Abstract
The effects of vitamin D and parathyroid hormone (PTH) levels on incident fracture remains uncertain. To test the hypothesis that increasing serum 25-hydroxyvitamin D (25(OH)D) and decreasing PTH levels are associated with decreased risk of hip and any non-spine fracture we conducted a prospective cohort study among 2614 community-dwelling white and black participants, aged ≥70 years, from the Health, Aging and Body Composition (Health ABC) study. Serum and plasma samples were drawn at year 2 which formed the baseline for this analysis. Serum 25(OH)D and intact PTH (1-84) were measured using radioimmunoassay with DiaSorin reagents and EDTA plasma with a two-site immunoradiometric assay kit, respectively. Incident fractures (hip and any non-spine) were assessed after year 2, every 6 months, by self-report and validated by radiology reports. The median (IQR) follow-up times for hip and any non-spine fractures were 6.4 (6.1–6.5) and 6.4 (5.5–6.5) years, respectively. Cox proportional hazards regression was used to estimate the hazard ratios (HR) with 95% confidence intervals (CI) for fracture. There were 84 hip and 247 non-spine fractures that occurred over the follow-up period. The multivariable adjusted HRs (95% CIs) of hip fracture for participants in the lowest (≤17.78 ng/ml), second (17.79-24.36 ng/ml) and third quartile (24.37-31.94 ng/ml) of 25(OH)D were 1.92 (0.97-3.83), 0.75 (0.32-1.72) and 1.86 (1.00-3.45), respectively; compared with participants in the highest 25(OH)D quartile (>31.94 ng/ml) (p-trend=0.217). Additional adjustment for IL-6 (p-value=0.107), PTH (p-value=0.124) and hip aBMD (p-value=0.137) attenuated HRs of hip fracture in the lowest quartile by 16.3%, 17.4%, and 26.1%, respectively. There was no evidence of an association between 25(OH)D and any non-spine fractures, or between PTH and hip or any non-spine fractures. We found limited evidence to support an association between calciotropic hormones and hip and non-spine fractures in older men and women.
Keywords: serum 25-hydroxyvitamin D, parathyroid hormone, hip fractures, non-spine fractures
Introduction
Serum 25-hydroxyvitamin D [25(OH)D] is the widely accepted indicator of vitamin D nutritional status1. Older adults are less likely to have sufficient serum 25(OH)D2. Low 25(OH)D in older adults has consequences for increased rates of bone loss and bone turnover, which subsequently lead to low bone mineral density (BMD)3. The optimal level of vitamin D sufficiency remains controversial. Recently published Institute of Medicine (IOM) guidelines recommended serum 25(OH)D levels ≥20 ng/ml for skeletal health in most US adults4; lower than the level of ≥30 ng/ml recommended by some experts5. The latter definition of 25(OH)D sufficiency was based on the level in which parathyroid hormone (PTH) values reached their nadir in relation to 25(OH)D. Identifying 25(OH)D inflection points based on fractures as outcome; might be a more clinically relevant way to define 25(OH)D sufficiency. Evidence linking low vitamin D status to higher fracture risk is inconsistent6-15, although prior cohort studies suggest an optimal 25(OH)D range of ≥12-24 ng/ml for decreasing risk of fracture 6-8,10,13-15. There is little evidence to suggest that PTH levels influence fracture risk16, despite a robust inverse correlation with 25(OH)D, and the known catabolic effects of PTH on increased bone turnover and decreased cortical BMD3.
Calciotropic hormones may influence fracture rates through various mediators in the causal pathway. Low levels of 25(OH)D have been linked independently to potential mediators such as falls, neuromuscular function, disability, and hip BMD17-19. Cauley and colleagues reported that the association between elevated 25(OH)D and hip fracture in the WHI study was attenuated by 18% after adjusting for C-terminal telopeptide, a marker of bone resorption6. Another report on 25(OH)D identified hip BMD and neuromuscular function measures as mediators of fracture risk7.
We conducted a prospective population-based cohort study of older adults to determine: (1) if low serum 25(OH)D or high PTH levels are associated with an increased risk of incident hip and any non-spine fracture in older adults; (2) the optimal threshold concentration of 25(OH)D based on fracture risk; and (3) if these associations are mediated by physical function, inflammatory markers, falls, kidney function, BMD, and serum calcium.
Methods
Study Population
The Health Aging and Body Composition (Health ABC) study enrolled 3,075 women and men, aged 70-79, from two field centers, Pittsburgh, PA and Memphis, TN, in 1997-1998. To be eligible to participate in Health ABC, participants had to report no difficulty walking at least 1/4 mile and or climbing a flight of stairs. Participants were identified from a random sample of white Medicare beneficiaries and all age-eligible black community residents in designated ZIP code areas surrounding Pittsburgh and Memphis. Exclusion criteria included reported difficulty performing basic activities of daily living, obvious cognitive impairment, inability to communicate with the interviewer, intention of moving within 3 years, or participation in a trial involving a lifestyle intervention. Follow-ups occurred on an annual basis and serum and plasma concentrations were collected during the first follow-up or year 2. Of the 2,998 participants at year 2, we excluded 205 (6.9%) for not having 25(OH)D or PTH measurements, 6 with 25(OH)D values (>187.5 ng/ml, based on statistical criteria (75th + 3IQR), 7 with PTH (>250 pg/mL), and 140 for using osteoporosis medications. An additional 26 participants were excluded for not completing a visit after year 2, resulting in an analytic sample of 2614 participants. Among the 2614 eligible participants, 21.8% died over the study period and 5.3% lost to follow-up. The institutional review board (IRB) at each center approved the study protocol, and written informed consent was obtained from all the participants.
Serum 25(OH)D and PTH
Both specimens (serum and plasma) were collected at one time point (year 2). Samples were drawn in the morning, after an overnight fast, after processing, the specimens were frozen at _70°C and shipped to the Core Laboratory at the University of Vermont for long term storage. Serum 25(OH)D and plasma PTH levels were subsequently assayed in 2008-2009. Serum 25(OH)D was measured in serum samples using a 2-step radioimmunoassay (25(OH)D 125I RIA Kit, DiaSorin, Stillwater, Minn., USA). The inter-assay coefficient of variation for 25(OH)D was 6.78% for log-transformed values. Intact PTH (1-84) was measured in EDTA plasma with a two-site immunoradiometric assay kit (N-tact PTHSP, DiaSorin, Stillwater, Minn., USA). The inter-assay coefficient of variation for PTH was 8.6%.
Hip and any Non-Spine Fractures
Participants were contacted every 6 months, alternating between clinic visits and telephone interviews. Only fractures that occurred after year 2 were included in the time to event analysis. Information on self-report of incident hip or any non-spine fractures was obtained by phone interview, in person, or from proxy respondents (i.e., family or friends). All fractures were validated by radiology reports (pathological fractures, fractures of unknown etiology, and traumatic fractures were excluded). Adjudication of 84 hip and 247 non-spine fractures was complete through June 30th, 2007 for the Pittsburgh clinic, and through December 31st, 2005 for the Memphis clinic. The median (IQR) follow-up times for hip and any non-spine fractures were 6.4 (6.1–6.5) and 6.4 (5.5–6.5) years, respectively. The follow-up times in person-years for hip and any non-spine fractures were 15136.4 and 14734.5, respectively.
Other Measurements
Demographic variables included self-report of age, sex, race (whites and blacks), and education (<high school (HS) or ≥HS). Season of blood draw was coded as winter, December–February; spring, March–May; summer, June–August; fall, September–November. Weight was measured on a standard balance beam scale to the nearest 0.1 kg, and height was measured by a stadiometer to the nearest 0.1 cm with BMI (kg/m2) calculated using the formula weight (kg)/height2(m2). Hip areal BMD (aBMD) was measured using DXA (QDR 4500A; software version 9.03; Hologic, Bedford, MA, USA). DXA quality assurance procedures were conducted at both study sites and monitored by the study Coordinating Center, ensuring scanner reliability and identical scan protocols. An anthropometric spine phantom was scanned daily, and a hip phantom, once per week to assess longitudinal performance of the scanners.
Lifestyle factors included self-report of smoking in pack-years, and alcohol consumption (none, <1 drink/week, 1-3 drinks/week, or ≥4 drinks/week). To assess supplementary intake for vitamin D and calcium, participants were asked to bring all prescription and over the counter medications, which were coded based on the Iowa Drug Information System.20 To estimate dietary intake of calcium, participants completed a 108-item interviewer-administered FFQ (Block Dietary Data Systems, Berkeley, CA). Time spent walking (min/week) was determined by self-report. History of fracture after age 45 was determined by self-report of doctor diagnosed fracture. To better understand the effect of comorbidities, a composite clinical comorbidity index was created centering on self-report of 7 chronic health conditions (cardiovascular disease, stroke, pulmonary disease, diabetes, kidney disease, arthritis, and depression). Respondents were asked to report the number of falls in the past 12 months. Physical function was determined by the Health ABC physical performance battery (HPPB) as described previously21. Briefly, the performance scale (0-4) includes five repeated chair stands, 6-meter walk time, 6-meter narrow walk, and a balance test.
Serum calcium was measured with direct quantitative colorimetric determination using Stanbio Total Calcium LiquiColor Procedure No. 0500 (Stanbio Laboratory, Boerne, TX, USA). The inter-assay coefficient of variation was 2.2%. Interleukin (IL)-6 was measured in duplicate by enzyme-linked immunosorbent assay. The detectable limit for IL-6 was 0.10 pg/ml. Blind duplicate analyses (n =150) for IL-6 showed an inter-assay coefficient of variation of 10.3%. Cystatin-C was measured using a BNII nephelometer (Dade Behring, Deerfield, IL) that used a particle-enhanced immunonepholometricassay (N Latex Cystatin C). The assay range was 0.195 to 7.330 mg/L with an intra-assay coefficient of variation of 7.7%. The estimated glomerular filtration rate (eGRF) was then calculated using a validated cystatin C-based equation22.
Statistical Analysis
Tests of trend were used to compare participant characteristics across quartiles of 25(OH)D and PTH. To further describe the relationship between PTH and 25(OH)D a LOESS scatterplot was created with PTH plotted across values of 25(OH)D. Tests of threshold were conducted to find the 25(OH)D points where PTH values begin to reach their nadir. To evaluate participant characteristics by fracture status two-sample t-tests, ANOVA, chi-square tests, Wilcoxon rank-sum and Kruskal-Wallis tests were used.
We assessed the association between 25(OH)D, PTH and incident hip and non-spine fracture using Semi-parametric Cox proportional hazards models. Hazard ratios (HR) and 95% confidence intervals (95% CI) comparing quartile 4 (top quartile) with quartiles 1, 2, and 3 were calculated for 25(OH)D and PTH with tests for trend across the quartiles. There was a non-linear association between 25(OH)D and incident hip fractures, thus consequently, we were unable to model 25(OH)D as a continuous variable. The proportional hazards assumption was confirmed graphically and formally using Schoenfeld residuals. The model fit was verified by plotting the Nelson-Aalen cumulative hazard of the Cox-Snell residuals.
To further test if there was a linear relationship between 25(OH)D and fracture we performed spline analysis. Restricted cubic spline linear regression was used with knots for 25(OH)D at the 5th, 25th, 75th and a reference group at the 95th percentile was set to create the spline plot. Threshold effects were evaluated by identifying potential inflection points on the spline and performing a test of equality to determine if the slopes above and below the cut point were equal.
For 25(OH)D and PTH, we first examined the unadjusted association with hip and non-spine fracture. For the base multivariable models, a variable would be considered for inclusion if it was associated with the exposure or outcome of interest. Backward elimination procedure (p=0.15 entry and removal) was then used with age, sex, race, season of blood draw, and the exposure of interest forced into all base multivariable models. As a result, the final base multivariable model for hip fracture included the forced variables, BMI, alcohol use, and fracture after age 45. The final base multivariable model for non-spine fracture included the same variables. To investigate mechanisms by which 25(OH)D and PTH might be associated with hip and non-spine fractures, we added the following variables one at a time to the base model to determine if they mediated this association: number of falls, time to complete five chair stands, IL-6, serum calcium, hip aBMD, and eGFR. We then adjusted for all variables simultaneously in the full multivariable model.
Sex, race, age (<75 vs. ≥75 years), and season did not modify the associations between the calciotropic hormones and incident hip or non-spine fracture for all models (p interaction>0.05), and thus we refrained from performing stratified analyses.
We conducted secondary analyses to determine if 25(OH)D is associated longitudinally with potential mediators (incident falls and BMD loss) in the causal pathway leading to fracture. Hip aBMD measurements were taken at baseline, years 3, 5 or 6, 8, and 10. Linear mixed effects models accounted for repeated measures of hip aBMD. We excluded participants with ≤1 measurement(s) of hip BMD (analytic sample n=2439). We included a random intercept for each subject and a random slope for time to account for within person correlation. Change in aBMD was estimated through an interaction of time with covariates of interest. We estimated the average annual change in hip aBMD by 25(OH)D quartiles. Incident number of falls for each participant was aggregated using data from years 3–10. The association between 25(OH)D quartiles and incident falls was then evaluated using Poisson regression with a robust variance estimator to account for overdispersion.
All statistical analyses were performed using the Statistical Analysis System (SAS, version 9.2; SAS Institute, Cary, NC).
Results
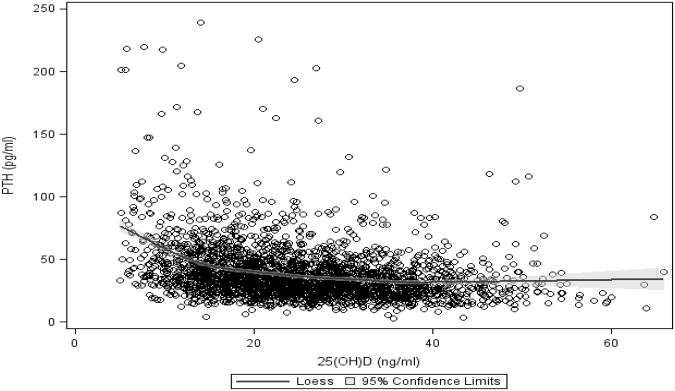
Table 1 shows the descriptive baseline characteristics by 25(OH)D and PTH quartiles. Lower 25(OH)D levels were associated with women, blacks, < high school education, greater BMI, winter, lower alcohol consumption, lower dietary and supplemental calcium intake, lower supplemental vitamin D intake, lower time spent walking, higher clinical comorbidity index, lower physical function, greater IL-6, lower hip aBMD, and greater eGFR. For PTH, the direction of these associations was reversed, with the exception of eGFR and hip aBMD, which were not significantly associated with PTH. Serum 25(OH)D was inversely associated with PTH. PTH concentrations began to reach their nadir at approximately 15 ng/ml of 25(OH)D (Figure 1).
Table 1. Descriptive baseline characteristics by 25(OH)D and PTH quartiles.
| 25 (OH)D | PTH | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
|
| ||||||||
| (n = 660) | (n = 660) | (n = 660) | (n = 660) | (n = 659) | (n = 659) | (n = 661) | (n = 660) | |
| Age, mean ± SD, yrs | 74.7 ± 2.9 | 74.6 ± 2.9 | 74.6 ± 2.9 | 74.7 ± 2.8 | 74.5 ± 2.8 | 74.6 ± 2.9 | 74.6 ± 2.8 | 74.9 ± 2.9 |
| Female gender, % | 61.2 | 45.9 | 44.6 | 43.9 | 45.1 | 47.2 | 50.2 | 53.0 |
| Black race, % | 69.1 | 47.4 | 29.2 | 18.3 | 31.4 | 37.8 | 41.0 | 53.8 |
| Education < high school, % | 34.5 | 26.5 | 21.4 | 16.8 | 22.6 | 23.4 | 25.2 | 28.1 |
|
|
||||||||
| BMI, mean ± SD, kg/m2 | 28.6 ± 5.6 | 27.7 ± 5.1 | 27.2 ± 4.2 | 26.0 ± 3.8 | 26.2 ± 4.0 | 27.0 ± 4.6 | 27.7 ± 5.1 | 28.4 ± 5.2 |
| Season of blood draw, % | ||||||||
| Winter | 31.0 | 28.2 | 24.2 | 19.1 | 22.5 | 24.9 | 28.1 | 27.1 |
| Spring | 33.2 | 33.5 | 30.6 | 30.1 | 29.7 | 31.7 | 32.4 | 33.5 |
| Summer | 11.4 | 17.1 | 21.1 | 19.9 | 19.0 | 18.2 | 16.3 | 15.9 |
| Fall | 24.4 | 21.2 | 24.1 | 30.9 | 28.8 | 25.2 | 23.2 | 23.5 |
| Pack-years exposure to cigarettes, median (IQR) | 3.0 (0.0 - 27.0) | 6.0 (0.0 - 33.0) | 3.0 (0.0 - 32.0) | 5.5 (0.0 - 32.5) | 4.0 (0.0 - 32.0) | 8.0 (0.0 - 33.0) | 3.0 (0.0 - 27.0) | 3.0 (0.0 - 31.0) |
| Current alcohol consumption, % | ||||||||
| None | 70.4 | 68.4 | 63.2 | 58.7 | 62.1 | 63.4 | 64.0 | 71.1 |
| < 1 drink/week | 4.7 | 5.6 | 6.5 | 7.1 | 7.5 | 6.1 | 4.5 | 5.8 |
| 1-3 drinks/week | 15.9 | 17.5 | 22.7 | 24.0 | 21.1 | 19.5 | 22.3 | 17.3 |
| 4+ drinks/week | 9.0 | 8.5 | 7.6 | 10.2 | 9.3 | 11.0 | 9.2 | 5.8 |
| Dietary calcium intake, median (IQR), mg/d | 650 (433 - 897) | 731 (517 - 971) | 725 (533 - 978) | 766 (556 - 1036) | 769 (547 - 1034) | 718 (527 - 955) | 719 (511 - 965) | 679 (466 - 950) |
| Supplemental calcium intake, % yes | 6.2 | 13.6 | 23.8 | 30.4 | 27.2 | 20.4 | 15.0 | 11.4 |
| Supplemental vitamin D intake, % yes | 2.0 | 5.6 | 11.2 | 15.0 | 12.3 | 8.8 | 7.4 | 5.3 |
| Time spent walking, median (IQR), minutes/week | 0 (0 - 90) | 40 (0.0 - 175) | 60 (0 - 180) | 70 (0 - 210) | 60 (0 - 210) | 45 (0 - 180) | 38 (0 - 180) | 20 (0 - 120) |
| Any fracture after age 45, % yes | 21.9 | 23.0 | 20.3 | 19.4 | 21.1 | 21.7 | 20.2 | 21.5 |
| Clinical Comorbidity Index (0-7), mean ± SD | 0.91± 0.98 | 0.78 ± 0.88 | 0.76 ± 0.85 | 0.73 ± 0.81 | 0.70 ± 0.80 | 0.77 ± 0.88 | 0.78 ± 0.87 | 0.93 ± 0.96 |
| Any falls in past 12 months, % yes | 26.1 | 21.4 | 23.8 | 22.1 | 22.2 | 22.4 | 25.0 | 23.7 |
| HABC Performance Score (0-4), mean ± SD | 2.0 ± 0.6 | 2.2 ± 0.5 | 2.3 ± 0.5 | 2.3 ± 0.5 | 2.3 ± 0.5 | 2.3 ± 0.5 | 2.2 ± 0.6 | 2.1 ± 0.6 |
| IL-6, median (IQR), pg/ml | 2.8 (1.7 - 4.7) | 2.4 (1.5 - 4.2) | 2.3 (1.5 - 3.7) | 2.2 (1.4 - 3.7) | 2.1 (1.4 - 3.6) | 2.3 (1.5 - 4.0) | 2.6 (1.6 - 4.3) | 2.7 (1.8 - 4.5) |
| Hip aBMD, mean ± SD, g/cm2 | 0.90 ± 0.17 | 0.91 ± 0.17 | 0.89 ± 0.16 | 0.88 ± 0.16 | 0.89 ± 0.16 | 0.90 ± 0.17 | 0.90 ± 0.17 | 0.90 ± 0.17 |
| Glomerular Filtration Rate, mean ± SD | 74.6 ± 17.1 | 73.8 ± 16.6 | 72.3 ± 15.8 | 69.7 ± 14.6 | 74.6 ± 15.8 | 74.0 ± 14.8 | 73.5 ± 14.6 | 68.2 ± 18.3 |
| Serum Calcium, mean ± SD, mg/dL | 8.9 ± 0.4 | 8.9 ± 0.4 | 8.9 ± 0.4 | 8.9 ± 0.4 | 8.9 ± 0.4 | 8.9 ± 0.4 | 8.8 ± 0.4 | 8.9 ± 0.5 |
| 25(OH)D, median (IQR), ng/ml | 29.1 (22.3 – 36.1) | 25.7 (19.5 – 32.3) | 22.9 (17.2 – 30.2) | 19.3 (13.8 – 26.3) | ||||
| PTH, median (IQR), pg/ml | 41.9 (31.7 – 58.3) | 35.2 (26.7 – 46.2) | 31.1 (24.4 – 41.0) | 28.6 (21.4 – 38.2) | ||||
25(OH)D quartile cut points are 17.777 ng/ml, 24.364 ng/ml and 31.9375 ng/ml
PTH quartile cut points are 25.17 pg/ml, 33.81 pg/ml and 45.92 pg/ml
Bold indicates p-value for trend < 0.05
Figure 1.
LOESS scatterplot showing PTH and 95% confidence limits (shaded area) across values of 25(OH)D.
Table 2 shows the descriptive characteristics by hip and any non-spine fracture status. Participants with hip and any non-spine fracture were significantly more likely to be older, women, white, have lower BMI, have a history of fracture after age 45, have a higher clinical comorbidity index, and have lower aBMD. Additional factors associated with any non-spine fracture were: more likely to have a high school education, have higher supplemental calcium and vitamin D intake, more likely to have fallen in the past 12 months, and have lower physical function and eGFR.
Table 2. Descriptive baseline characteristics by hip and non-spine fracture status.
| Hip fracture | Any non-spine fracture | |||
|---|---|---|---|---|
|
| ||||
| No (n = 2556) | Yes (n = 84) | No (n = 2393) | Yes (n = 247) | |
| Age, mean ± SD, yrs | 74.6 ± 2.8 | 76.4 ± 2.8 | 74.6 ± 2.8 | 75.3 ± 3.0 |
| Female gender, % | 48.5 | 60.7 | 46.7 | 70.5 |
| Black race, % | 41.4 | 28.6 | 42.3 | 28.3 |
| Education < high school, % | 24.8 | 25.0 | 25.6 | 17.0 |
| BMI, mean ± SD, kg/m2 | 27.4 ± 4.8 | 25.8 ± 4.3 | 27.4 ± 4.8 | 26.7 ± 4.8 |
| Season of blood draw, % | ||||
| Winter | 25.5 | 29.8 | 25.4 | 28.3 |
| Spring | 32.1 | 23.8 | 32.1 | 29.2 |
| Summer | 17.3 | 19.0 | 17.3 | 17.4 |
| Fall | 25.1 | 27.4 | 25.2 | 25.1 |
| Pack-years exposure to cigarettes, median (IQR) | 4.0 (0.0 - 30.0) | 4.0 (0.0 - 36.5) | 4.5 (0.0 - 30.0) | 1.5 (0.0 - 30.0) |
| Current alcohol consumption, % | ||||
| None | 65.0 | 71.1 | 65.1 | 65.8 |
| < 1 drink/week | 6.0 | 6.0 | 6.1 | 4.9 |
| 1-3 drinks/week | 20.1 | 18.1 | 19.6 | 23.9 |
| 4+ drinks/week | 8.9 | 4.8 | 9.2 | 5.4 |
| Dietary calcium intake, median (IQR), mg/d | 717 (515 - 973) | 736 (532 - 995) | 719 (517 - 978) | 716 (501 - 940) |
| Supplemental calcium intake, % yes | 18.3 | 25.0 | 17.4 | 28.7 |
| Supplemental vitamin D intake, % yes | 8.3 | 13.1 | 8.1 | 12.2 |
| Time spent walking, median (IQR), minutes/week | 40 (0 - 180) | 60 (0 - 195) | 40 (0 - 180) | 36 (0 - 150) |
| Any fracture after age 45, % yes | 20.7 | 33.3 | 20.0 | 32.4 |
| Clinical Comorbidity Index (0-7), mean ± SD | 0.79 ± 0.88 | 1.06 ± 0.97 | 0.78 ± 0.87 | 0.95 ± 0.99 |
| Any falls in past 12 months, % yes | 23.1 | 28.9 | 22.7 | 28.9 |
| HABC Performance Score (0-4), mean ± SD | 2.2 ± 0.5 | 2.1 ± 0.6 | 2.2 ± 0.5 | 2.1 ± 0.6 |
| IL-6, median (IQR), pg/ml | 2.4 (1.5 - 4.1) | 2.7 (1.7 - 4.4) | 2.4 (1.5 - 4.1) | 2.4 (1.6 - 4.0) |
| Hip aBMD, mean ± SD, g/cm2 | 0.90 ± 0.16 | 0.74 ± 0.14 | 0.91 ± 0.16 | 0.78 ± 0.14 |
| Glomerular Filtration Rate, mean ± SD | 72.7 ± 16.1 | 70.2 ± 16.6 | 72.9 ± 16.2 | 69.8 ± 15.5 |
| Serum Calcium, mean ± SD, mg/dL | 8.9 ± 0.43 | 8.9 ± 0.42 | 8.9 ± 0.43 | 8.9 ± 0.41 |
| 25(OH)D, median (IQR), ng/ml | 24.3 (17.8 – 31.9) | 26.3 (15.9 – 31.9) | 24.3 (17.9 – 31.8) | 24.7 (16.5 – 32.2) |
| PTH, median (IQR), pg/ml | 33.8 (25.2 – 45.9) | 34.3 (24.8 – 46.9) | 33.8 (25.2 – 45.7) | 34.3 (24.9 – 47.0) |
Bold indicates p-value < 0.05
25(OH)D and Fracture
Table 3 shows the associations between 25(OH)D and hip and non-spine fracture. The HR (95% CIs) of hip fracture for participants in the lowest quartile (≤17.78 ng/ml) was 1.92 (0.97–3.83) after adjusting for age, sex, race, season of blood draw, BMI, alcohol use, fracture after age 45, and the clinical comorbidity index compared with participants in the highest 25(OH)D quartile (≥31.93 ng/ml) . There was no association [0.75(0.32–1.72], between 25(OH) D and hip fracture among participants in the second quartile (>17.78-24.36 ng/ml), however participants in the third quartile (>24.36–<31.93 ng/ml) had an elevated risk [1.86(1.00, 3.45)] of fracture. The overall trend was not statistically significant (p trend=0.217). Adjusting individually for IL-6 (p-value=0.107), PTH (p-value=0.124) and hip aBMD (p-value=0.137) in addition to the aforementioned covariates attenuated these associations further by 16.3%, 17.4%, and 26.1%, respectively.
Table 3. Hazard Ratios (95% Risk limits) of Hip and Non-vertebral Fracture across 25(OH)D Quartiles.
| Model | N | Q1 | Q2 | Q3 | Q4 | p-trend |
|---|---|---|---|---|---|---|
| Hip Fractures | ||||||
| Base MV Model a | 2501 | 1.92 (0.97, 3.83) | 0.75 (0.32, 1.72) | 1.86 (1.00, 3.45) | Ref | 0.217 |
| Base MV Modela + Falls | 2426 | 1.99 (0.99, 3.96) | 0.68 (0.29, 1.61) | 1.86 (1.01, 3.46) | Ref | 0.230 |
| Base MV Modela + Health ABC Performance Score (0-4) | 2468 | 1.89 (0.93, 3.81) | 0.79 (0.34, 1.84) | 2.04 (1.09, 3.83) | Ref | 0.112 |
| Base MV Modela + IL-6 | 2477 | 1.77 (0.88, 3.58) | 0.78 (0.34, 1.79) | 1.74 (0.93, 3.26) | Ref | 0.269 |
| Base MV Modela + Serum Calcium | 2492 | 1.89 (0.94, 3.78) | 0.74 (0.32, 1.70) | 1.83 (0.98, 3.40) | Ref | 0.245 |
| Base MV Modela + Hip aBMD | 2480 | 1.68 (0.85, 3.34) | 0.66 (0.29, 1.51) | 1.49 (0.80, 2.79) | Ref | 0.632 |
| Base MV Modela + eGFR | 2501 | 1.95 (0.97, 3.90) | 0.76 (0.33, 1.77) | 1.88 (1.01, 3.50) | Ref | 0.198 |
| Base MV Modela + PTH | 2500 | 1.76 (0.86, 3.60) | 0.74 (0.32, 1.70) | 1.82 (0.98, 3.39) | Ref | 0.210 |
| Full MV Model (Base MV Modela + all mediators) | 2347 | 1.73 (0.80, 3.75) | 0.74 (0.31, 1.79) | 1.60 (0.83, 3.10) | Ref | 0.430 |
|
| ||||||
| Non-vertebral Fractures | ||||||
| Base MV Model b | 2494 | 1.21 (0.83, 1.75) | 1.01 (0.68, 1.49) | 1.12 (0.78, 1.60) | Ref | 0.752 |
| Base MV Modelb + Falls | 2419 | 1.17 (0.80, 1.71) | 0.98 (0.66, 1.45) | 1.12 (0.78, 1.61) | Ref | 0.739 |
| Base MV Modelb + Health ABC Performance Score (0-4) | 2461 | 1.31 (0.89, 1.93) | 1.09 (0.73, 1.63) | 1.20 (0.83, 1.74) | Ref | 0.522 |
| Base MV Modelb + IL-6 | 2470 | 1.21 (0.83, 1.75) | 1.02 (0.69, 1.52) | 1.11 (0.77, 1.59) | Ref | 0.777 |
| Base MV Modelb + Serum Calcium | 2485 | 1.21 (0.83, 1.75) | 0.99 (0.66, 1.46) | 1.12 (0.78, 1.60) | Ref | 0.774 |
| Base MV Modelb + Hip aBMD | 2473 | 1.16 (0.79, 1.68) | 1.02 (0.69, 1.52) | 1.11 (0.77, 1.59) | Ref | 0.743 |
| Base MV Modelb + eGFR | 2494 | 1.26 (0.87, 1.83) | 1.06 (0.71, 1.57) | 1.16 (0.81, 1.67) | Ref | 0.606 |
| Base MV Modelb + PTH | 2493 | 1.16 (0.79, 1.70) | 1.00 (0.68, 1.49) | 1.11 (0.78, 1.60) | Ref | 0.727 |
| Full MV Model (Base MV Modelb + all mediators) | 2340 | 1.36 (0.90, 2.07) | 1.16 (0.76, 1.77) | 1.23 (0.85, 1.80) | Ref | 0.430 |
Quartile cut points are 17.777 ng/ml, 24.364 ng/ml and 31.9375 ng/ml
Base MV (multivariable) model adjusted for Age, Gender, Race, Season of blood draw, BMI, Current drinking, Fracture after age 45 and Clinical comorbidity index.
Base MV (multivariable) model adjusted for Age, Gender, Race, Education level, Season of blood draw, BMI, Current drinking, Fracture after age 45 and Clinical comorbidity index.
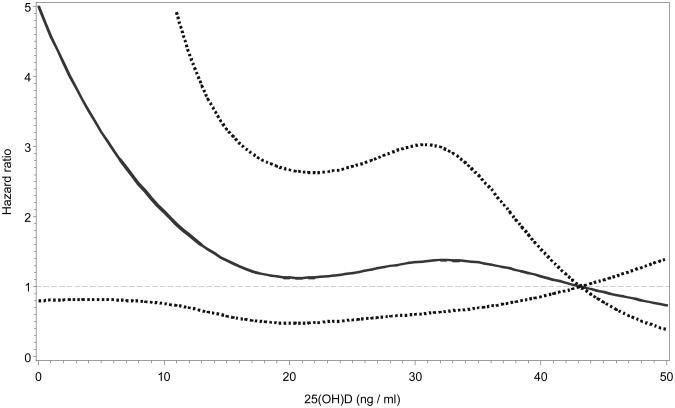
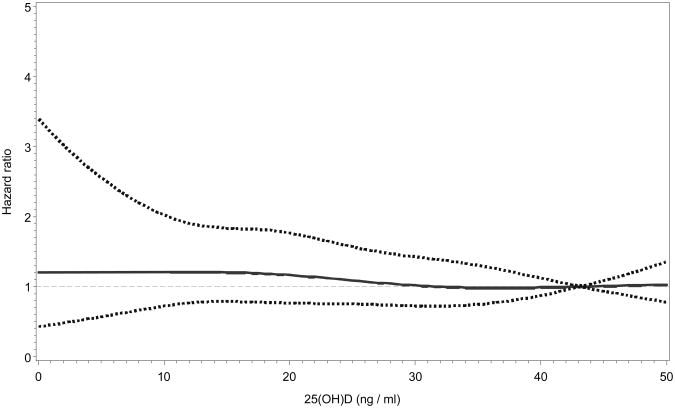
The risk of any non-spine fracture was highest among those in the lowest 25(OH)D quartile, however there was no significant association in any model. There was no evidence of a threshold (p for test of threshold>0.05; Figures 2 and 3) or trend for serum 25(OH)D concentrations and incident hip or non-spine fracture.
Figure 2.
Restricted cubic-spline Cox regression plot showing the hazard ratios and 95% confidence limits (dotted lines) of incident hip fractures by serum 25(OH)D. Regression was adjusted for age, gender, race, season of blood draw, BMI, alcohol use, fracture after age 45, falls, health ABC performance score, IL-6, serum calcium, hip aBMD, eGFR, and PTH.
Figure 3.
Restricted cubic-spline Cox regression plot showing the hazard ratios and 95% confidence limits (dotted lines) of incident non-spine fractures by serum 25(OH)D. Regression was adjusted for age, gender, race, season of blood draw, BMI, alcohol use, fracture after age 45, falls, health ABC performance score, IL-6, serum calcium, hip aBMD, eGFR, and PTH
PTH and Fracture
Table 4 shows the associations between PTH and hip and non-spine fracture. In the base multivariable model, the adjusted HR (95% CI) of hip fracture was 0.68 (0.35-1.31) among participants in the lowest quartile of PTH compared with those in the highest quartile of PTH (p trend=0.94). Similarly, there were also no significant trends or differences across quartiles for the association of PTH with non-spine fracture.
Table 4. Hazard Ratios (95% Risk limits) of Hip and Non-vertebral Fractures across PTH Quartiles.
| Model | N | Q1 | Q2 | Q3 | Q4 | p-trend |
|---|---|---|---|---|---|---|
| Hip Fractures | ||||||
| Base MV Model a | 2500 | 0.68 (0.35, 1.31) | 0.73 (0.38, 1.37) | 0.96 (0.52, 1.79) | Ref | 0.942 |
| Base MV Modela + Falls | 2425 | 0.65 (0.34, 1.27) | 0.72 (0.38, 1.35) | 0.90 (0.48, 1.68) | Ref | 0.798 |
| Base MV Modela + Health ABC Performance Score (0-4) | 2467 | 0.71 (0.36, 1.40) | 0.78 (0.41, 1.49) | 1.05 (0.56, 1.98) | Ref | 0.814 |
| Base MV Modela + IL-6 | 2476 | 0.69 (0.35, 1.36) | 0.73 (0.38, 1.39) | 0.91 (0.48, 1.73) | Ref | 0.810 |
| Base MV Modela + Serum Calcium | 2491 | 0.68 (0.35, 1.33) | 0.73 (0.39, 1.38) | 0.95 (0.51, 1.78) | Ref | 0.927 |
| Base MV Modela + Hip aBMD | 2479 | 0.84 (0.43, 1.64) | 0.86 (0.45, 1.64) | 1.03 (0.54, 1.95) | Ref | 0.930 |
| Base MV Modela + eGFR | 2500 | 0.68 (0.35, 1.32) | 0.73 (0.38, 1.38) | 0.96 (0.52, 1.80) | Ref | 0.956 |
| Base MV Modela + 25(OH)D | 2500 | 0.76 (0.38, 1.51) | 0.79 (0.41, 1.51) | 1.00 (0.54, 1.88) | Ref | 0.956 |
| Full MV Model (Base MV Modela + all mediators) | 2347 | 0.94 (0.44, 2.02) | 0.94 (0.47, 1.90) | 1.02 (0.51, 2.06) | Ref | 0.951 |
|
| ||||||
| Non-vertebral Fractures | ||||||
| Base MV Model a | 2500 | 0.99 (0.68, 1.42) | 0.85 (0.58, 1.23) | 0.95 (0.66, 1.36) | Ref | 0.588 |
| Base MV Modela + Falls | 2425 | 1.00 (0.69, 1.44) | 0.85 (0.58, 1.23) | 0.94 (0.65, 1.35) | Ref | 0.534 |
| Base MV Modela + Health ABC Performance Score (0-4) | 2467 | 0.95 (0.66, 1.38) | 0.83 (0.57, 1.20) | 0.90 (0.63, 1.31) | Ref | 0.444 |
| Base MV Modela + IL-6 | 2476 | 1.01 (0.70, 1.47) | 0.87 (0.59, 1.26) | 0.96 (0.67, 1.39) | Ref | 0.644 |
| Base MV Modela + Serum Calcium | 2491 | 1.00 (0.69, 1.45) | 0.86 (0.59, 1.24) | 0.96 (0.67, 1.39) | Ref | 0.641 |
| Base MV Modela + Hip aBMD | 2479 | 1.17 (0.81, 1.71) | 1.00 (0.68, 1.46) | 1.05 (0.72, 1.52) | Ref | 0.980 |
| Base MV Modela + eGFR | 2500 | 1.01 (0.70, 1.46) | 0.90 (0.62, 1.31) | 0.98 (0.68, 1.41) | Ref | 0.782 |
| Base MV Modela + 25(OH)D | 2500 | 1.01 (0.69, 1.49) | 0.86 (0.59, 1.25) | 0.96 (0.67, 1.38) | Ref | 0.614 |
| Full MV Model (Base MV Modela + all mediators) | 2347 | 1.29 (0.85, 1.94) | 1.11 (0.74, 1.66) | 1.07 (0.72, 1.58) | Ref | 0.944 |
Quartile cut points are 25.17 pg/ml, 33.81 pg/ml and 45.92 pg/ml
Base MV (multivariate) model adjusted for Age, Gender, Race, Season of blood draw, BMI, Current drinking, Fracture after age 45 and Clinical comorbidity index.
Secondary Analyses
Lower 25(OH)D was associated with greater aBMD loss (p trend=0.024). Participants in the top quartile (−0.55%; 95 CI: −0.48, −0.62) of 25(OH)D had significantly lower annualized hip aBMD loss compared to those in the lowest quartile (−0.65%; 95 CI: −0.58, −0.72). Serum 25(OH)D levels were not associated with incident falls (p trend=0.284). The multivariable adjusted HRs (95% CIs) of falls among participants in the lowest, second and third quartile of 25(OH)D were 0.91 (0.77-1.08), 0.96 (0.81-1.13) and 0.98 (0.83-1.15), respectively; compared with participants in the top 25(OH)D quartile. Both secondary analyses adjusted for age, sex, race, BMI and season.
Discussion
In our prospective cohort study, we found limited evidence to support an association between 25(OH)D and hip fracture and no evidence of an association with non-spine fracture. The lack of a clear trend complicates the interpretation of these data. We were also unable to identify an optimal threshold concentration of 25(OH)D based on fracture risk. In addition, PTH concentrations were not associated with hip or non-spine fractures.
The lack of a clear trend between 25(OH)D and hip fracture has implications for the cause and effect relationship, as well as potential biological plausibility. Though we observed that subjects with the lowest 25(OH)D and those in the third quartile had a borderline increased risk of fracture, there was no evidence of a clear trend. Thus, it is possible that some of the hazard rate variations of hip fracture across 25(OH)D quartiles are a result of random error or chance.
Our mostly null finding for 25(OH)D and hip fracture supports the results from a prior prospective study9 among participants from the study of osteoporotic fractures (SOF). However, comparing these two studies is difficult, mainly as a result of the SOF cohort's use of a much older assay that has been shown to misclassify vitamin D status23.
Previous studies showed an increased risk of hip fracture among older adults with low serum 25(OH)D levels6-8,14. The National Health and Nutrition Examination Survey (NHANES III) reported the highest risk of hip fracture was at levels ≤17.2 ng/ml of 25(OH)D.8 Similarly, Cauley and colleagues reported that men7 and women6 with estimated 25(OH)D values of <19 ng/ml were at the greatest risk of hip fracture. The Cardiovascular Health Study showed that serum concentrations of <15 ng/ml were associated with a 61% greater risk of hip fracture14. The number of hip fractures (n=84) in our study has implications for power (estimated post-hoc power when comparing Q4 and Q1 was 54%) and an increased chance of type II errors. However, the MrOS study7 found a dose-response relationship with an even lower number of hip fractures (n=81).
We found no association between 25(OH)D and any incident non-spine fracture consistent with two prospective studies in older men7 and postmenopausal women11. Fractures in bones with a larger proportion of trabecular bone (i.e., wrist and ankle fractures) may explain this consistent null association for non-spine fractures. The association between 25(OH)D and trabecular BMD has been shown to be largely null25,26, and excess PTH may actually help maintain or even have anabolic effects on trabecular bone27. In addition, the association between hip aBMD and non-spine fracture was slightly weaker than for hip fractures in our population.
Serum 25(OH)D concentrations may affect fracture risk through various mediators in the causal pathway. Individual adjustment for IL-6, PTH and hip aBMD attenuated HRs of hip fracture in the lowest quartile by 16.3%, 17.4%, and 26.1%, respectively. Low 25(OH)D has been linked to increased inflammatory activity in vivo28 and in a population based cohort29, and high levels of inflammatory markers (i.e., IL-6) have been shown to increase fracture risk30. In our study, there was a robust inverse association between 25(OH)D and IL-6. PTH was also inversely correlated with 25(OH)D in our population. This is expected, since low serum 25(OH)D causes increased stimulation of the parathyroid gland, and subsequent release of excess PTH5. The effects of 25(OH)D on incident hip fracture were mainly explained by hip aBMD. This is not surprising given the robust positive trend we observed between 25(OH)D and hip aBMD, and the large difference in hip aBMD (0.16 g/cm2) between hip fracture cases and non-cases. Our cross-sectional findings were also supported by our longitudinal analysis which showed that lower 25(OH)D was associated with higher hip aBMD loss.
Plasma PTH levels were not associated with hip or non-spine fracture consistent with a recent prospective cohort study14. There was robust inverse correlation between PTH levels and 25(OH)D; however PTH was not associated with hip aBMD, which may explain our null findings. Our findings are also consistent with a meta-analysis of 905 hip fractures cases and 924 controls from population-based case-control studies16. Of the 10 case-control studies identified, only two showed significantly higher PTH levels in hip fracture patients compared to controls31,32.
Though we were unable to identify a statistically significant optimal 25(OH)D threshold for fracture risk, there was a marginal elevated risk of hip fracture among participants with a 25(OH)D level of ≤17.8 ng/ml. This is consistent with the current IOM recommendations for 25(OH)D sufficiency4 and results from other longitudinal studies on 25(OH)D and hip fracture6-8. Optimal 25(OH)D concentrations were previously defined based on the nadir of PTH levels,33 leading to a wide range of optimal 25(OH)D thresholds, and thus resulting in a lack of a consensus regarding a defined level of sufficiency. In our study, we found that PTH values reached their lowest point of 25(OH)D at 15 ng/ml, consistent with our findings on 25(OH)D and incident hip fracture.
Strengths of our study include the large sample size, long follow-up and thus the ability to reliably assess temporality, control for many potential confounders, exploration of several mechanisms potentially underlying these associations, and the use of a valid and reliable 25(OH)D assay. Our study also has several limitations. Blacks are more likely to be in the lowest quartiles of 25(OH)D; however, most of the fractures occurred among whites. We previously showed that the relationship between 25(OH)D and fracture differed in black women34, but we had too few hip fractures in blacks to carry out race specific analyses. Second, serum 25(OH)D was measured at one point and may not reflect vitamin D status over the follow-period. Nonetheless, a strong correlation (r=0.7) was reported for 25(OH)D values measured after 3 years35. Third, even though the blood test was taken during visit 2, the actual assay of 25(OH)D and PTH occurred around 10 years later. Hence, participants wouldn't have known their vitamin D status, and thus, their behavior regarding vitamin D and calcium supplementary intake would be unaffected by the serum samples taken at year 2. Nevertheless they still could have been tested by their own doctors and initiated vitamin and calcium supplementation. Fourth, we considered many potential confounders and mediators in our analyses; however there may be residual confounding by unmeasured factors. Finally, we did not have measures of bone turnover which have been shown to mediate the relationship between 25(OH)D and fracture6.
In summary, we found limited evidence to support an association between calciotropic hormones and hip and non-spine fractures in a community based sample of older men and women.
Acknowledgments
Role of Funding Source: The Health Aging and Body Composition Study (Health ABC) includes the contract numbers: N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, RO1-AG028050, RO1-NR012459, and WFUHS11200.This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Conflict of Interest: All authors have no conflict of interest
Contributor Information
Denise K. Houston, Email: dhouston@wfubmc.edu.
Steven R. Cummings, Email: SCummings@sfcc-cpmc.net.
Robert Boudreau, Email: boudreaur@edc.pitt.edu.
Tanushree Prasad, Email: tap55@pitt.edu.
Yahtyng Sheu, Email: sheuy@edc.pitt.edu.
Douglas C. Bauer, Email: dbauer@psg.ucsf.edu.
Janet A. Tooze, Email: jtooze@wfubmc.edu.
Stephen B. Kritchevsky, Email: skritche@wfubmc.edu.
Frances A. Tylavsky, Email: ftylavsk@uthsc.edu.
Tamara B. Harris, Email: harrist@gw.nia.nih.gov.
Jane A. Cauley, Email: jcauley@edc.pitt.edu.
References
- 1.Foulkes RG. Dietary reference intakes - Calcium, phosphorus, magnesium, vitamin D, and fluoride. Fluoride. 1997;30:252–257. [PubMed] [Google Scholar]
- 2.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988-1994 compared with 2000-2004. American Journal of Clinical Nutrition. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 4.Ross AC, Manson JE, Abrams SA, et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. Journal of Clinical Endocrinology & Metabolism. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6.Cauley JA, Lacroix AZ, Wu L, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149:242–250. doi: 10.7326/0003-4819-149-4-200808190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cauley JA, Parimi N, Ensrud KE, et al. Serum 25-hydroxyvitamin D and the risk of hip and nonspine fractures in older men. J Bone Miner Res. 2010;25:545–553. doi: 10.1359/jbmr.090826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Looker AC, Mussolino ME. Serum 25-hydroxyvitamin D and hip fracture risk in older US white adults. Journal of Bone and Mineral Research. 2008;23:143–150. doi: 10.1359/jbmr.071003. [DOI] [PubMed] [Google Scholar]
- 9.Cummings SR, Browner WS, Bauer D, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. New England Journal of Medicine. 1998;339:733–738. doi: 10.1056/NEJM199809103391104. [DOI] [PubMed] [Google Scholar]
- 10.Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int. 2005;16:1425–1431. doi: 10.1007/s00198-005-1860-1. [DOI] [PubMed] [Google Scholar]
- 11.Garnero P, Munoz F, Sornay-Rendu E, Delmas PD. Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY study. Bone. 2007;40:716–722. doi: 10.1016/j.bone.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Roddam AW, Neale R, Appleby P, Allen NE, Tipper S, Key TJ. Association between plasma 25-hydroxyvitamin D levels and fracture risk - The EPIC-oxford study. American Journal of Epidemiology. 2007;166:1327–1336. doi: 10.1093/aje/kwm210. [DOI] [PubMed] [Google Scholar]
- 13.Melhus H, Snellman G, Gedeborg R, et al. Plasma 25-hydroxyvitamin D levels and fracture risk in a community-based cohort of elderly men in Sweden. J Clin Endocrinol Metab. 2010;95:2637–2645. doi: 10.1210/jc.2009-2699. [DOI] [PubMed] [Google Scholar]
- 14.Robinson-Cohen C, Katz R, Hoofnagle AN, et al. Mineral Metabolism Markers and the Long-Term Risk of Hip Fracture: The Cardiovascular Health Study. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2010-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Schoor NM, Visser M, Pluijm SM, Kuchuk N, Smit JH, Lips P. Vitamin D deficiency as a risk factor for osteoporotic fractures. Bone. 2008;42:260–266. doi: 10.1016/j.bone.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Lai JKC, Lucas RM, Clements MS, Roddam AW, Banks E. Hip fracture risk in relation to vitamin D supplementation and serum 25-hydroxyvitamin D levels: a systematic review and meta-analysis of randomised controlled trials and observational studies. Bmc Public Health. 2010;10 doi: 10.1186/1471-2458-10-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhesi JK, Jackson SH, Bearne LM, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33:589–595. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 18.Semba RD, Garrett E, Johnson BA, Guralnik JM, Fried LP. Vitamin D deficiency among older women with and without disability. Am J Clin Nutr. 2000;72:1529–1534. doi: 10.1093/ajcn/72.6.1529. [DOI] [PubMed] [Google Scholar]
- 19.Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 20.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 21.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 22.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binkley N, Krueger D, Cowgill CS, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89:3152–3157. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]
- 24.Stone KL, Seeley DG, Lui LY, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 25.Barbour KE, Zmuda JM, Horwitz MJ, et al. The association of serum 25-hydroxyvitamin D with indicators of bone quality in men of Caucasian and African ancestry. Osteoporos Int. 2010 doi: 10.1007/s00198-010-1481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauretani F, Bandinelli S, Russo CR, et al. Correlates of bone quality in older persons. Bone. 2006;39:915–921. doi: 10.1016/j.bone.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan Y, De LV, Seeman E. Parathyroid hormone deficiency and excess: similar effects on trabecular bone but differing effects on cortical bone. J Clin Endocrinol Metab. 1999;84:718–722. doi: 10.1210/jcem.84.2.5498. [DOI] [PubMed] [Google Scholar]
- 28.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–1720S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 29.Miller RR, Hicks GE, Shardell MD, et al. Association of serum vitamin D levels with inflammatory response following hip fracture: the Baltimore Hip Studies. J Gerontol A Biol Sci Med Sci. 2007;62:1402–1406. doi: 10.1093/gerona/62.12.1402. [DOI] [PubMed] [Google Scholar]
- 30.Cauley JA, Danielson ME, Boudreau RM, et al. Inflammatory markers and incident fracture risk in older men and women: the Health Aging and Body Composition Study. J Bone Miner Res. 2007;22:1088–1095. doi: 10.1359/jbmr.070409. [DOI] [PubMed] [Google Scholar]
- 31.Boonen S, Broos P, Verbeke G, et al. Calciotropic hormones and markers of bone remodeling in age-related (type II) femoral neck osteoporosis: alterations consistent with secondary hyperparathyroidism-induced bone resorption. J Gerontol A Biol Sci Med Sci. 1997;52:M286–M293. doi: 10.1093/gerona/52a.5.m286. [DOI] [PubMed] [Google Scholar]
- 32.Sakuma M, Endo N, Oinuma T, et al. Vitamin D and intact PTH status in patients with hip fracture. Osteoporos Int. 2006;17:1608–1614. doi: 10.1007/s00198-006-0167-1. [DOI] [PubMed] [Google Scholar]
- 33.Bischoff-Ferrari HA. The 25-hydroxyvitamin D threshold for better health. J Steroid Biochem Mol Biol. 2007;103:614–619. doi: 10.1016/j.jsbmb.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Cauley JA, Danielson ME, Boudreau R, et al. Serum 25 hydroxyvitamin (OH)D and clinical fracture risk in a multiethnic Cohort of women: The Women's health initiative (WHI) J Bone Miner Res. 2011 doi: 10.1002/jbmr.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15:255–265. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]