, Hoves S, Kurth S, Stevenson JR, Schauenstein K, et al. Effects of social stress on blood leukocyte distribution: the role of α- and β-adrenergic mechanisms. J Neuroimmunol. 2004;156:153–162. doi: 10.1016/j.jneuroim.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Shirato K, Motohashi N, Tanihata J, Tachiyashiki K, Tomoda A, Imaizumi K. Effects of two types of inactivity on the number of white blood cells in rats. Eur J Appl Physiol. 2006;98:590–600. doi: 10.1007/s00421-006-0306-6. [DOI] [PubMed] [Google Scholar]
- 14.Shirato K, Tanihata J, Motohashi N, Tachiyashiki K, Tomoda A, Imaizumi K. β2-agonist clenbuterol induced changes in the distribution of white blood cells in rats. J Pharmacol Sci. 2007;104:146–152. doi: 10.1254/jphs.FP0070267. [DOI] [PubMed] [Google Scholar]
- 15.Higashino-Matsui Y, Shirato K, Suzuki Y, Kawashima Y, Someya Y, Sato S, et al. Age-related effects of fasting on ketone body production during lipolysis in rats. Environ Health Prev Med. 2012;17:157–163. doi: 10.1007/s12199-011-0231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Someya Y, Tanihata J, Sato S, Kawano F, Shirato K, Sugiyama M, et al. Zinc-deficiency induced changes in the distribution of rat white blood cells. J Nutr Sci Vitaminol. 2009;55:162–169. doi: 10.3177/jnsv.55.162. [DOI] [PubMed] [Google Scholar]
- 17.Ubbink JB, Hayward Vermaak WJ, Bissbort S. Rapid high-performance liquid chromatographic assay for total homocysteine levels in human serum. J Chromatogr. 1991;565:441–446. doi: 10.1016/0378-4347(91)80407-4. [DOI] [PubMed] [Google Scholar]
- 18.Rogers EJ, Chen S, Chan A. Folate deficiency and plasma homocysteine during increased oxidative stress. N Engl J Med. 2007;357:421–422. doi: 10.1056/NEJMc066569. [DOI] [PubMed] [Google Scholar]
- 19.Cacciapuoti F. Hyper-homocysteinemia: a novel risk factor or a powerful marker for cardiovascular diseases? Pathogenetic and therapeutical uncertainties. J Thromb Thrombolysis. 2011;32:82–88. doi: 10.1007/s11239-011-0550-4. [DOI] [PubMed] [Google Scholar]
- 20.Dhur A, Galán P, Christides JP, Polier de Courcy G, Preziosi P, Hercberg S. Effect of folic acid deficiency upon lymphocyte subsets from lymphoid organs in mice. Comp Biochem Physiol Comp Physiol. 1991;98:235–240. doi: 10.1016/0300-9629(91)90526-I. [DOI] [PubMed] [Google Scholar]
- 21.Courtemanche C, Elson-Schwab I, Mashiyama ST, Kerry N, Ames BN. Folate deficiency inhibits the proliferation of primary human CD8+ T lymphocytes in vitro. J Immunol. 2004;173:3186–3192. doi: 10.4049/jimmunol.173.5.3186. [DOI] [PubMed] [Google Scholar]
- 22.Lin HL, Chen CJ, Tsai WC, Yen JH, Liu HW. In vitro folate deficiency induces apoptosis by a p53, Fas (Apo-1, CD95) independent, bcl-2 related mechanism in phytohaemagglutinin-stimulated human peripheral blood lymphocytes. Br J Nutr. 2006;95:870–878. doi: 10.1079/BJN20051579. [DOI] [PubMed] [Google Scholar]
- 23.Kim YI, Hayek M, Mason JB, Meydani SN. Severe folate deficiency impairs natural killer cell-mediated cytotoxicity in rats. J Nutr. 2002;132:1361–1367. doi: 10.1093/jn/132.6.1361. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan SS, Basford RE. Effect of vitamin B12 and folic acid deficiencies on neutrophil function. Blood. 1976;47:801–805. [PubMed] [Google Scholar]