Abstract
Fluid efflux across the region of the amnion overlying the placenta is an essential component of the intramembranous absorption pathway that maintains amniotic fluid volume homeostasis. Dysregulation of this pathway may result in adverse pregnancy outcomes, however the factors controlling amnion permeability are unknown. Here, we report a novel mechanism that increases placental amnion permeability. Pre-B Cell Colony Enhancing Factor (PBEF) is a stress-responsive cytokine expressed by the human amnion, and is known to induce Vascular Endothelial Growth Factor (VEGF) production by other cell types. Interestingly, VEGF is up-regulated in the ovine amnion when intramembranous absorption is augmented. In this study, we show that PBEF induced VEGF secretion by primary human amniotic epithelial cells (AEC) derived from the placental amnion, as well as from the reflected amnion that lines the remainder of the gestational sac. Further, PBEF treatment led to the increased expression of VEGFR2 in placental AEC, but not reflected AEC. To test the hypothesis that PBEF and VEGF increase placental amnion permeability, we monitored the transfer of 2′,7′-dichlorofluorescein (DCF) from the fetal to the maternal side of human amnion explants. A treatment regimen including both PBEF and VEGF increased the rate of DCF transfer across the placental amnion, but not the reflected amnion. In summary, our results suggest that by augmenting VEGFR2 expression in the placental amnion, PBEF primes the tissue for a VEGF-mediated increase in permeability. This mechanism may have important implications in amniotic fluid volume control throughout gestation.
Keywords: Pre-B cell colony enhancing factor (PBEF), Visfatin, Nicotinamide phosphoribosyltransferase (NAMPT), Vascular endothelial growth factor (VEGF), Amnion, Permeability
1. Introduction
To ensure proper fetal development, amniotic fluid turns over constantly and is regulated by the intramembranous absorption pathway of fluid efflux to maintain an ideal volume [1]. Intramembranous absorption (IA) is defined as the transfer of AF from the gestational sac into the blood vessels lining the fetal surface of the placenta, thus allowing the AF to re-enter the fetal circulation [2,3]. Dysregulated AF volume results in the pathologies polyhydramnios (too much AF) or oligohydramnios (too little AF), both associated with preterm birth and perinatal morbidity or mortality. Although there are fetal abnormalities or maternal conditions recognized to cause poly- or oligohydramnios, most incidents of these AF volume disorders are of an unknown etiology [4,5]. These idiopathic cases may result from dysregulated IA, however, the mechanisms governing this important pathway are unknown.
The amnion is the innermost layer of the fetal membranes, and is thought to be the primary site that limits the efflux of amniotic fluid [6]. Composed of a single layer of amniotic epithelial cells (AEC) and its underlying collagen matrix embedded with mesenchymal cells, the amnion is divided into multiple discrete, yet continuous regions: the “placental” amnion lies at the interface between the amniotic fluid (AF) and placenta, whereas the “reflected” amnion lines the remainder of the fetal sac [7]. Since the placental amnion is situated such that AF and solutes must transfer through it to access the fetal placental vessels, factors influencing the permeability of the placental amnion may ultimately regulate AF volume by controlling IA.
Pre-B cell colony enhancing factor (PBEF) is an ubiquitous, evolutionarily conserved protein that is constitutively expressed in the fetal membranes where its greatest expression is in the amnion [8]. PBEF was originally identified as a cytokine that potentiated the clonal expansion and differentiation of pre-B cells [9], but it is also acknowledged to be the ubiquitous intracellular enzyme nicotinamide phosphoribosyltranferase (NAMPT) [10] and the adipokine “visfatin” [11]. A stress-responsive gene, PBEF is up-regulated in hypoxic [12] and inflammatory conditions [13]. In such conditions, PBEF can prevent apoptosis in several cell types [14,15] and mediate an increase in lung epithelial permeability [16]. Interestingly, PBEF/visfatin also stimulates angiogenesis in endothelial cells by up-regulating their production of vascular endothelial growth factor (VEGF) [17].
Studies in pregnant sheep have shown that amniotic VEGF expression is up-regulated in vivo simultaneous with increases in IA [18,19], qualifying VEGF as a candidate factor affecting amnion permeability. Although VEGF is classically recognized to mediate the proliferation and permeability of the endothelium, it can also increase the permeability of epithelial tissues [20,21]. VEGF increases caveolin-1 activity in ovine AECs, implicating a transcytotic caveolae-mediated mechanism of amniotic fluid transport across the amnion [22]. However, VEGF treatment alone does not alter the permeability of ovine AEC monolayers in vitro [23], suggesting other factors may be involved. Given that PBEF induces VEGF production in endothelial cells, and the concomitant up-regulation of VEGF and its receptors has been noted in several tissue types [24,25] including the amnion in pre-term labor [26], we sought to determine if these same phenomena occur in human AEC. In addition, as VEGF is likely involved in the regulation of IA, we tested the hypothesis that PBEF could augment a VEGF-mediated increase in human placental amnion permeability.
2. Materials and methods
2.1. Tissue collection and amniotic epithelial cell culture
Fetal membranes were collected anonymously, immediately following singleton, 38–40 week gestation, elective Cesarean sections prior to labor at Kapi’olani Medical Center for Women and Children (Honolulu, HI, USA) with approval from the Hospital Institutional Review Board.
Leaving a 1–2 cm margin around the placenta, the reflected fetal membranes were removed and the placental amnion was peeled from the chorionic plate. If the membranes were utilized for explant permeability studies, two sutures were inserted in the placental amnion during removal for orientation purposes.
Primary AEC were isolated as previously described [14,27]. Laboratory observations revealed that reflected AEC attached to cultureware and proliferated more efficiently than placental AEC, so the reflected were plated on tissue culture-treated dishes (BD Falcon) at 70, 000 cell/cm2 and the placental at 80,000 cells/cm2. AEC were cultured in complete DMEM:F12 with 10% FBS (Invitrogen, Carlsbad, CA), penicillin (10 U/ml)-streptomycin (50 μg/ml) and incubated at 37 °C in 95% air/5% CO2. Media was changed every 2–3 days and the cells were utilized without passage upon reaching confluence (6–10 days).
2.2. Recombinant PBEF production
Hexahistidine-tagged PBEF was prepared as previously reported [8].
2.3. PBEF treatment of isolated AEC
Upon reaching confluence, primary AEC were serum-starved for 16 h in 0.5% FBS-containing DMEM:F12 medium then treated with 0,10,100, and 250 ng/ml PBEF for 24 h. The media was collected, centrifuged 16,000 g for 10 min at 4 °C, and the supernatant was stored at −80 °C. Whole cell lysates were collected for western blotting (below) and stored at −80 °C.
2.4. VEGF ELISA
Sandwich ELISA assays were conducted using the Quantikine VEGF Immunoassay (#DVE00, R&D Systems) according to the manufacturer instructions. Each sample was tested in duplicate, and the optical density was measured on a VersaMax plate reader (Molecular Devices, Sunnyvale, CA).
2.5. Immunohistochemistry
Placenta and fetal membranes were washed in PBS and placed in formalin within 1 h following collection. Overnight fixation was followed by daily changes to 70% ethanol for 3 days and subsequent paraffin embedding. Prior to staining, the slides were deparaffinized and hydrated through xylenes and a graded alcohol series, antigen unmasked for 30 min in steam using 10 mM sodium citrate pH 6.0 for VEGFR1 labeling or 1 mM EDTA pH 8.0 for VEGFR2, and peroxidase-quenched with 0.3% hydrogen peroxide for 30 min. Sections were incubated with VEGFR1 (1:250) antibody (#ab32152, Abcam, Cambridge, MA) and the VEGFR2 (1:300) antibody (#2479, Cell Signaling Technology, Danvers, MA). All slides were stained using Vectastain ABC Elite kits (Vector Laboratories, Burlingame, CA) according to the provided protocol, and developed using the DAB Peroxidase Substrate Kit (Vector Laboratories) and mounted using Pro-Texx (Lerner Laboratories, Pittsburgh, PA).
2.6. Western blotting
Whole cell lysates were prepared by washing the cells with ice-cold PBS and scraping into a modified RIPA buffer containing EDTA-Free Protease Cocktail Inhibitor and PhosSTOP Phosphatase Inhibitor Cocktail (Roche, Indianapolis, IN). Protein concentrations were determined using the BCA Protein Assay Kit (Thermo Pierce Scientific, Rockford, IL).
Cell lysates (25 μg) were heated at 95 °C for 7 min in 1 × SDS buffer with 5% betamercaptoethanol. Electrophoretically separated proteins were transferred to nitrocellulose membranes. Immunoblotting was performed by blocking with either 5% non-fat milk in PBS with 0.01% Tween-20 or Odyssey Blocking Buffer (Li-Cor Biosciences, Lincoln, NE) containing 0.01% Tween-20. The membranes were incubated with primary antibodies to VEGFR1 (1:10,000), VEGFR2 (1:1000), or Betaactin (1:5000) (Abcam #ab8227, Cambridge, MA) for 1 h at room temperature in blocking buffer. The membranes were incubated with IRDye 800CW goat anti-rabbit IgG secondary antibody (1:5000, Licor) for 45 min at room temperature in blocking buffer. The membranes were scanned and the signals were quantitated (Odyssey Infrared Imaging System, LiCor Biosciences). The relative levels of receptor expression were normalized to the actin signal for each sample.
2.7. Permeability assays
The manufacturer’s synthetic membranes were removed from 12-well transwell inserts (Corning, Lowell, MA), the plastic scored and re-sterilized in 70% ethanol and dried under UV overnight. 2.5 cm2 pieces of placental (n = 12) and reflected amnion (n = 7) were cut and attached to the transwell inserts using sterile 5/16 inch dental bands to ensure a complete seal. The tissues were oriented so the fetal side of the membrane faced up in the 12-well dishes. Explants were allowed to equilibrate in a 37 °C, 95% air/5% CO2 incubator for a minimum of 2 h before experimentation. Explants were separated into 4 treatment groups: 1) untreated controls; 2) PBEF-pretreatment (100 ng/ml) for 18 h; 3) acute VEGF treatment (20 ng/ml); 4) PBEF-pretreatment (100 ng/ml) for 18 h plus acute VEGF treatment (20 ng/ml). All treatments were performed in triplicate. PBEF-pretreated explants were exposed to PBEF in both the top and bottom wells for 18 h. Subsequently, the media in the top and bottom wells were exchanged for fresh media with additives according to the treatment group. Culture medium added to the top well of all treatment groups contained 25 μM 2,7-dichlorosfluorescein (DCF) (Sigma), and contained VEGF for treatment groups 3 and 4. Medium in the bottom wells of treatment groups 3 and 4 also contained VEGF. Ten microliter aliquots were transferred from the bottom well into black walled 96-well plates (Corning) at 2.5, 5, 10, 15, 20, 30, 40, 60, 80, 100, 120, 140, and 160 min following DCF ± VEGF addition. At the end of the time course, 40 μl of PBS was added to each well and the fluorescent values were measured (SpectraMAX Gemini XS, Molecular Devices) at 484/535 excitation/emission. DCF μM values were calculated using a DCF standard curve (n = 52, average r2 = 0.999). Damaged or improperly sealed tissue explants were identified after the experiment; if the fluorescent readings within the first 10 min of the experiment were greater than 10-fold the average of all the values at any given time-point, these explants were considered compromised and excluded from analysis.
2.8. Statistics and data analyses
2.8.1. ELISAs and westerns
The ELISA data were normalized to the untreated control to reflect the fold change in VEGF secretion following PBEF treatment. Normalization was due to the wide variation in baseline VEGF production, which is normal for primary cells derived from different patients. The data were analyzed by repeated-measures ANOVA with a Dunnett post test using Prism 5.0 (GraphPad Software, La Jolla, CA). Western blot densitometry values also varied among experiments, therefore the values were adjusted to fold change compared to untreated controls and analyzed by Mann–Whitney tests.
2.8.2. Analysis of the explant permeability data
The average μM DCF value was calculated at each time point for each treatment group and correlating averages from all experiments were pooled for statistical analysis. Prism 5.0 was used for all statistical and regression analyses.
Assuming transport was constant across the membranes, a linear regression analysis was performed on the data sets from both the placental and reflected amnion. We used a runs test to evaluate whether the data sets deviated systematically from linearity; a non-linear fit implied that DCF transfer approached equilibrium. By excluding late time points from the analysis and repeating a runs test, we determined through which time-point every treatment from both tissues fell into the linear range. Restricting the data sets to the linear range to examine early conditions when DCF transport was not saturated, Prism compared the slopes of these lines using a method equivalent to an analysis of covariance (ANCOVA) to detect significant differences in the rate of DCF accumulation on the maternal side of the explants. We also compared the slopes of the non-treated tissues using an unpaired T-test to determine whether the DCF transport characteristics were the same for the placental and reflected tissues under control conditions.
To interpret how membrane permeability changed between each time-point, and to show the maximal capacity for DCF transport in response to the treatment regimens, we transformed the data by calculating the average rate of DCF transport (pmoles/min) between each time point. The average rates were then graphed over time using a one-phase exponential association nonlinear regression model to visualize the absolute maximum rate of DCF transfer in response to each treatment and when this maximum rate was achieved. This provided a visual comparison of how transport rates increased following treatments (slope of linear phase) and when transport was saturated (plateau) in the naive and treated tissues.
3. Results
3.1. PBEF induces secretion of VEGF from both placental and reflected AEC
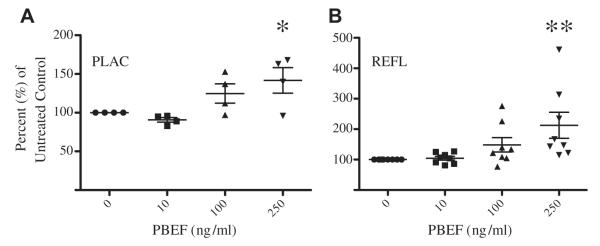
Amniotic epithelial cells from both the placental (n = 4, p < 0.05) and reflected amnion (n = 8, p < 0.005) signficantly increased the secretion of VEGF following 250 ng/ml PBEF treatment (Fig. 1A and B). Untreated placental and reflected AEC constitutively secreted VEGF. Although untreated reflected AEC produced an average of 2.5 fold more VEGF compared to placental AEC from the same patient (n = 4, data not shown), there was no significant difference between the two regions (p = 0.10).
Fig. 1.
PBEF treatment induces secretion of VEGF from amniotic epithelial cells. Primary AEC were treated with 0, 10, 100 and 250 ng/ml PBEF for 24 h, and VEGF levels from the cell supernatant were measured by ELISA. PBEF up-regulated VEGF secretion by both A) placental AEC and B) reflected AEC. Data are displayed as the percent of untreated control cells, and the line represents the mean percent change. (*P < 0.05, **P < 0.005).
3.2. VEGF receptors 1 and 2 are expressed in the human placental and reflected amnion
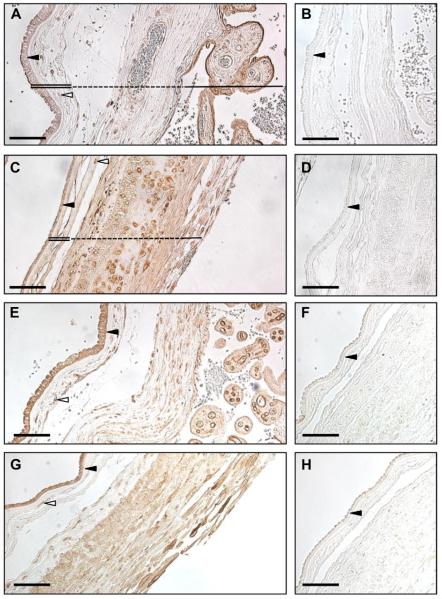
Considering that VEGF may function in an autocrine/paracrine fashion in the amnion, we characterized the protein expression of VEGF receptors 1 and 2 in the human fetal membranes using immunohistochemistry (Fig. 2) and western blotting (Fig. 3). Observation of hematoxylin & eosin stained sections at 40× magnification (data not shown) revealed morphological differences in the AEC from different regions of the amnion (n = 5). Placental AEC were larger and more columnar cells as compared to the reflected AEC. Some folding of the apical epithelial surface in the negative control images of the placental amnion is seen in Fig. 2. This appears as dense cell surface stain, which is accepted as a common artifact of epithelial staining.
Fig. 2.
The VEGF receptors are expressed in the human fetal membranes and placenta. Immunohistochemistry was performed on formalin-fixed tissues from both the placental (A, B, E, F) and reflected (C, D, G, H) regions of the fetal membranes. The tissues sections were stained for VEGFR1 (A, C) and VEGFR2 (E, G). The respective negative controls for VEGFR1 (B, D) and VEGFR2 (F, H) are also displayed. In A and C, the amnion is indicated with a double line and the chorion with a dashed line. The solid line denotes the placenta (Pl) in A and the decidua in C. Dark arrowheads point to the amnion epithelial cell layer, and empty arrowheads identify amnion mesenchymal cells. The pictures are taken at 20× magnification and the scale bar represents 100 μm.
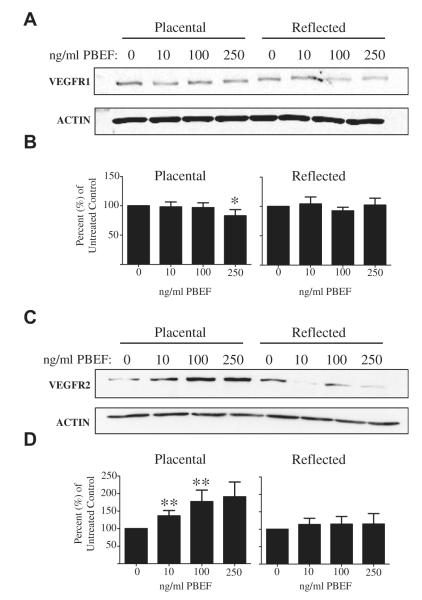
Fig. 3.
PBEF treatment increases VEGFR2 and decreases VEGFR1 expression in placental AEC, but not in reflected AEC. Primary AEC were exposed to 0, 10, 100 and 250 ng/ml PBEF for 24 h, and (A) VEGFR1 (n = 7), (C) VEGFR2 (n = 12), and actin protein levels were measured by Western blot. The densitometry values of the B) VEGFR1 and D) VEGFR2 blots were normalized to their respective untreated controls, and the mean values are displayed as the percent of untreated control (*P < 0.05, **P < 0.005).
VEGFR1 was expressed in the AEC and mesenchymal cells of the placental (Fig. 2A) and reflected amnion (Fig. 2C). The fibroblasts embedded in the reticular layer of the chorion were also stained in both regions. The chorionic trophoblasts of the reflected amnion were densely stained, while those of the placental amnion were stained to a lesser degree. The decidua parietalis of the reflected amnion expressed VEGFR1 throughout, including the endothelial cells of the decidual vessels (vessels not shown in Fig. 2C). In the placenta, the syncytiotrophoblasts were the predominant cell type expressing VEGFR1 (Fig. 2A).
VEGFR2 consistently stained the strongest in the AEC of both the placental (Fig. 2E) and reflected amnion (Fig. 2G). The mesenchymal cells of both amnion regions were also positive. In the chorionic plate overlying the placenta, the reticular layer fibroblasts, chorionic trophoblasts, and the endothelial cells of the fetal vessels within the chorion (vessel not shown in Fig. 2E) expressed VEGFR2. The chorionic trophoblasts of the reflected amnion were stained. The decidua parietalis of the reflected amnion expressed VEGFR2, with a strong stain observed in the decidual vessel endothelial cells (vessel not shown in section). In the placenta, the endothelial cells of the villous tree stained strongly for VEGFR2.
3.3. PBEF up-regulates VEGFR2 and down-regulates VEGFR1 in the placental, but not reflected AEC
Western blotting of the PBEF-treated AEC whole cell lysates revealed that PBEF increased expression of VEGFR2 (n = 12) in the placental AEC at the 10 and 100 ng/ml doses (P < 0.005) (Fig. 3C, D). Additionally, the 250 ng/ml PBEF treatment led to the significant down-regulation of placental AEC VEGFR1 expression (n = 7, P < 0.05, Fig. 3A, B). PBEF had no effect on VEGFR expression in the reflected amnion.
3.4. The treatment of explants with both PBEF and VEGF increases the permeability of the placental, but not reflected amnion
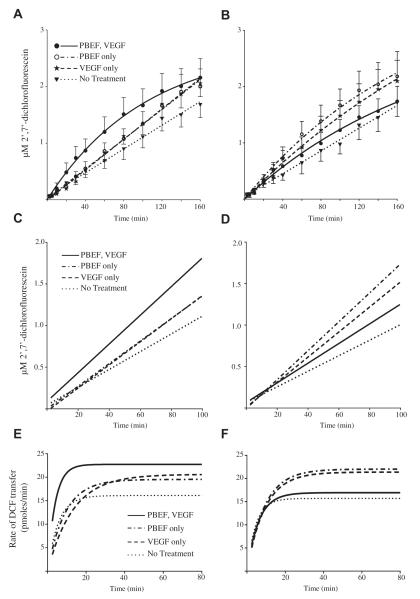
To determine if PBEF and VEGF would cooperate to increase the permeability of the amnion, we monitored the movement of DCF from the fetal to the maternal side of both placental and reflected amnion explants following PBEF and/or VEGF treatments. The DCF concentration approached an equilibrium in PBEF-pretreated and VEGF-treated placental amnion explants, as evidenced by the plateauing curve displayed in Fig. 4A. The same effect was seen in the reflected amnion explants that were treated with PBEF, VEGF, or both (Fig. 4B). The approximation of equilibrium indicates that we successfully monitored early changes in membrane permeability within the observed time course.
Fig. 4.
PBEF and VEGF dual treatment increases the permeability of placental amnion explants, but not reflected. Amnion explants were separated into 4 treatment groups: untreated controls, PBEF (100 ng/ml) for 18 h, acute VEGF treatment (20 ng/ml), or PBEF (100 ng/ml) for 18 h with subsequent acute VEGF treatment (20 ng/ml). Concurrent with acute VEGF treatment, the transfer of 2′,7′-dichlorofluorescein (DCF) from the fetal to the maternal side of amnion explants was monitored in all treatment groups to observe changes in membrane permeability. The mean concentration of DCF that accumulated on the maternal side of the A) placental amnion (n = 12) and the B) reflected amnion (n = 7) at each time time point is displayed with standard error bars. The lines for each data set were derived from linear or nonlinear regression modeling as best fit the data. C and D are the linear regression lines fit to the initial 100 min of each data set from the placental amnion and reflected amnion respectively. To interpret the change in membrane permeability per unit time, the data were transformed to reveal the average rate of DCF transfer between each time point. This data were modeled using nonlinear regression and the curves are displayed for the placental (E) and reflected (F) amnion. As the maximum rate was constant in most experimental conditions past 80-min, the data are only displayed through 80 min.
Subsequent linear regression analyses allowed us to utilize the slopes to compare differences in the rates of DCF transfer in response to the treatments before saturation of transport processes occurred, verify the sensitivity of the assay, and to justify the direct comparison of the placental amnion results to the reflected amnion results. Using the runs test in parallel with linear regression modeling, we determined that the data sets for both tissues fell into the linear range when restricting analysis to the initial 100 min of the experiment. This allowed us to compare the slopes of the lines to analyze DCF accumulation on the maternal side of the explants. In the placental amnion, the slope of the PBEF plus VEGF treatment data was significantly increased compared to non-treated controls (P < 0.005), as was that of the VEGF alone treatment (P < 0.05) (Fig. 4C, Table 1), indicating significantly increased DCF accumulation. Following PBEF treatment alone, the increase in slope approached statistical significance (P = 0.07). For the reflected amnion, we found that the slopes of both the VEGF and PBEF individual treatments were significantly increased from the nontreated controls (p < 0.005) (Fig. 4D, Table 1). However, unlike the placental amnion, the PBEF and VEGF coupled treatment had no such effect on the reflected amnion. These findings reveal that our assay was sensitive enough to detect statistically significant changes in our system in response to the treatments. Additionally, the identical slopes (P = 0.6587) of the non-treated reflected (n = 7) and placental (n = 12) amnion revealed that the transport of DCF had the same characteristics for both tissues under control conditions. This similarity supports our claim that the placental and reflected amnion each respond differently to the PBEF and VEGF treatments.
Table 1.
PBEF and VEGF dual treatment increases the permeability of placental amnion explants, but not reflected. Placental and reflected amnion explants were chronically treated with PBEF (100 ng/ml) and/or acutely exposed to VEGF (20 ng/ml) in parallel with untreated controls. The transfer of 2′,7′-dichlorofluorescein (DCF) through the explants was monitored over 160 min. Best fit regression modeling was performed on the data over the full 160 min of DCF collection and the data for each treatment was defined as either linear or non-linear. Linear regression modeling was then used to analyze the initial 100 min of the data sets for all of the treatment conditions. The respective slopes of each regression line are displayed +/− the standard error.
| Treatment | Best fit regression model of complete 160 minute data set |
Timepoint at which all treatments in linear range |
Slope of linearized 100 min data set (×10−2) |
|
|---|---|---|---|---|
| Placental | No Treatment | Linear | 100 min | 1.07 ± 0.03 |
| PBEF-only | Linear | 100 min | 1.36 ± 0.03 | |
| VEGF-only | Linear | 100 min | 1.39 ± 0.05b | |
| PBEF, VEGF | Non-linear | 100 min | 1.73 ± 0.10a | |
| Reflected | No Treatment | Linear | 100 min | 0.94 ± 0.04 |
| PBEF-only | Non-linear | 100 min | 1.75 ± 0.06a,c | |
| VEGF-only | Non-linear | 100 min | 1.51 ± 0.05a | |
| PBEF, VEGF | Non-linear | 100 min | 1.19 ± 0.06 |
Statistically significant from “No Treatment” P < 0.005.
Statistically significant from “No Treatment” P < 0.05.
Statistically significant from “PBEF, VEGF” P < 0.01.
To interpret the change in membrane permeability per unit time, the data were transformed to graph the average rate of DCF transfer between each time point (Fig. 4E and F). The PBEF and VEGF coupled treatment in the placental amnion resulted in an upward shift of the plateau as compared to the untreated controls, suggesting that the maximal rate of DCF transfer is higher in treated tissues. Additionally, these membranes may have achieved this maximum rate sooner, as evidenced by the increased slope in comparison to controls. Both the VEGF-only and PBEF-only treatments demonstrate moderate effects on the permeability of the placental amnion, as shown by their elevated maximum transfer rates. It is notable that the PBEF and VEGF coupled treatment did not appear to effect the reflected amnion as it did the placental (Fig. 4F), as the maximal DCF transfer rate in this sample set was comparable to the non-treated controls. The maximum rates of DCF transfer following PBEF-only, VEGF-only, and untreated controls were similar between the reflected and placental amnion.
4. Discussion
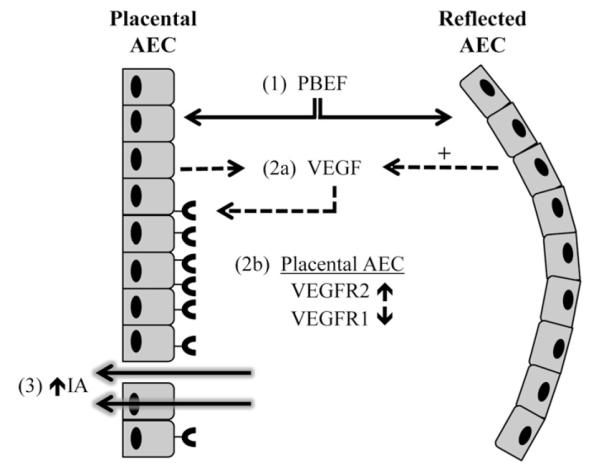
These studies demonstrate that PBEF and VEGF act in concert to increase the permeability of the human placental amnion (Fig. 5). To accomplish these functional effects, our data suggest that PBEF induces the placental amnion to secrete VEGF and up-regulate the expression of VEGF receptor 2. This increase in amnion permeability did not occur in the reflected amnion. While the gene expression profiles of the placental and reflected amnion have previously been reported to contrast at term [7], this study provides the first evidence that distinct regions of the amnion differentially respond to the same stimuli.
Fig. 5.
Model: Stimulation of intramembranous absorption by PBEF and VEGF. PBEF (1) induces the production of VEGF (2a) by both regions of the amnion. The reflected amnion may produce more VEGF than the placental amnion, as indicated by the “+” symbol. Simultaneous with VEGF secretion, the placental AEC up-regulates the expression of VEGFR2 while down-regulating VEGFR1 (2b). The functional result of these events is an increase in placental amnion permeability, thus allowing amniotic fluid efflux via paracellular or transcellular fluid transport mechanisms, and subsequent intramembranous absorption (3).
Although fluid efflux occurs specifically through the placental amnion during IA [1], prior research in this field had utilized human reflected, or mixed amnion tissue to describe placental amnion function [28,29]. Our study, along with the fact that the reflected amnion contains a zone of altered morphology fated to become the rupture site during labor [30], affirms that the amnion functions differently at distinct locales. Our novel approach focused on human placental amnion biology translates directly to human AF volume control, and our findings are cautionary for other investigators, who should define and justify the specific region of the amnion from which they utilize tissue or cells.
For VEGF to exert its effects on a target cell, it binds to and stimulates signaling from transmembrane receptor homo- or heterodimers [31]. The heterodimerization of VEGFR1 and 2 is considered influential in regulating the permeability of human tissues [31,32]. The VEGFR1 has a higher affinity for VEGFA (the prototypical VEGF family member used in this study) but very weak tyrosine kinase activity following ligand binding compared to VEGFR2 [33]. It is interesting to note that while PBEF up-regulated VEGFR2 expression in the placental AEC, it down-regulated the expression of VEGFR1. Such a response to PBEF may shift the balance toward VEGFR2 homodimerization, permitting a strong downstream signal to increase amnion permeability.
By demonstrating the expression of both VEGF receptors throughout the fetal membranes and placenta, this report confirms and extends previous findings [26,34–36]. In sheep, VEGF is upregulated in the fetal membranes and placenta at times of experimentally induced intramembranous absorption [18,19,37]. If a similar mechanism is stimulated in humans, the VEGF receptors expressed throughout the tissues may coordinate a response to VEGF that both permits AF efflux from the fetal sac and controls its subsequent resorption into the fetal and maternal blood vessels.
Our study aimed to test the hypothesis that PBEF and VEGF cooperate to increase the permeability of the placental amnion, not to define the mechanism of fluid transport. To monitor tissue explant permeability, we used DCF, a slightly polar, small molecular weight (400 kD) compound that has no known mammalian transporter. The measured equilibrium concentration of DCF was below that which was expected by mass balance, and we believe that the incomplete recovery was due to DCF binding membrane and culture medium proteins [38] as well plastic culture-ware. DCF may cross the monolayer through altered intercellular tight junctions or any number of transcellular channels. Since the physicochemistry of the DCF molecule is not changed, this implies an upregulation of transporters or transport processes and/or enhanced rates or activation of constitutively present transporters by VEGF and PBEF (Fig. 4E). Although we cannot define how DCF transfers through the membrane, PBEF and VEGF exposure clearly enhances one or more of these pathways to increase placental amnion permeability. Thus, the cellular processes involved in fluid transport are the subjects of further investigation.
The combined effects of PBEF and VEGF significantly increased placental amnion permeability (Fig. 4C, Table 1). The same double treatment may have had minor, though ambiguous effects on reflected amnion permeability, as suggested by the non-linear fit of that data compared to the linear fit of the non-treated controls (Fig. 4B, Table 1). Being that the vast majority of AF efflux across the fetal membranes is known to occur through the placental amnion, our results demonstrating the regional differences in permeability change align with the known biology of AF volume regulation. Mechanistic components like receptors, transporters, and channels that may be present in the placental amnion are likely absent or at a much lower concentration in the reflected amnion. The increased expression of VEGFR2 by placental AEC following PBEF treatment may be one such component. Alternatively, the double treatment may have had a dampening effect on reflected amnion permeability, as evidenced by the decreased DCF transfer rate compared to either PBEF or VEGF individual treatments (Fig. 4D, Table 1). However, we do not wish to over-analyze the effects of the individual treatments on amnion permeability as both PBEF and VEGF are simultaneously present in AF in vivo [39,40].
PBEF represents a critical node that may explain idiopathic cases of AF volume disorders. We previously demonstrated that PBEF is a cytokine secreted by AEC in response to stretch forces [14,27]. The fetal membranes of pregnant women with polyhydramnios are under intensified, chronic stretch. If the PBEF-stimulated permeability pathway is impaired in these cases, it could lead to the accumulation of AF. We speculate that an increased stretch force on the fetal membranes due to the daily accumulation of amniotic fluid could be a normal biomechanical trigger initiating PBEF release, eventually leading to VEGF production and intramembranous absorption. Also, it is well documented that hypoxic conditions are major risk factor for oligohydramnios. As PBEF is a hypoxia-responsive gene in other tissues, we speculate that it may be overproduced during such conditions, leading to increased IA and a failure to maintain appropriate AF volume.
This study reveals, for the first time, a pathway that alters human placental amnion permeability. Additionally, our novel region-specific model system establishes a new paradigm for AF transport studies and adds to the anatomical understanding of the human amnion. By illuminating the obscure mechanisms that control AF volume homeostasis, our findings provide insight into the pathologies of idiopathic, dysregulated AF volume and may ultimately provide new therapeutic targets to treat these conditions.
Acknowledgments
This study was supported by grants from the Hawaii Community Foundation Straub Trust #49269 and NIMHD #P20MD00006084 awarded to CEKW. We thank Sandy Yamamoto and the Histology and Imaging Core Facility from the John H. Burns School of Medicine of the University of Hawaii for their technical support, and we greatly appreciate the nurses and staff of the labor and delivery ward of Kapi’olani Medical Center for Women and Children for their help with the tissue collection.
Footnotes
Author roles JA, ACC and CEKW conceived and designed the experiments, and analyzed data. JA conducted the experiments. JA and CEKW wrote the manuscript.
References
- [1].Brace RA. Physiology of amniotic fluid volume regulation. Clin Obstet Gynecol. 1997;40:280–9. doi: 10.1097/00003081-199706000-00005. [DOI] [PubMed] [Google Scholar]
- [2].Gilbert WM, Eby-Wilkens E, Tarantal AF. The missing link in rhesus monkey amniotic fluid volume regulation: intramembranous absorption. Obstet Gynecol. 1997;89:462–5. doi: 10.1016/S0029-7844(96)00509-1. [DOI] [PubMed] [Google Scholar]
- [3].Gilbert WM, Brace RA. The missing link in amniotic fluid volume regulation: intramembranous absorption. Obstet Gynecol. 1989;74:748–54. [PubMed] [Google Scholar]
- [4].Harman CR. Amniotic fluid abnormalities. Semin Perinatol. 2008;32:288–94. doi: 10.1053/j.semperi.2008.04.012. [DOI] [PubMed] [Google Scholar]
- [5].Volante E, Gramellini D, Moretti S, Kaihura C, Bevilacqua G. Alteration of the amniotic fluid and neonatal outcome. Acta Biomed. 2004;75(Suppl. 1):71–5. [PubMed] [Google Scholar]
- [6].Adams EA, Choi HM, Cheung CY, Brace RA. Comparison of amniotic and intramembranous unidirectional permeabilities in late-gestation sheep. Am J Obstet Gynecol. 2005;193:247–55. doi: 10.1016/j.ajog.2004.12.001. [DOI] [PubMed] [Google Scholar]
- [7].Han YM, Romero R, Kim JS, Tarca AL, Kim SK, Draghici S, et al. Region-specific gene expression profiling: novel evidence for biological heterogeneity of the human amnion. Biol Reprod. 2008;79:954–61. doi: 10.1095/biolreprod.108.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol. 2002;187:1051–8. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- [9].Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–7. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–34. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- [11].Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Sci. Vol. 307. New York, N.Y: 2005. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin; pp. 426–30. [DOI] [PubMed] [Google Scholar]
- [12].Bae SK, Kim SR, Kim JG, Kim JY, Koo TH, Jang HO, et al. Hypoxic induction of human visfatin gene is directly mediated by hypoxia-inducible factor-1. FEBS Lett. 2006;580:4105–13. doi: 10.1016/j.febslet.2006.06.052. [DOI] [PubMed] [Google Scholar]
- [13].Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, Bryant-Greenwood GD. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J Mol Endocrinol. 2001;26:107–17. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- [14].Kendal-Wright CE, Hubbard D, Bryant-Greenwood GD. Chronic stretching of amniotic epithelial cells increases pre-B cell colony-enhancing factor (PBEF/visfatin) expression and protects them from apoptosis. Placenta. 2008;29:255–65. doi: 10.1016/j.placenta.2007.12.008. [DOI] [PubMed] [Google Scholar]
- [15].Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–27. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu P, Li H, Cepeda J, Zhang LQ, Cui X, Garcia JGN, et al. Critical role of PBEF expression in pulmonary cell inflammation and permeability. Cell Biol Int. 2009;33:19–30. doi: 10.1016/j.cellbi.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res. 2008;78:356–65. doi: 10.1093/cvr/cvm111. [DOI] [PubMed] [Google Scholar]
- [18].Cheung CY. Vascular endothelial growth factor activation of intramembranous absorption: a critical pathway for amniotic fluid volume regulation. J Soc Gynecol Investig. 2004;11:63–74. doi: 10.1016/j.jsgi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- [19].Daneshmand SS, Cheung CY, Brace RA. Regulation of amniotic fluid volume by intramembranous absorption in sheep: role of passive permeability and vascular endothelial growth factor. Am J Obstet Gynecol. 2003;188:786–93. doi: 10.1067/mob.2003.160. [DOI] [PubMed] [Google Scholar]
- [20].Ablonczy Z, Crosson CE. VEGF modulation of retinal pigment epithelium resistance. Exp Eye Res. 2007;85:762–71. doi: 10.1016/j.exer.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Antony AB, Tepper RS, Mohammed KA. Cockroach extract antigen increases bronchial airway epithelial permeability. J Allergy Clin Immunol. 2002;110:589–95. doi: 10.1067/mai.2002.127798. [DOI] [PubMed] [Google Scholar]
- [22].Cheung CY, Li S, Chen D, Brace RA. Regulation of caveolin-1 expression and phosphorylation by VEGF in ovine amnion cells. Reprod Sci. 2010;17:1112–9. doi: 10.1177/1933719110378175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cheung CY, Brace RA. Unidirectional transport across cultured ovine amniotic epithelial cell monolayer. Reprod Sci. 2008;15:1054–8. doi: 10.1177/1933719108322426. [DOI] [PubMed] [Google Scholar]
- [24].Kremer C, Breier G, Risau W, Plate KH. Up-regulation of flk-1/vascular endothelial growth factor receptor 2 by its ligand in a cerebral slice culture system. Cancer Res. 1997;57:3852–9. [PubMed] [Google Scholar]
- [25].Hammes HP, Lin J, Bretzel RG, Brownlee M, Breier G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes. 1998;47:401–6. doi: 10.2337/diabetes.47.3.401. [DOI] [PubMed] [Google Scholar]
- [26].Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Expression of angiogenic and neurotrophic factors in the human amnion and choriodecidua. Am J Obstet Gynecol. 2002;187:728–34. [PubMed] [Google Scholar]
- [27].Kendal-Wright CE, Hubbard D, Gowin-Brown J, Bryant-Greenwood GD. Stretch and inflammation-induced pre-B cell colony-enhancing factor (PBEF/Visfatin) and interleukin-8 in amniotic epithelial cells. Placenta. 2010;31:665–74. doi: 10.1016/j.placenta.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Leontic EA, Tyson JE. Prolactin and fetal osmoregulation: water transport across isolated human amnion. Am J Phys. 1977;232:R124–7. doi: 10.1152/ajpregu.1977.232.3.R124. [DOI] [PubMed] [Google Scholar]
- [29].Raabe MA, McCoshen JA. Epithelial regulation of prolactin effect on amnionic permeability. Am J Obstet Gynecol. 1986;154:130–4. doi: 10.1016/0002-9378(86)90408-4. [DOI] [PubMed] [Google Scholar]
- [30].El Khwad M, Stetzer B, Moore RM, Kumar D, Mercer B, Arikat S, et al. Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol Reprod. 2005;72:720–6. doi: 10.1095/biolreprod.104.033647. [DOI] [PubMed] [Google Scholar]
- [31].Olsson A-K, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- [32].Kiba A, Sagara H, Hara T, Shibuya M. VEGFR-2-specific ligand VEGF-E induces non-edematous hyper-vascularization in mice. Biochem Biophys Res Commun. 2003;301:371–7. doi: 10.1016/s0006-291x(02)03033-4. [DOI] [PubMed] [Google Scholar]
- [33].Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–83. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- [34].Brace RA, Gilbert WM, Thornburg KL. Vascularization of the ovine amnion and chorion: a morphometric characterization of the surface area of the intramembranous pathway. Am J Obstet Gynecol. 1992;167:1747–55. doi: 10.1016/0002-9378(92)91770-b. [DOI] [PubMed] [Google Scholar]
- [35].Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M, et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11. doi: 10.1186/1471-213X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vuckovic M, Ponting J, Terman BI, Niketic V, Seif MW, Kumar S. Expression of the vascular endothelial growth factor receptor, KDR, in human placenta. J Anat. 1996;188(Pt 2):361–6. [PMC free article] [PubMed] [Google Scholar]
- [37].Matsumoto LC, Bogic L, Brace RA, Cheung CY. Prolonged hypoxia upregulates vascular endothelial growth factor messenger RNA expression in ovine fetal membranes and placenta. Am J Obstet Gynecol. 2002;186:303–10. doi: 10.1067/mob.2002.119806. [DOI] [PubMed] [Google Scholar]
- [38].Klugerman MR. Chemical and physical variables affecting the properties of fluorescein isothiocyanate and its protein conjugates. J Immunol. 1965;95:1165–73. doi: 10.21236/ad0459385. [DOI] [PubMed] [Google Scholar]
- [39].Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med. 2008;36:485–96. doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chow SSW, Craig ME, Jones CA, Hall B, Catteau J, Lloyd AR, et al. Differences in amniotic fluid and maternal serum cytokine levels in early midtrimester women without evidence of infection. Cytokine. 2008;44:78–84. doi: 10.1016/j.cyto.2008.06.009. [DOI] [PubMed] [Google Scholar]