Abstract
Vismodegib (Erivedge) for advanced basal cell carcinoma
INTRODUCTION
Basal cell carcinoma (BCC), first described in 1827,1 is the most common form of skin cancer,2–5 accounting for approximately 80% of all skin malignancies.6 An estimated 2.8 million new cases of BCC are diagnosed each year in the U.S.7
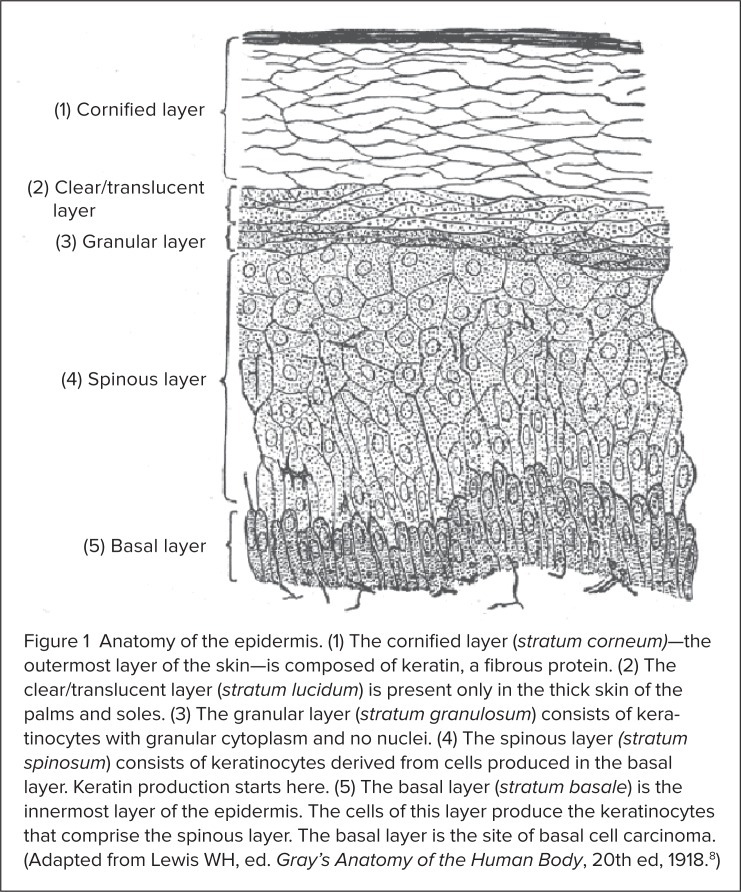
As its name implies, BCC develops in the basal, or lowest, layer of the epidermis (Figure 1).6,8 Cells in this layer of the skin continually divide to form keratinocytes, the predominant cell type in the epidermis. Keratinocytes, in turn, produce the protein keratin, which helps the skin protect the rest of the body.8–10 Although BCC can occur anywhere, it most commonly develops on the head and neck.11 BCC is an indolent disease,11 but in rare cases the tumors can invade local tissue (stage III disease) or metastasize to other parts of the body (stage IV disease).6,12,13 BCC can be highly disfiguring, involving extensive areas of soft tissue, cartilage, and bone.7,9,12,13 The disease, however, is rarely fatal.6,7
Figure 1.
Anatomy of the epidermis. (1) The cornified layer (stratum corneum)—the outermost layer of the skin—is composed of keratin, a fibrous protein. (2) The clear/translucent layer (stratum lucidum) is present only in the thick skin of the palms and soles. (3) The granular layer (stratum granulosum) consists of keratinocytes with granular cytoplasm and no nuclei. (4) The spinous layer (stratum spinosum) consists of keratinocytes derived from cells produced in the basal layer. Keratin production starts here. (5) The basal layer (stratum basale) is the innermost layer of the epidermis. The cells of this layer produce the keratinocytes that comprise the spinous layer. The basal layer is the site of basal cell carcinoma. (Adapted from Lewis WH, ed. Gray’s Anatomy of the Human Body, 20th ed, 1918.8)
The major risk factor for the development of any type of skin cancer is excessive exposure to ultraviolet radiation from the sun or indoor tanning.14,15 Additional risk factors for BCC include fair skin, light hair, a family history of skin cancer, and a weakened immune system (Table 1).6,14–16
Table 1.
Risk Factors for the Development of Basal Cell Carcinoma (BCC)
|
The absolute incidence of BCC is difficult to determine because non-melanoma skin cancers are rarely reported to cancer registries.14 Nevertheless, available data indicate that BCC may have an annual incidence of 0.1% to 0.5% in the U.S.17,18 Incidence rates of BCC have steadily increased throughout the world during the past 30 years.17,19 During that period, the number of women younger than 40 years of age with BCC has more than doubled in the U.S.7 In 1994, it was estimated that Caucasian populations in North America had nearly a one-in-three (30%) risk of developing BCC over their lifetimes. In addition, age-standardized yearly rates in the U.S. were estimated at up to 407 cases of BCC per 100,000 Caucasian men and 212 cases per 100,000 Caucasian women.20 In South Wales, the United Kingdom, the age-standardized incidence of BCC was estimated at 114 per 100,000 population in 1998.21 The reported incidence of BCC in Australia was 726 per 100,000 in 1993,22 and Australia continues to have the highest rate of BCC in the world.17
Although BCC most commonly occurs in elderly men, patients with this disease are increasingly likely to be young women.23 BCC is rarely seen in individuals younger than 20 years of age19 or in dark-skinned races.16,19 Skin cancers affect only 1% to 2% of all African Americans.7 Approximately six or seven cases of BCC are diagnosed by primary care physicians in the U.S. each year.24
Because of its high incidence and significant morbidity, BCC imposes a substantial economic burden on health care systems.19,25 In 2004, the total direct cost associated with the treatment of non-melanoma skin cancer (i.e., BCC and squamous cell carcinoma) was $1.5 billion.26
Surgery and radiation therapy are the mainstays of treatment for localized BCC.11,15,27–29 Topical therapies, such as 5-fluorouracil, imiquimod (Aldara, 3M/Medicis), photodynamic therapy, and cryotherapy, may be used in patients for whom surgery or radiation is contraindicated or impractical, although these approaches are less effective than primary treatment.11 BCC is generally curable if it is restricted to a small area of skin. Advanced BCC, however, cannot be effectively treated with surgery or radiation.12,13 The median survival time for patients with metastatic BCC is 8 months.30,31
In January 2012, the FDA approved vismodegib (Erivedge, Genentech), the first oral medication for adults with meta-static BCC or locally advanced BCC that has recurred after surgery or for patients who are not candidates for surgery or radiation.32,33
CHEMICAL AND PHYSICAL PROPERTIES
Vismodegib is a crystalline free base with a dissociation constant (pKa) acidity rating (pyridinium cation) of 3.8. Vismodegib appears as a white powder. Its solubility is pH-dependent, with 0.1 mcg/mL at pH 7 and 0.99 mg/mL at pH 1. The structural formula of vismodegib is shown in Figure 2.33
Figure 2.
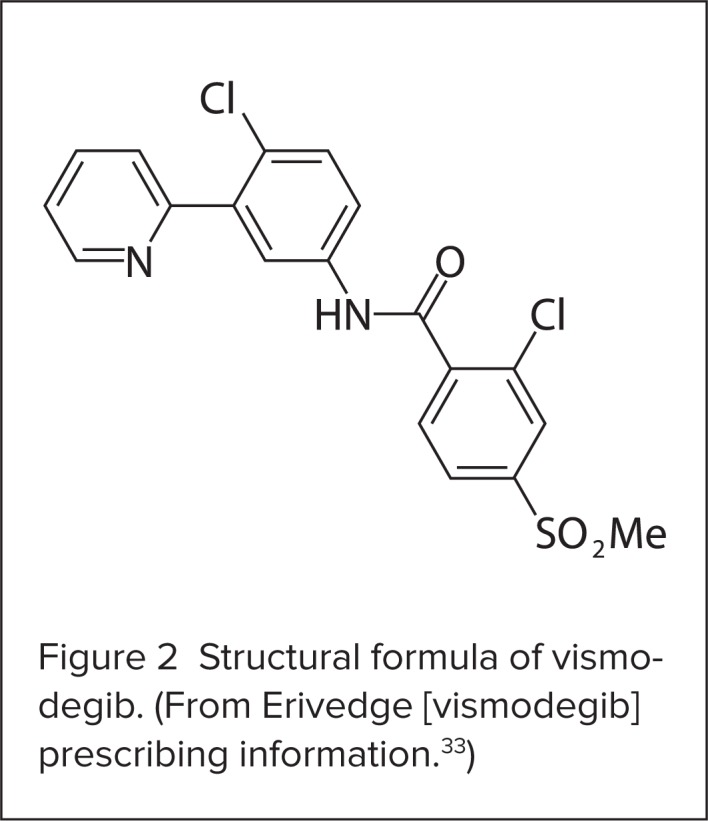
Structural formula of vismodegib. (From Erivedge [vismodegib] prescribing information.33)
Each capsule contains 150 mg of vismodegib and the following inactive ingredients: microcrystalline cellulose, lactose monohydrate, sodium lauryl sulfate, povidone, sodium starch glycolate, talc, and magnesium stearate (non-bovine).33
The capsules have a pink opaque body and a gray opaque cap, with “150 mg” printed on the capsule body and “VISMO” printed on the capsule cap in black ink. The capsule shell contains gelatin, titanium dioxide, red iron oxide, and black iron oxide. The black printing ink contains shellac and black iron oxide.33
MECHANISM OF ACTION
Vismodegib is a small-molecule systemic inhibitor of the Hedgehog (Hh) intracellular signaling pathway.33,34 During embryogenesis, this pathway plays an important role in the growth and development of tissues, including the promotion of primitive hematopoietic, neural, and mammary stem cells.35–38 The Hh signaling pathway is initiated when the Hh protein binds to its cell–surface receptor (Patched). Both the Hh and the Patched proteins then move inside the cell. Binding of the Patched protein liberates an intracellular protein (Smooth-ened), which moves to the cell surface. There, Smoothened activates the GLI protein family, leading to the activation of Hh target genes involved in cell growth.39–41 Normally, the Hh pathway is quiescent in adults.39,41
Reactivation of the Hh pathway has been implicated in several cancers, including BCC and medulloblastoma.39,41,42 Mutations in the Smoothened or Patched proteins, which encode the Hh pathway, are believed to lead to cancer cell growth in BCC.41,43,44 Vismodegib selectively binds to the Smoothened protein, thereby blocking intracellular signaling and deactivating the Hh pathway. This activity, in turn, interferes with tumor cell growth and survival.33,41,43,45
Because of its mechanism of action, vismodegib can cause severe injury or death to an embryo or fetus, and the product labeling includes a boxed warning to that effect.33
PHARMACOKINETICS
Orally administered, vismodegib demonstrates nonlinear, time-dependent pharmacokinetics resulting from differential plasma protein binding, solubility-limited absorption, and slow metabolic elimination properties.46
Absorption and Distribution
Vismodegib is a highly permeable compound with low aqueous solubility (Biopharmaceutics Classification System Class 2). The drug’s absolute bioavailability is low (32%) after a single dose. Absorption is saturable, as indicated by the absence of a dose–proportional increase in exposure after a single dose of 270 mg or 540 mg.33
The systemic exposure of vismodegib at steady state is not affected by food; therefore, vismodegib capsules may be taken without regard to meals.33
The volume of distribution ranges from 16.4 to 26.6 L, and the medication’s plasma protein binding is greater than 99%. Vismodegib binds to both human serum albumin and alpha-1-acid glyco-protein (AAG), and binding to AAG is saturable.33,47
Metabolism and Elimination
The parent drug accounts for more than 98% of the total circulating drug-related components of vismodegib. The metabolic pathways in humans include oxidation, glucuronidation, and pyridine ring cleavage.33
In vitro, the two most abundant oxidative metabolites recovered in feces are produced by recombinant cytochrome P450 (CYP) 2C9 and CYP3A4/5.33
Vismodegib and its metabolites are eliminated primarily by the liver; 82% of the administered dose is recovered in feces and 4.4% is recovered in urine. The estimated elimination half-life of vismodegib is 12 days after a single dose and 4 days after continuous once-daily administration.33
Specific Populations
The effect of hepatic or renal impairment on the systemic exposure of vismodegib has not been studied.33 In pharmacokinetic analyses, weight (range, 41–140 kg), age (range, 26–89 years), creatinine clearance (range, 30–80 mL/minute), and the patient’s sex did not have clinically meaningful effects on the systemic exposure of vismodegib.33
SAFETY PROFILE
Boxed Warning
The labeling for vismodegib includes a boxed warning regarding the potential for embryofetal death or severe birth defects. Both male and female patients must be advised of this risk. In addition, before initiating treatment with vismodegib, physicians must verify a female patient’s pregnancy status and must advise female patients of the need for contraception. Male patients must be informed of the potential risk of exposing their partners to vismodegib through semen.33
Warnings and Precautions
As noted previously, vismodegib can cause fetal harm when administered to pregnant women, based on its mechanism of action. Vismodegib is teratogenic, embryotoxic, and fetotoxic in rats at maternal exposures lower than human exposures at the recommended dose of 150 mg/day. In rats, malformations included craniofacial anomalies, an open perineum, and absent or fused digits. Fetal retardations and variations were also noted.33
Patients should contact their health care provider immediately if pregnancy is suspected. Female and male patients of reproductive age should be counseled regarding pregnancy prevention and planning.
If vismodegib is used during pregnancy or if a female patient becomes pregnant while taking vismodegib, the patient should be informed of the potential hazard to the fetus.33
Patients should not donate blood or blood products during treatment and for at least 7 months after they receive the last dose of vismodegib.33
Common Adverse Reactions
Four open-label clinical trials were conducted to evaluate vismodegib monotherapy at doses of 150 mg or greater once daily in 138 patients with advanced BCC. Patients’ median age was 61 years (range, 21–101 years). All patients were Caucasian (including Hispanic individuals), and most (64%) were men.
The median duration of treatment was approximately 10 months (range, 0.7–36 months). A total of 111 patients were treated with vismodegib for 6 months or longer.33
Common adverse reactions (all grades) included muscle spasms (71.7%), alopecia (63.8%), dysgeusia (55.1%), and weight loss (44.9%) (Table 2).33 The most common serious adverse reactions (grade 3 or 4) included weight loss (7.2%), fatigue (5.8%), muscle spasms (3.6%), and decreased appetite (2.2%) (see Table 2).33
Table 2.
Adverse Reactions Occurring With Vismodegib in 10% of Patients or More With Advanced Basal Cell Carcinoma
| MedDRA Preferred Term | All Patients (N = 138) | ||
|---|---|---|---|
|
| |||
| All Grades (%) | Grade 3 (%) | Grade 4 (%) | |
|
| |||
| Gastrointestinal disorders | |||
| • Nausea | 30.4 | 0.7 | – |
| • Diarrhea | 29.0 | 0.7 | – |
| • Constipation | 21.0 | – | – |
| • Vomiting | 13.8 | – | – |
|
| |||
| General disorders and administration-site conditions | |||
| • Fatigue | 39.9 | 5.1 | 0.7 |
|
| |||
| Investigations | |||
| • Weight loss | 44.9 | 7.2 | – |
|
| |||
| Metabolism and nutrition disorders | |||
| • Decreased appetite | 25.4 | 2.2 | – |
|
| |||
| Musculoskeletal and connective tissue disorders | |||
| • Muscle spasms | 71.7 | 3.6 | – |
| • Arthralgias | 15.9 | 0.7 | – |
|
| |||
| Nervous system disorders | |||
| • Dysgeusia | 55.1 | – | – |
| • Ageusia | 10.9 | – | – |
|
| |||
| Skin and subcutaneous tissue disorders | |||
| • Alopecia | 63.8 | – | – |
MedDRA = Medical Dictionary for Regulatory Activities.
Adapted from Erivedge (vismodegib) prescribing information.33
In clinical trials, amenorrhea developed in three of 10 premenopausal women during vismodegib treatment.33
Serious (grade 3) treatment-emergent laboratory abnormalities included hyponatremia in six patients (4%), azotemia in three patients (2%), and hypokalemia in two patients (1%).33
Safety
Vismodegib is a Pregnancy Category D drug, and it can cause fetal harm when administered to a pregnant woman, based on its mechanism of action. It is teratogenic in rats at doses corresponding to 20% of the exposure at the recommended human dose.33
It is not known whether vismodegib is excreted in human breast milk. Because of the potential for serious adverse reactions in nursing infants from exposure to vismodegib, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.33
The safety and efficacy of vismodegib have not been established in pediatric patients or in patients with hepatic or renal impairment.33 Clinical studies of vismodegib did not include sufficient numbers of patients 65 years of age or older to determine whether they respond differently from younger patients.33
DRUG INTERACTIONS
Effects of Other Drugs on Vismodegib
The metabolism of vismodegib involves multiple pathways (oxidation, glucuronidation, and pyridine ring cleavage). Vismodegib is excreted predominantly as an unchanged drug, and several minor metabolites are produced by multiple CYP enzymes.33
Although vismodegib is a substrate of CYP2C9 and CYP3A4 in vitro, CYP inhibition is not expected to affect systemic exposure, because similar steady-state plasma concentrations of vismodegib were observed in patients concomitantly treated with CYP3A4 inducers, such as carbamazepine (e.g., Carbatrol, Shire), modafinil (Provigil, Cephalon/Teva), and phenobarbital or CYP3A4 inhibitors such as erythromycin and fluconazole (Diflucan, Pfizer) in clinical trials.33
In vitro studies indicate that vismodegib is a substrate of the efflux transporter P-glycoprotein (P-gp). When vismodegib is given with a drug that inhibits P-gp, such as clarithromycin (Biaxin, Abbott), erythromycin, or azithromycin (Zithromax, Pfizer), the systemic exposure of vismodegib and the incidence of adverse events with vismodegib may be increased.33
Drugs that alter the pH of the upper gastrointestinal tract (e.g., proton pump inhibitors, histamine H2-receptor antagonists, and antacids) may alter the solubility of vismodegib and may reduce its bioavailability. No clinical studies have evaluated the effect of gastric pH-altering drugs on the systemic exposure of vismodegib. When vismodegib is taken with such agents, increasing the vismodegib dose is not likely to compensate for the loss of exposure.
Coadministration of vismodegib with a proton pump inhibitor, an H2-receptor antagonist, or an antacid may reduce the systemic exposure of vismodegib. The effect on the efficacy of vismodegib in this situation is unknown.33
Effects of Vismodegib on Other Drugs
Results of a drug–drug interaction study in cancer patients showed that the systemic exposure of rosiglitazone (Avandia, GlaxoSmithKline), a CYP2C8 substrate, or of an oral contraceptive (ethinyl estradiol or norethindrone) was not altered when either drug was administered with vismodegib.33,48
In vitro studies indicate that vismodegib inhibits the hepatic enzymes CYP2C8, CYP2C9, and CYP2C19 and the drug efflux transporter Bcrp. Vismodegib does not induce CYP1A2, CYP2B6, or CYP3A4/5 in human hepatocytes.33
CLINICAL EFFICACY
A phase 1 study was conducted to evaluate vismodegib in patients with solid tumors that were refractory to current therapies or for which no standard treatment existed.39,49 The malignancies included BCC, pancreatic cancer, medulloblastoma, and 17 other types of cancer.
This was the first clinical trial that investigated the efficacy and safety of oral vismodegib, given at escalating doses (150, 270, and 540 mg/day), in patients with advanced solid malignancies. Of the 68 patients in this study, 33 had advanced BCC. Seventeen of these patients received vismodegib 150 mg/day; 15 patients received 270 mg/day; and one patient received 540 mg/day, for a median period of 9.8 months.
Of the 33 patients with BCC, 18 (54.5%) demonstrated an objective response to vismodegib: seven according to imaging assessments, and 11 on physical examination (one patient was rated on both). Two patients (6.0%) had a complete response, and 16 (48.5%) had a partial response.39
Eight grade 3 adverse events that were considered to be related to vismodegib occurred in six patients, including four patients with fatigue, two with hyponatremia, one with muscle spasm, and one with atrial fibrillation. One patient withdrew from the study because of adverse events.39
The results of this study established the recommended phase 2 dosage of vismodegib at 150 mg/day, because pharmacokinetic analyses indicated that higher doses did not result in higher steady-state plasma concentrations of vismodegib and because no dose-limiting toxic effects were observed.49
The investigators found evidence of Hh signaling in tumors that responded to vismodegib treatment as well as evidence of down-modulation of the GLI1 oncogene in non-involved skin, indicating inhibition of the Hh pathway.39,49
Subsequent FDA approval of vismodegib was based on results from a pivotal phase 2 single-arm, open-label, two-cohort clinical study (ERIVANCE BCC) that included 104 patients with locally advanced (n = 71) or metastatic (n = 33) BCC.32,33,50
In this pivotal trial, patients with locally advanced BCC had to have lesions that had recurred after radiotherapy unless radiotherapy was contraindicated or inappropriate; the lesions were unresectable; or surgical resection would result in substantial deformity. Patients received vismodegib 150 mg once daily until disease progression or unacceptable toxicity occurred.33,50
The primary efficacy measure was the objective response rate (ORR). In the cohort with metastatic BCC, tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0. In the cohort with locally advanced BCC, the evaluation of tumor response included measurement of externally assessable lesions (including scars), assessment of ulceration in photographs, radiographic assessment of target lesions (if appropriate), and tumor biopsy.33,50
An objective response in locally advanced BCC required at least one of the following criteria and the absence of any criterion for disease progression:33,50
a reduction of 30% of more in lesion size (the sum of the longest diameter [SLD]) from baseline in target lesions on radiography
a reduction of 30% or more in SLD from baseline in the externally visible dimension of target lesions
complete resolution of ulceration in all target lesions
A complete response was defined as an objective response with no residual BCC on sampling tumor biopsy. Disease progression was defined as any of the following:33,50
an increase of 20% or more in SLD from nadir in target lesions, determined either by radiography or by an increase in visible dimensions
new ulceration of target lesions persisting without evidence of healing for at least 2 weeks
new lesions detected by radiography or physical examination
progression of non-target lesions, as defined by RECIST parameters
Of the 104 patients enrolled in this study, 96 were evaluable for ORR. Nevoid basal cell carcinoma (Gorlin syndrome), a rare, inherited genetic disorder, had been diagnosed in 21% of the patients. The median age of the evaluable population was 62 years (46% were at least 65 years of age). Most of the patients (61%) were men, and all were Caucasian.
In the cohort with locally advanced BCC (n = 63), 94% of the patients had received prior therapy, including surgery (89%), radiotherapy (27%), and systemic or topical therapies (11%). In the cohort with metastatic disease (n = 33), 97% of the patients had received prior treatment, including surgery (97%), radiotherapy (58%), and systemic therapies (30%).33,50
The median duration of treatment was 10.2 months.33,50 Key results are presented in Table 3. Vismodegib shrank lesions (ORR) in 43% of patients (27/63) with locally advanced BCC and in 30% of patients (10/33) with metastatic disease.33,50
Table 3.
Key Efficacy Results in Evaluable Patients* Treated With Vismodegib
| Metastatic BCC (n = 33) | Locally Advanced BCC (n = 63) | |
|---|---|---|
|
| ||
| IRF-confirmed ORR (%) | 30.3 | 42.9 |
| • 95% confidence interval | (15.6–48.2) | (30.5–56.0) |
| • Complete response (%)† | 0 | 20.6 |
| • Partial response (%) | 30.3 | 22.2 |
|
| ||
| Median duration of response (months) | 7.6 | 7.6 |
| • 95% confidence interval | (5.6–NE) | (5.7–9.7) |
Evaluable patients were those who received at least one dose of vismodegib with an independent pathologist-confirmed diagnosis of BCC.
For patients with locally advanced BCC, a complete response was defined as an objective response with no residual BCC on sampling tumor biopsy.
BCC = basal cell carcinoma; IRF = independent review facility; NE = not estimable; ORR = objective response rate.
Adapted from Erivedge (vismodegib) prescribing information.33
Genentech is continuing to investigate vismodegib in several ongoing studies of BCC (Table 4).
Table 4.
Ongoing Clinical Trials of Vismodegib in Basal Cell Carcinoma
| Study Title | Primary Endpoint | Enrollment (No.) | Estimated Completion Date |
|---|---|---|---|
| Phase 2 Multicenter, Single-Arm, Two-Cohort Trial Evaluating the Efficacy and Safety of GDC-0449 in Patients With Advanced Basal Cell Carcinoma | Overall response rate | 15 | December 2012 |
| Phase 2 Multicenter, Open-Label, Two-Cohort Trial Evaluating the Efficacy and Safety of GDC-0449 in Operable Basal Cell Carcinoma (BCC) | Complete clearance rate (CCR) and durable complete clearance rate (DCCR) of target nodular BCC lesions at time of excision | 49 | December 2012 |
| Randomized Phase 2 Multicenter Trial Evaluating the Efficacy and Safety of a Systemic Hedgehog Pathway Antagonist (GDC-0449) in Patients With Basal Cell Nevus Syndrome (BCNS) | Reduction of new surgically eligible BCCs in BCNS patients during months 3–18 of ingestion of vismodegib 150 mg/day | 41 | March 2013 |
| Single-Arm, Open-label, Phase 2 Multicenter Study to Assess the Safety of Vismodegib (GDC-0449) in Patients With Locally Advanced or Metastatic Basal Cell Carcinoma (BCC) | Incidence of adverse events | 150 | September 2013 |
Data from ClinicalTrials.gov.
DOSAGE AND ADMINISTRATION
The recommended dose of vismodegib is one capsule (150 mg) once daily until disease progression or unacceptable toxicity occurs.33 Vismodegib may be taken with or without food. The capsules should be swallowed whole.33
DRUG DEVELOPMENT AND APPROVAL
Vismodegib was discovered by Genen-tech, a South San Francisco–based member of the Roche Group, and was jointly validated by Genentech and Curis, Inc., in a series of preclinical studies. Roche proceeded to develop vismodegib under a collaboration agreement with Curis. Through this collaboration, Genentech, Roche, and Chugai Pharmaceuticals, respectively, are responsible for the clinical development and commercialization of vismodegib inside the U.S., outside the U.S. (excluding Japan and Korea), and in Japan. Under the agreement with Roche, Curis is eligible to receive cash payments upon the successful achievement of specified clinical development and regulatory approval milestones as well as royalties upon commercialization of vismodegib.51
Genentech filed an Investigational New Drug (IND) application with the FDA in September 2006. In May 2011, a pre-submission meeting was held between representatives of the FDA and Genentech to reach agreement on the content and format of a New Drug Application (NDA) for registration of vismodegib in advanced BCC. In September 2011, Genentech submitted the NDA for vismodegib for use in adults with advanced BCC for whom surgery was inappropriate, based on results from the pivotal phase 2 ERIVANCE BCC study.52
The FDA granted Genentech’s application priority review status in November and set the action date for March 8, 2012.53,54 On January 30, 2012—6 weeks ahead of the action date—the FDA approved oral vismodegib for the treatment of adults with metastatic BCC, or with locally advanced BCC that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation.32,33 The FDA approval triggered a $10 million payment to Curis, Inc.55
COST AND AVAILABILITY
Genentech has set the price of vismodegib at $7,500 for a month’s supply of once-daily capsules––or approximately $250 per capsule. Although the duration of treatment with vismodegib can vary, patients are expected to be using the therapy for about 10 months, for an average cost of $75,000 for a total course of treatment.56 Financial analysts have predicted that sales of vismodegib, including sales in Europe (where the drug is expected to launch in 2013), will reach $401 million in 2015 and will peak at $533 million in 2022.55 Vismodegib is available only through specialty pharmacies.51,57
CONCLUSION
The Hh pathway inhibitor vismodegib (Erivedge), the first medication for advanced BCC, provides an important new therapy for this disfiguring and potentially life-threatening disease and offers new possibilities for long-term clinical management. The Roche Group is pursuing further development of vismodegib for BCC and other cancers.
Footnotes
Disclosure: The author reports that he has no financial, commercial, or industrial relationships to disclose in regard to this article.
REFERENCES
- 1.Jacob A. Observations respecting an ulcer of peculiar character, which attacks the eyelids and other parts of the face. Dublin Hosp Rep Commun Med Surg. 1827;4:232–239. [Google Scholar]
- 2.Miller SJ. Biology of basal cell carcinoma (part I) J Am Acad Dermatol. 1991;24:1–13. doi: 10.1016/0190-9622(91)70001-i. [DOI] [PubMed] [Google Scholar]
- 3.Miller SJ. Biology of basal cell carcinoma (part II) J Am Acad Dermatol. 1991;24:161–175. doi: 10.1016/0190-9622(91)70022-t. [DOI] [PubMed] [Google Scholar]
- 4.Miller SJ. Aetiology and pathogenesis of basal cell carcinoma. Clin Dermatol. 1995;13:527–536. doi: 10.1016/0738-081x(95)00062-k. [DOI] [PubMed] [Google Scholar]
- 5.Lacour JP. Carcinogenesis of basal cell carcinomas: Genetics and molecular mechanisms. Br J Dermatol. 2002;146(Suppl 61):17–19. doi: 10.1046/j.1365-2133.146.s61.5.x. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society Skin cancer: Basal and squamous cell. Jan 31, 2012. Available at: http://documents.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Accessed February 22, 2012.
- 7.Skin Cancer Foundation Skin cancer facts. Available at: www.skincancer.org/skin-cancer-information/skin-cancer-facts. Accessed February 27, 2012.
- 8.Lewis WH, editor. Gray’s Anatomy of the Human Body. 20th ed. Philadelphia: Lea & Febiger; 1918. [Google Scholar]
- 9.McGrath JA, Eady RAJ, Pope FM. Anatomy and organization of human skin. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook’s Textbook of Dermatology. 7th ed. Oxford, UK: Blackwell Science Ltd; 2004. pp. 3.1–3.84. [Google Scholar]
- 10.Williams AC. Transdermal and Topical Drug Delivery: From Theory to Clinical Practice. London, U.K.: Pharmaceutical Press; 2004. Structure and function of skin; pp. 1–25. [Google Scholar]
- 11.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology. (NCCN Guidelines): Basal cell and squamous cell skin cancers, Version 1.2012. Available at: www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Accessed February 27, 2012.
- 12.Neville JA, Welch E, Leffell DJ. Management of nonmelanoma skin cancer in 2007. Nat Clin Pract Oncol. 2007;8:462–469. doi: 10.1038/ncponc0883. [DOI] [PubMed] [Google Scholar]
- 13.Walling HW, Fosko SW, Geraminejad PA, et al. Aggressive basal cell carcinoma: Presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389–402. doi: 10.1023/B:CANC.0000031775.04618.30. [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Dermatology Skin cancer: Who gets and causes. 2012. Available at: www.aad.org/skin-conditions/dermatology-a-to-z/skin-cancer/who-gets-causes. Accessed February 27, 2012.
- 15.Stulberg DL, Crandell B, Fawcett RS. Diagnosis and treatment of basal cell and squamous cell carcinoma. Am Fam Physician. 2004;70:1481–1488. [PubMed] [Google Scholar]
- 16.Lear JT, Smith AG. Basal cell carcinoma. Postgrad Med J. 1997;73:538–542. doi: 10.1136/pgmj.73.863.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262–2269. doi: 10.1056/NEJMra044151. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute, Surveillance Epidemiology and End Results (SEER) SEER stat fact sheets: Other non-epithelial skin. Available at: http://seer.cancer.gov/statfacts/html/othskin.html. Accessed February 28, 2012.
- 19.Wong CSM, Strange RC, Lear JT. Basal cell carcinoma. BMJ. 2003;327:794–798. doi: 10.1136/bmj.327.7418.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: Incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 21.Holmes SA, Malinovszky K, Roberts DL. Changing trends in non-melanoma skin cancer in South Wales, 1988–1998. Br J Dermatol. 2000;143:1224–1229. doi: 10.1046/j.1365-2133.2000.03892.x. [DOI] [PubMed] [Google Scholar]
- 22.Marks R, Staples M, Giles G. Trends in non-melanocytic skin cancer treated in Australia: The second national survey. Int J Cancer. 1993;53:585–590. doi: 10.1002/ijc.2910530410. [DOI] [PubMed] [Google Scholar]
- 23.de Vries E, Louwman M, Bastiaens M, et al. Rapid and continuous increases in incidence rates of basal cell carcinoma in the southeast Netherlands since 1973. J Invest Dermatol. 2004;123:634–638. doi: 10.1111/j.0022-202X.2004.23306.x. [DOI] [PubMed] [Google Scholar]
- 24.Strayer SM, Reynolds PL. Diagnosing skin malignancy: Assessment of predictive clinical criteria and risk factors. J Fam Pract. 2003;52:210–218. [PubMed] [Google Scholar]
- 25.Crowson AN. Basal cell carcinoma: Biology, morphology, and clinical implications. Modern Pathol. 2006;19:S127–S149. doi: 10.1038/modpathol.3800512. [DOI] [PubMed] [Google Scholar]
- 26.Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004: A joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490–500. doi: 10.1016/j.jaad.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Institute Basal cell carcinoma of the skin: Treatment. Jul 29, 2011. Available at: www.cancer.gov/cancertopics/pdq/treatment/skin/HealthProfessional. Accessed February 27, 2012.
- 28.Telfer NR, Colver GB, Morton CA. Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35–48. doi: 10.1111/j.1365-2133.2008.08666.x. [DOI] [PubMed] [Google Scholar]
- 29.Samarasinghe V, Madan V, Lear JT. Focus on basal cell carcinoma. J Skin Cancer. 2011;2011:328615. doi: 10.1155/2011/328615. (online). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadhera A, Fazio M, Bricca G, Stanton O. Metastatic basal cell carcinoma: A case report and literature review. How accurate is our incidence data? Dermatol Online J. 2006;12(5):7. [PubMed] [Google Scholar]
- 31.Raszewski RL, Guyuron B. Long-term survival following nodal metastases from basal cell carcinoma. Ann Plast Surg. 1990;24:170–175. doi: 10.1097/00000637-199002000-00012. [DOI] [PubMed] [Google Scholar]
- 32.FDA approves new treatment for most common type of skin cancer. Jan 30, 2012. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnounce-ments/ucm289545.htm. Accessed February 28, 2012.
- 33.Erivedg (vismodegib), prescribing information. South San Francisco, Calif.: Genentech; Jan, 2012. Available at: www.gene.com/gene/products/information/erivedge/pdf/erivedge_prescribing.pdf. Accessed February 22, 2012. [Google Scholar]
- 34.Prous Science Molecule of the month: Vismodegib. Feb 16, 2012. Available at: www.prous.com/molecules/default.asp?ID=210. Accessed February 28, 2012.
- 35.Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- 36.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic Hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 37.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of Hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 39.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the Hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 40.Daya-Grosjean L, Couvé-Privat S. Sonic hedgehog signaling in basal cell carcinomas. Cancer Lett. 2005;225:181–192. doi: 10.1016/j.canlet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with Hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 44.Xie J, Murone M, Luoh SM, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 45.Epstein EH. Basal cell carcinoma: Attack of the Hedgehog. Nat Rev Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graham RA, Hop CECA, Lum BL, et al. Single and multiple-dose IV and oral pharmacokinetics of the Hedgehog pathway inhibitor vismodegib (GDC-0449) during a phase 1 study in healthy female subjects. Poster, American Society for Clinical Pharmacology and Therapeutics (ASCPT) Annual Meeting; Dallas. March 2–5, 2011; Available at: www.xceleron.com. Accessed March 1, 2012. [Google Scholar]
- 47.Graham RA, Lum BL, Cheeti S, et al. Pharmacokinetics of Hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors: The role of alpha-1-acid glycoprotein binding. Clin Cancer Res. 2011;17:2512–2520. doi: 10.1158/1078-0432.CCR-10-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LoRusso PM, Piha-Paul SA, Colevas AD, et al. Pharmacokinetic assessment of drug–drug interaction potential when rosiglitazone or combined oral contraceptive is coadministered with vismodegib in patients with locally advanced or meta-static solid tumors (Abstract No. B188) Mol Cancer Ther. 2011;10(Suppl 1) [Google Scholar]
- 49.LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.FDA approves Erivedge (vismodegib) capsule, the first medicine for adults with advanced basal cell carcinoma. Roche; Jan 30, 2012. Available at: www.roche.com/media/media_releases/med-cor-2012-01-30.htm. Accessed February 29, 2012. [Google Scholar]
- 52.Vismodegib Briefing Package: Pediatric ODAC Committee Meeting. South San Francisco, Calif.: Genentech; Nov 1, 2011. Available at: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM277585.pdf. Accessed February 29, 2012. [Google Scholar]
- 53.FDA accepts Roche’s New Drug Application for vismodegib in advanced form of skin cancer. Roche Group; Nov 9, 2011. Available at: www.roche.com/media/media_releases/medcor-2011-11-09.htm. Accessed March 5, 2011. [Google Scholar]
- 54.FDA grants priority review for vismodegib for advanced skin cancer. Oncology Times. 2011;33(23):5. [Google Scholar]
- 55.Grogan K. FDA approves Roche’s skin cancer drug Erivedge. Pharma Times Online. 2012 Jan 31; Available at: www.pharmatimes.com/article/12-01-31/FDA_approves_Roche_s_skin_cancer_drug_Erivedge.aspx. Accessed February 29, 2012. [Google Scholar]
- 56.Corbett Dooren J, Winslow R. New type of cancer drug gets approval. The Wall Street Journal. Jan 31, 2012. (online). Available at: http://online.wsj.com. Accessed February 29, 2012.
- 57.Access, coverage, and patient assistance for Erivedge (vismodegib) capsule. Genentech; 2012. Available at: www.erivedge.com. Accessed March 5, 2012. [Google Scholar]