Abstract
Objective
To determine if migraineurs have evidence of interictal cutaneous sensitisation.
Subjects and methods
Thermal and mechanical pain thresholds in 20 episodic migraineurs, 20 chronic migraineurs, and 20 non-migraine control subjects were compared. Quantitative sensory testing was conducted when subjects had been migraine-free for at least 48 h. Heat, cold and mechanical pain thresholds, and heat and cold pain tolerance thresholds were measured.
Results
Thermal pain thresholds and thermal pain tolerance thresholds differed significantly by headache group (P=0.001). During the interictal period, episodic and chronic migraineurs were more sensitive to thermal stimulation than non-migraine controls.
Conclusions
Interictal sensitisation may predispose the migraineur to development of headaches, may be a marker of migraine activity, and a target for treatment.
Keywords: migraine, cutaneous allodynia, central sensitisation, quantitative sensory testing, pain thresholds
Introduction
Sensitisation of peripheral and central trigeminal nociceptors is implicated in migraine pathophysiology. Central sensitisation involves activity-dependent changes in the excitability and synaptic strength of neurons. Sensitised neurons have receptive field expansion, increased responsiveness to noxious stimuli, increased spontaneous activity, and recruitment of novel low-threshold inputs (1). During a migraine attack, trigeminal sensitisation may result in reductions in cutaneous pain thresholds and in symptoms of cutaneous allodynia (2–5). The majority of migraineurs develop cutaneous allodynia during a migraine (2). The patient with allodynia may report discomfort with light touch of the skin, wearing eyeglasses and earrings, tight collars, shaving, and laying one’s head on a pillow.
Although there have been several studies of cutaneous pain thresholds and cutaneous allodynia during an individual migraine attack, there have been fewer investigations of cutaneous pain thresholds between migraine attacks (2,3,6–9). Furthermore, cutaneous pain thresholds in subjects with chronic migraine have not been adequately investigated. Interictal investigation of pain thresholds could provide information regarding the propensity for future migraine attacks. If the migraineur maintains a state of persistent sensitisation, activation of the trigeminovascular system would presumably occur with more ease. Thus, there could be a cyclical process of migraine headaches causing sensitisation and sensitisation allowing for easier production of future migraine attacks. If so, interictal cutaneous pain thresholds may serve as a measure of migraine activity and interictal sensitisation as a target for migraine prevention.
This investigation was conducted to compare cutaneous thermal pain thresholds, thermal pain tolerance thresholds and mechanical pain thresholds between migraine attacks (interictal) in episodic migraine subjects, chronic migraine subjects, and non-migraine controls. We hypothesised that subjects with migraine would have reduced pain and pain tolerance thresholds, suggestive of interictal sensitisation, compared to non-migraine controls.
Subjects and methods
Participants
Institutional Review Board approval was obtained from the Washington University Human Studies Committee. Twenty participants were enrolled into each of the three subject groups. Subjects were recruited from the Washington University Headache Center, from the community, and from an institutional database of research volunteers. Headache diagnoses were made according to International Classification of Headache Disorders II criteria (10). Subjects were men and women aged 18–65 years who did not have a medical or neurological disorder that would directly affect quantitative sensory testing (QST) results (e.g. peripheral neuropathy, other sensory disorder), who were free from other chronic pain disorders (e.g. fibromyalgia, reflex sympathetic dystrophy, chronic back pain) and free from current acute pain disorders (e.g. bone or muscle injury, postoperative).
Controls were studied when in their usual state of health and headache-free (tension-type headache) for at least 48 h. Episodic migraine participants were studied after being headache-free (migraine and non-migraine) for at least 48 h. Chronic migraine subjects were studied when migraine-free for at least 48 h. Subjects had not used opiate medication or migraine-specific abortive medications during the 48 h prior to testing.
Measurements
Subjects completed the Migraine Disability Assessment (MIDAS), the State-Trait Anxiety Inventory (STAI), the Allodynia Symptom Checklist (ASC-12), and the Beck Depression Inventory (BDI) (11–14). Subjects completed the ASC-12 twice; once for current symptoms (on the day of QST) and again according to their recall of symptoms during their most severe headaches.
QST was performed to determine heat, cold, and mechanical pain thresholds and heat and cold pain tolerance thresholds. Standardised instructions were delivered to the subjects prior to QST testing. ‘Pain threshold’ was defined as the first instant that the stimulus felt painful. ‘Pain tolerance threshold’ was defined as the first instant that the subject could no longer tolerate the stimulus. Each subject underwent testing at the right and left forehead and the right and left ventral medial forearm.
Thermal testing was performed using the Medoc Pathway platform with a 30mm × 30mm thermode. The thermode was applied to the skin and fastened with a Velcro strap. The method of limits was used for testing. The thermode adjusts to a baseline temperature of 32°C. Depending upon the modality being tested (heat or cold), thermode temperature increases or decreases by 1°C/s. The subject presses a button on the subject response unit (computer mouse) when the threshold being tested (heat pain, heat tolerance, cold pain, cold tolerance) is reached. The heating or cooling process stops immediately and the thermode returns to the baseline temperature. This test is performed three times for each modality in each body location. The mean of the three trials for each modality in each body location is the pain threshold.
Pressure pain threshold was tested using a set of 20 calibrated von Frey filaments (Bioseb). In an ascending pattern of increasing tensile strength, each filament was applied three times until the subject reported pain on two of three applications. Each filament was held in place for 2 s. Mechanical pain tolerance threshold was not tested in order to avoid bruising.
Statistical analysis
Demographics, headache symptoms, and anxiety, disability, depression and allodynia scores were compared between each of the three groups using the Wilcoxon Mann–Whitney test for continuous variables and Pearson chi-squared or Fisher’s Exact tests, as appropriate, for categorical variables.
Descriptive statistics were used to describe pain thresholds and pain tolerance thresholds. von Frey hair filament numbers were converted to grams of pressure for analysis of mechanical pain. Measures on the left and right sides of the body were averaged. To determine if thresholds for each type of pain and tolerance (cold, heat, pressure) differed among headache groups (control, episodic migraine and chronic migraine) and between body region of QST testing (arm vs head), mixed model analysis of variance (SAS PROC MIXED, SAS Institute, Cary, NC, USA) was used with a between-subjects factor (headache group), a within-subjects factor (body region), and the interaction of the two factors. Pair-wise group comparisons were used to test the primary research questions of whether significant differences existed in QST thresholds between (i) episodic migraine and controls, (ii) chronic migraine and controls, and (iii) chronic migraine and episodic migraine.
In addition to group comparisons, we compared individual subjects’ QST pain thresholds to our control subject normal ranges for skin pain thresholds, defined as the control subject mean ±2 SD. Cutaneous sensitisation was considered present if, after averaging by body region (head and arm), skin pain thresholds fell below that which was considered normal in reference to controls for heat or mechanical pressure, or above that which was considered normal in reference to controls for cold. Subjects who met the criteria for any one of the three modalities (heat, cold, pressure) in at least one of the regions (head or arm) were considered to have sensitisation. To determine whether average QST scores for each modality were correlated with scores from the ASC-12, Spearman’s rank order correlation coefficient was used.
For all analyses where pair-wise comparisons are concerned, P-values were adjusted for multiple comparisons using the Tukey method. For all tests, P<0.05 was considered to be statistically significant. SAS v.9.2 (SAS Institute) was used for all analyses.
Results
Subject demographics, pain level at the time of testing, headache frequency, days since last headache, use of migraine prophylactics, MIDAS score, STAI scores, BDI scores, and ASC-12 scores (at the time of testing and recalled during headache) are shown in Table 1. Groups did not differ significantly by age, sex, anxiety or depression scores. Pain level at the time of testing was higher in chronic migraine compared to controls (P<0.001) and episodic migraine (P<0.001). Chronic migraineurs had fewer number of days since last headache than episodic migraineurs (P<0.001). MIDAS scores were higher in chronic migraineurs than episodic migraineurs (P<0.001). More chronic migraineurs were taking prophylactic medications than controls (P<0.001) and episodic migraineurs (P=0.008). Episodic migraineurs reported more symptoms of cutaneous allodynia at the time of testing than controls (P=0.046). Episodic migraineurs and chronic migraineurs reported more cutaneous allodynia symptoms during headache than controls (12 had infrequent tension type headache; both P<0.001).
Table 1.
Demographics and other variables by headache group Controls
| Controls (n = 20) median (min–max) |
Episodic migraine (n = 20) median (min–max) |
Chronic migraine (n = 20) median (min–max) |
|
|---|---|---|---|
| Age years | 35.5 (23–62) | 31.5 (18–52) | 31 (19–47) |
| Female n (%) | 16 (80) | 15 (75) | 18 (90) |
| Current pain level | 0 (0–0) | 0 (0–0) | 1.5 (0–8)a,b |
| Headache frequency (days/month) | 1 (0–2) | 5.5 (1–8)a | 20 (15–30)a,b |
| Days between last headache and QSTc | 14 (3–30) | 7 (2–20)a | 0 (0–7)a,b |
| Prophylactic medications n (%) | 0 (0) | 4 (20) | 13 (65)a,b |
| MIDAS | 0 (0–4) | 10.5 (1–34)a | 56.5 (5–195)a,b |
| State anxiety | 49 (39–58) | 49 (34–56) | 48 (32–56) |
| Trait anxiety | 46 (39–52) | 45 (41–53) | 45.5 (32–58) |
| BDI | 2 (0–9) | 4 (0–13) | 5.5 (0–32) |
| ASC-12 at testing | 0 (0–4) | 0.5 (0–10)a | 0 (0–7) |
| ASC-12 during headachec | 0 (0–6) | 6.5 (0–18)a | 7 (0–16)a |
P<0.05 versus control subjects.
P<0.05 chronic versus episodic migraine subjects.
Twelve control subjects had very infrequent tension-type headaches; thus data for allodynia symptoms during headache and for days between last headache and QST testing were restricted to these 12 control subjects.
Mean thresholds of QST testing for heat and cold are shown by headache group and body region in Figures 1 and 2. Detailed results of mixed models can be found in the figure captions. Heat pain and tolerance thresholds as well cold pain and tolerance thresholds differed significantly by headache group (all P=0.001), but mechanical pain thresholds did not (P=0.119).
Figure 1.
(A) Mean heat pain thresholds and (B) mean heat tolerance thresholds (with 95% confidence interval) by headache group and body region. The main effect of headache group was significant for heat pain (F(2,57)=11.4; P<0.001) and heat tolerance (F(2,57)=11.7; P<0.001). For heat pain, significant headache group pair-wise comparisons include EM (42.7°C) vs CTL (46.4°C; P<0.001) and CM (43.4°C) vs CTL (P=0.002). For heat tolerance, significant differences were seen for EM (44.3°) vs. CTL (47.4°C; P<0.001) and CM (44.8°C) vs CTL (P=0.001). The main effect of body region (head vs arm) was not significant for heat pain (F(1,57)=3.8; P=0.057) or heat tolerance (F(1,57)=0.4; P=0.525). The interaction of body region was not significant for heat pain (F(2,57)=0.6; P=0.561) or heat tolerance (F(2,57)=0.1; P=0.945).
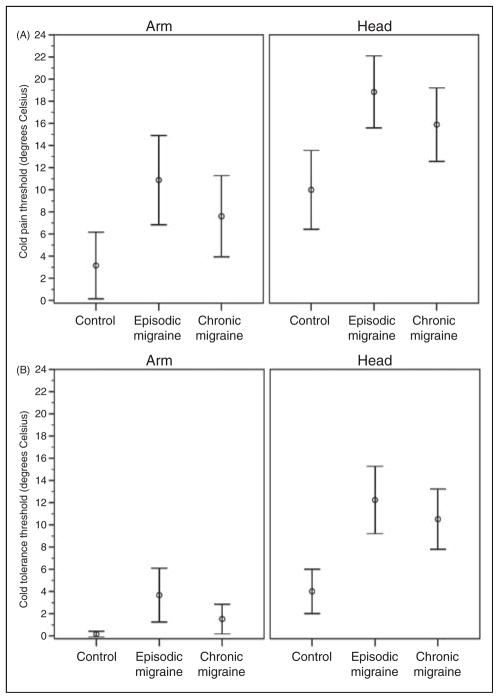
Figure 2.
(A) Mean cold pain thresholds and (B) mean cold tolerance thresholds (with 95% confidence interval) by headache group and body region. The main effect of headache group was significant for cold pain (F(2,57)=7.40; P=0.001) and cold tolerance (F(2,57)=10.4; P<0.001). For cold pain, significant headache group pair-wise comparisons include EM (14.9°C) vs CTL (6.6°C; P=0.001); CM (11.7°C) vs CTL did not quite reach statistical significance (P=0.053). For cold tolerance, CM (10.5°C) vs CTL (4.0°C) was only significant in the head region (P=0.007). EM (head 12.2°C, arm 3.7°C) differed from CTL (head 4.0°C, arm 0.2°C) in both the head (P<0.001) and arm (P=0.023). The main effect of body region (head vs arm) was significant for cold pain (F(1,57)=105.7; P<0.001; mean difference 7.7°C) and cold tolerance (F(1,57)=177.3; P<0.001; mean difference 7.1°C). The interaction of headache group and region was not significant for cold pain (F(2,57)=0.34; P=0.711) but was significant for cold tolerance (F(2,57)=9.4; P<0.001).
Episodic migraineurs were significantly more sensitive to heat and cold stimuli than controls. Chronic migraineurs were more sensitive than controls regarding heat pain, heat tolerance, and cold tolerance (head region only). There were no significant differences between chronic migraineurs and episodic migraineurs for any of the modalities. When use of migraine prophylactic medications was added to the mixed models, prophylactics were not significantly associated with QST thresholds for any of the modalities, and results for group and region comparisons were similar to those reported above. When chronic migraine subjects who were experiencing non-migraine headache at the time of QST were excluded from the analysis, the mean thresholds between chronic migraine and control subjects became slightly larger. This resulted in the chronic migraine to control comparison for cold pain to reach statistical significance (difference in mean values=7.7°C; P=0.020).
We compared QST pain thresholds for episodic and chronic migraineurs to normal thresholds based on the control subjects. If the skin pain threshold was below two standard deviations from the control mean for heat (head 42.8°C, arm 42.4°C), above two standard deviations from the control mean for cold (head 25.2°C, arm 16.1°C), or below two standard deviations from the control mean for pressure (15 g for both head and arm), the subject was considered to have cutaneous sensitisation. Using this definition, 10 (50%) episodic migraine and 12 (60%) chronic migraine subjects had cutaneous sensitisation between migraines.
The correlation between pain and pain tolerance thresholds for each modality and ASC-12 scores at the time of testing and during headache was examined. Overall, there were significant negative correlations of heat pain threshold and heat tolerance threshold with ASC-12 scores at the time of testing (Spearman’s ρ=−0.27, P=0.035; Spearman’s ρ=−0.37, P=0.004), and with ASC-12 scores recalled during headache (Spearman’s ρ=−0.31, P=0.025; Spearman’s ρ=−0.30, P=0.029). There were no significant correlations between cold pain or tolerance thresholds or pressure thresholds with ASC-12 scores.
Discussion
Migraineurs process sensory stimuli abnormally during (ictal) and between migraine attacks (interictal). Prior studies have demonstrated that migraineurs are hypersensitive to light, sound and odours, both ictally and interictally (15–17). There is also evidence that migraineurs process somatosensory stimuli abnormally during a migraine, and may be hypersensitive to somatosensory stimuli interictally (2,3,6–8,18). Central sensitisation develops in about 75% of migraineurs during an individual migraine attack, leading to symptoms of cutaneous allodynia in some (2,3). Prior studies have documented progressive and spreading allodynia during a migraine attack and lower intrasubject pain thresholds during a migraine compared to the interictal state (2–5,19). Only a few studies, with contradictory results, have compared interictal pain thresholds in migraine subjects to non-migraine controls (6,8,20). Furthermore, the majority of investigations have studied subjects with episodic migraine, making the role of sensitisation in chronic migraine less clear (7,8).
In our study, episodic and chronic migraine subjects were significantly more sensitive to thermal stimulation when tested interictally compared to non-migraine control subjects. We theorise that these findings suggest an interictal sensitised state present in groups of episodic and chronic migraine subjects. Pain thresholds correlated with current cutaneous allodynia symptom scores (ASC-12), further supporting this assertion. We believe that lower pain thresholds in subjects with migraine are due to interictal central sensitisation. Interictal central sensitisation may be secondary to increased facilitation of ascending nociceptive signals, reduced activity in descending antinociceptive pathways, and/or an increase in descending facilitation (20–22). Although we feel it is less likely, one could hypothesise that our findings are explained by peripheral sensitisation of nociceptors in the absence of central sensitisation. This could be due to spontaneous activity of peripheral nociceptors, an increased sensitivity of peripheral nociceptors to inflammatory mediators, and/or a greater concentration of circulating inflammatory mediators, a phenomenon for which there is evidence in prior migraine studies (19,23,24). However, identification of lower pain thresholds at the arms of migraine subjects suggests sensitisation of third-order neurons, above the level of the trigeminal nucleus caudalis.
We hypothesise that interictal sensitisation may lower the activation threshold of the trigeminal system, thus predisposing the migraine patient to future migraine attacks. We expected that a small proportion of migraine patients would report interictal symptoms of cutaneous allodynia, but that a greater proportion would have reduced cutaneous pain thresholds compared to non-migraine controls. These subjects could be considered to have ‘asymptomatic’ sensitisation, a process previously termed ‘suballodynia’ (6,20). Even if asymptomatic (not causing symptoms of allodynia), reductions in interictal pain thresholds may be indicative of a mildly sensitised state which could predispose the migraine patient to their next headache. In support of this idea, Sand and colleagues (20) have recently shown that cutaneous pain thresholds decrease within a 24-h period prior to the next migraine attack. In that study, 11 migraine subjects with infrequent episodic attacks (2–6 per month) studied within 24 h of their next migraine had lower heat and cold pain thresholds compared to their interictal thresholds (more than 24 h from previous attack and more than 24 h to next attack). These data suggest that central sensitisation is part of the process of migraine development, and not simply a secondary phenomenon.
In addition to lower pain thresholds, we found lower pain tolerance thresholds in our episodic and chronic migraine subjects. Whether pain tolerance is a primarily a result of physiological or psychological factors is a matter of debate. It is possible that the lower pain tolerance thresholds we identified in migraine subjects are explained by the affective response to noxious stimuli (25–27). However, we believe that lower pain tolerance thresholds in migraine subjects are directly correlated with lower pain thresholds and are explained by shared physiological factors (25,28,29).
We did not find significant differences in mechanical pain thresholds between subjects with migraine and controls, nor did we find significant differences in pain or pain tolerance thresholds between episodic and chronic migraine subjects. A post-hoc power analysis using standard deviations from our study revealed that with 20 subjects per group and α=0.05, we had 80% power to detect the following minimum differences when comparing two of the groups to each other: 65.6 g of pressure for mechanical pain, 2.2°C for heat pain, 1.6°C for heat tolerance, 5.5°C for cold pain, and 3.4°C for cold tolerance. With greater sample size, we may have been able to detect smaller differences in mean mechanical pain thresholds. Future studies with larger sample sizes are needed to address this possibility. Furthermore, we hypothesised, but did not find, that chronic migraine subjects would be more sensitive to cutaneous stimulation than episodic migraine subjects. Additional comparisons of episodic and chronic migraineurs are needed in order to support or refute these findings.
A few aspects of our study may have resulted in underestimating the degree of interictal sensitisation present in migraineurs. Enrolment of subjects using migraine prophylactic medications may have increased our likelihood of Type-II error, since it is possible they reduce allodynia/sensitisation when effective (30). However, use of prophylactic medications was not significantly associated with QST thresholds when added to the mixed models. Thus, this limitation does not invalidate our results. Type II error may also have been increased due to the placement of testing safety parameters. Maximum and minimum temperatures and maximum mechanical force used for testing is limited to avoid injuries. However, some subjects (controls, most commonly) have thresholds that exceed the limits of testing. Since these subjects were assigned the maximum or minimum stimuli tested (e.g. 0°C for cold, 446.7 g for pressure), this study may underestimate the amplitude of interictal sensitisation in migraineurs. Furthermore, we did not collect data on the timing of the next migraine attack in our subjects. A recent publication suggests episodic migraine subjects have reduced pain thresholds in the 24 h prior to migraine onset (20). Although we cannot entirely exclude this as a confounder, it is unlikely that a substantial proportion of our episodic migraine subjects (median of 5.5 headache days per month) developed a migraine within 24 h of testing.
Conclusions
This study suggests that episodic and chronic migraine subjects are more sensitive to thermal stimulation during the interictal period. A mildly sensitised state may predispose some migraineurs to future migraine headaches, may be a marker of migraine activity, and could be a target for migraine treatment. Further comparisons of cutaneous pain thresholds among episodic migraineurs, chronic migraineurs and non-migraine controls are indicated. Longitudinal studies may help to define better the relationship between sensitisation, migraine frequency, and migraine progression.
Acknowledgments
The authors acknowledge the assistance of Dr Evan Kharasch with the design of this study and for reviewing this manuscript. Dr Kharasch’s work on this project was funded by NIH K24DA00417. This publication was made possible by the following grants: NIH UL1 RR024992, NIH KL2 RR024994, and NIH RO1NS48602.
Footnotes
Reprints and permissions: sagepub.co.uk/journalsPermissions.nav
References
- 1.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 2.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–624. [PubMed] [Google Scholar]
- 3.Jakubowski M, Silberstein S, Ashkenazi A, Burstein R. Can allodynic migraine patients be identified interictally using a questionnaire? Neurology. 2005;65:1419–1422. doi: 10.1212/01.wnl.0000183358.53939.38. [DOI] [PubMed] [Google Scholar]
- 4.Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89:107–110. doi: 10.1016/s0304-3959(00)00478-4. [DOI] [PubMed] [Google Scholar]
- 5.Bigal ME, Ashina S, Burstein R, et al. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. 2008;70:1525–1533. doi: 10.1212/01.wnl.0000310645.31020.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weissman-Fogel I, Sprecher E, Granovsky Y, Yarnitsky D. Repeated noxious stimulation of the skin enhances cutaneous pain perception of migraine patients in-between attacks: clinical evidence for continuous sub-threshold increase in membrane excitability of central trigeminovascular neurons. Pain. 2003;104:693–700. doi: 10.1016/S0304-3959(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 7.Kitaj MB, Klink M. Pain thresholds in daily transformed migraine versus episodic migraine headache patients. Headache. 2005;45:992–998. doi: 10.1111/j.1526-4610.2005.05179.x. [DOI] [PubMed] [Google Scholar]
- 8.Cooke L, Eliasziw M, Becker WJ. Cutaneous allodynia in transformed migraine patients. Headache. 2007;47:531–539. doi: 10.1111/j.1526-4610.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 9.Filatova E, Latysheva N, Kurenkov A. Evidence of persistent central sensitization in chronic headaches: a multi-method study. J Headache Pain. 2008;9:295–300. doi: 10.1007/s10194-008-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. Cephalalgia. (2) 2004;24(Suppl 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 11.Stewart WF, Lipton RB, Kolodner KB, Sawyer J, Lee C, Liberman JN. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain. 2000;88:41–52. doi: 10.1016/S0304-3959(00)00305-5. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy BL, Schwab JJ, Morris RL, Beldia G. Assessment of state and trait anxiety in subjects with anxiety and depressive disorders. Psychiatr Q. 2001;72:263–276. doi: 10.1023/a:1010305200087. [DOI] [PubMed] [Google Scholar]
- 13.Lipton RB, Bigal ME, Ashina S, Burstein R, Silberstein S, Reed ML, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148–158. doi: 10.1002/ana.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnau RC, Meagher MW, Norris MP, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol. 2001;20:112–119. doi: 10.1037//0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- 15.Main A, Dowson A, Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache. 1997;37:492–495. doi: 10.1046/j.1526-4610.1997.3708492.x. [DOI] [PubMed] [Google Scholar]
- 16.Ashkenazi A, Mushtaq A, Yang I, Oshinsky ML. Ictal and interictal phonophobia in migraine – a quantitative controlled study. Cephalalgia. 2009;29:1042–1048. doi: 10.1111/j.1468-2982.2008.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanchin G, Dainese F, Trucco M, Mainardi F, Mampreso E, Maggioni F. Osmophobia in migraine and tension-type headache and its clinical features in patients with migraine. Cephalalgia. 2007;27:1061–1068. doi: 10.1111/j.1468-2982.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 18.Lovati C, D’Amico D, Bertora P, et al. Acute and interictal allodynia in patients with different headache forms: an Italian pilot study. Headache. 2008;48:272–277. doi: 10.1111/j.1526-4610.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- 19.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123:1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 20.Sand T, Zhitniy N, Nilsen KB, Helde G, Hagen K, Stovner LJ. Thermal pain thresholds are decreased in the migraine preattack phase. Eur J Neurol. 2008;15:1199–1205. doi: 10.1111/j.1468-1331.2008.02276.x. [DOI] [PubMed] [Google Scholar]
- 21.Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- 22.de Tommaso M, Difruscolo O, Sardaro M, et al. Effects of remote cutaneous pain on trigeminal laser-evoked potentials in migraine patients. J Headache Pain. 2007;8:167–174. doi: 10.1007/s10194-007-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrari MD, Odink J, Tapparelli C, Van Kempen GM, Pennings EJ, Bruyn GW. Serotonin metabolism in migraine. Neurology. 1989;39:1239–1242. doi: 10.1212/wnl.39.9.1239. [DOI] [PubMed] [Google Scholar]
- 24.Fusayasu E, Kowa H, Takeshima T, Nakaso K, Nakashima K. Increased plasma substance P and CGRP levels, and high ACE activity in migraineurs during headache-free periods. Pain. 2007;128:209–214. doi: 10.1016/j.pain.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Woodrow KM, Friedman GD, Siegelaub AB, Collen MF. Pain tolerance: differences according to age, sex and race. Psychosom Med. 1972;34:548–556. doi: 10.1097/00006842-197211000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63:316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Benedetti F, Vighetti S, Ricco C, et al. Pain threshold and tolerance in Alzheimer’s disease. Pain. 1999;80:377–382. doi: 10.1016/s0304-3959(98)00228-0. [DOI] [PubMed] [Google Scholar]
- 28.Clark JW, Bindra D. Individual differences in pain thresholds. Can J Psychol. 1956;10:69–76. doi: 10.1037/h0083660. [DOI] [PubMed] [Google Scholar]
- 29.Bendtsen L, Jensen R, Olesen J. Decreased pain detection and tolerance thresholds in chronic tension-type headache. Arch Neurol. 1996;53:373–376. doi: 10.1001/archneur.1996.00550040113021. [DOI] [PubMed] [Google Scholar]
- 30.Berry JD, Petersen KL. A single dose of gabapentin reduces acute pain and allodynia in patients with herpes zoster. Neurology. 2005;65:444–447. doi: 10.1212/01.wnl.0000168259.94991.8a. [DOI] [PubMed] [Google Scholar]