Summary
Nocturnal enuresis in children and nocturia in the elderly are two highly prevalent clinical conditions characterized by a mismatch between urine production rate in the kidneys and storage in the urinary bladder during the sleep phase. Here we demonstrate, using a novel method for automated recording of mouse micturition, that connexin43 (Cx43), a bladder gap junction protein, is a negative regulator of functional bladder capacity. Bladder Cx43 levels and functional capacity show circadian oscillations in wild-type mice, but such rhythms are completely lost in Cry-null mice having a dysfunctional biological clock. Bladder muscle cells have an internal clock, and show oscillations of Cx43 and gap junction function. A clock regulator, Rev-erbα, upregulates Cx43 transcription as a co-factor of Sp1 using Sp1 cis-elements of the promoter. Therefore, circadianoscillation of Cx43 is associated with the biological clock and contributes to diurnal changes in bladder capacity, which avoids disturbance of sleep by micturition.
Nocturnal enuresis is the involuntary loss of urine during sleep in childhood, and nocturia is the undesired waking at night for micturition later in life. Studies show that at least 10% of school children have nocturnal enuresis1,2, and 60–90% of the elderly over 60 years suffer from nocturia3,4. These conditions are detrimental to the quality of life by interfering with patients’ self-esteem or sleeping habits, and they are the major diseases found in urology clinics. Nocturnal enuresis and nocturia are characterized by a mismatch between urine production rate in the kidneys and storage in the urinary bladder5,6. During the sound sleep of a healthy person, a smaller volume of urine is produced than that during the daytime, and more urine is stored during the sleep phase than during the active phase7–9. Although it is unknown how such temporal variation is generated, these phenomena could be related to biological rhythms because behaviour, physiology and metabolism in mammals are subject to a well-controlled daily rhythm, generated by an internal self-sustained molecular oscillator referred to as the circadian clock10–13.
Circadian oscillations are driven by a transcription-translation feedback loop consisting of PER and CRY as negative components and CLOCK and BMAL1 as positive components. Rhythmic oscillations of this core loop are followed by the clock-associated oscillations of Dbp and Rev-erbα, whose products regulate oscillations of a number of clock controlled genes regulating cell- or organ-specific physiology through D-box and RORE sites, respectively14–16. These molecular oscillators exist in most body cells and organs13, but little is known about the role of the clock in urinary bladder function.
Micturition occurs by the contraction of smooth muscles of the urinary bladder upon a sensation of fullness, which is precisely controlled by regulation of the central and peripheral nerves17,18. We and others have reported that an increase in connexin43 (Cx43), a gap junction protein in the urinary bladder, enhances intercellular electrical and chemical transmission and sensitizes the response of bladder muscles to cholinergic neural stimuli. This results in a decrease in functional bladder capacity and an increase in micturition frequency in rats19–22. These phenomena mimic some aspects of an overactive bladder, a human pathological condition characterized by urinary urgency and increased micturition frequency23; however, the involvement of Cx43 in regulating normal bladder function remains unclear.
To further elucidate the role of Cx43 in bladder function, we investigated the effect of genetic ablation of Cx43 on micturition behaviour in mice and the implication of Cx43 for circadian micturition rhythm. The circadian micturition rhythm in free-moving mice still remains elusive, since the urine volume voided per micturition (UVVM) in mice is so minute (sometimes <50 μl)24,25. To overcome this problem, we designed a novel system, called the automated voided stain on paper (aVSOP) method, which can accurately record micturition of mice for several days. Using this system, we demonstrated the role of Cx43 and the circadian clock as regulators of functional bladder capacity in mice. We also showed that bladder muscle has internal rhythms of the clock and Cx43, which are correlated with oscillation in gap junction function. Further, we propose a novel paradigm that links the circadian clock with Cx43, in which Rev-erbα protein transactivates the Cx43 promoter through interaction with Sp1.
RESULTS
Cx43 is involved in control of functional bladder capacity
We began our study by developing an automated machine called aVSOP (Fig. 1a). The conversion of UVVM by mice from a drop area on filter paper has been reported to be an accurate method24,26, and this principle was applied to the automated system by using a laminated filter paper pre-treated to turn the edge of urine stains deep purple (Supplementary Fig. S1a). This modification enabled us to record the micturition of free-moving mice fed ad libitum for several successive days, for a UVVM as little as 10 μl (Supplementary Fig. S1b).
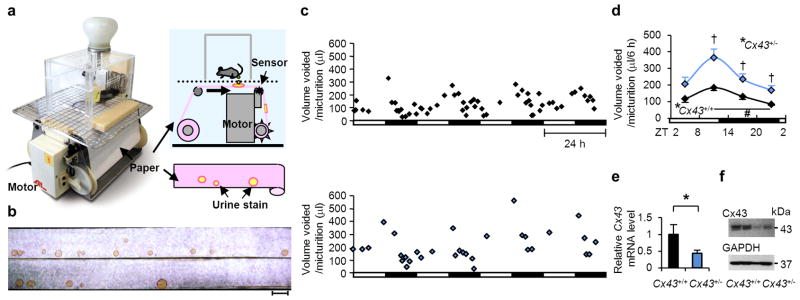
Figure 1. aVSOP reveals an association between functional bladder capacity andCx43.
(a) A photograph and diagram showing the aVSOP method. Each stain was traced, scanned and quantified by Image J 1.42 software. (b–d) Female Cx43+/− mice had larger functional bladder capacity than sex-matched Cx43+/+ littermates. (b) A photograph of urine spots on paper made by Cx43+/+ (top) and Cx43+/− (bottom) mice. The scale bar indicates 10 cm, corresponding to 1 hour. (c) Representative charts of UVVM of Cx43+/+ (top) and Cx43+/− (bottom) mice under light/dark conditions for 4 days. UVVM, urine volume voided per micturition. (d) UVVM per 6 hours in Cx43+/+ and Cx43+/− mice had diurnal variation (F(3[degrees of freedom (DF) for the time factor],9[error DF])=12.3 and 10.9, respectively; *P < 0.005 by one-way repeated measures ANOVA; #P < 0.05 in the late light [sleep] phase vs. late dark [active] phase, followed by Bonferroni’s post hoc test). Maximal correlations from a cosine curve (MaxCorr) of Cx43+/+ and Cx43+/− mice were 0.949 and 0.989, respectively. ZT, zeitgeber time: light-on at ZT0 and off at ZT12. UVVM was significantly different between Cx43+/+ and Cx43+/− mice (F(1[DF for the strain factor],6[error DF])=11.2, P < 0.05 by two-way repeated measures ANOVA; †P < 0.05 vs. Cx43+/+ by Bonferroni’s post hoc test; n=4 for each group, with a total of 296 micturitions). Error bars represent s.e.m. (e) Relative Cx43 mRNA levels of the urinary bladder in Cx43+/− and Cx43+/+ mice used in the micturition analysis by real-time RT-PCR. Error bars represent s.d., n=4 for each mice. The value of Cx43+/+ was set as 1. *P < 0.05 by Student’s t-test. (f) Cx43 protein expression of the urinary bladder in Cx43+/+ and Cx43+/− mice.
To assess the effect of genetic Cx43 ablation on micturition, we compared heterozygous Cx43+/− and wild-type (WT) Cx43+/+ littermate mice by the aVSOP method (Fig. 1b–d) because homozygous Cx43−/− mice die shortly after delivery27. Cx43 mRNA and protein levels in the urinary bladder of Cx43+/− mice were decreased compared with those in Cx43+/+ mice (Fig. 1e,f). In both genotypes, UVVM showed temporal variation under 12-hour light and 12-hour dark (LD) conditions (Fig. 1b–d), consistent with the nocturnal characteristics of the mice28. UVVM was higher in Cx43+/−mice than that in Cx43+/+ mice (Fig. 1b–d), while total urine volume was not significantly different (Supplementary Fig. S1c).
These data demonstrate that the expression level of Cx43 is crucial for determining the functional capacity of the urinary bladder. This finding and the temporal variation in the micturition behaviour of the mice led us to focus on its association with Cx43 expression in the bladder and the circadian clock.
Association of bladder clock with bladder capacity and Cx43
It has been reported that micturition shows a diurnal change in rodents as well as in humans8,22,28,29, although the day-night change is inverted because of the difference in diurnal versus nocturnal habits. However, it is unknown whether these changes are induced by an endogenous oscillator or only by a reflection of external light-dark changes. To address this in mice, we first examined micturition of WT mice under both LD and constant dark (DD) conditions. A distinct rhythm of UVVM was recorded, peaking at zeitgeber time (ZT) or circadian time (CT) 8–12 (Fig. 2a,b). Chi-square analysis showed a circadian periodicity under both LD and DD conditions (Supplementary Fig. S2a). No significant difference between LD and DD was found in the circadian amplitude (0.042 cycles per hour) quantified as relative power calculated by Fourier transform30 (Supplementary Fig. S2b). This demonstrates that the mouse could be a model animal for micturition rhythmicity, and that the rhythm could be related to an internal biological rhythm that is also operational in the absence of environmental change in light/dark cycles. The involvement of the circadian clock in micturition behaviour was tested in mice with a dysfunctional circadian clock by deletion of Cryptochrome-1 (Cry1) and Cryptochrome-2 (Cry2) (Cry-null mice); accordingly, these mice have completely arrhythmic behaviour and metabolism11,31. We placed our aVSOP system in a box with an infrared activity sensor and measured the micturition and locomotor activity simultaneously. Similar to behavioural locomotor rhythms (Supplementary Fig. S3a), circadian characteristics of UVVM observed in WT mice were abolished in Cry-null mice (Fig. 2c,d) analysed by Chi-square and Fourier transform (Supplementary Fig. S3b) as well as those of total urine volume and frequency (Supplementary Fig. S3c,d). These findings further support the notion that the circadian clock regulates UVVM.
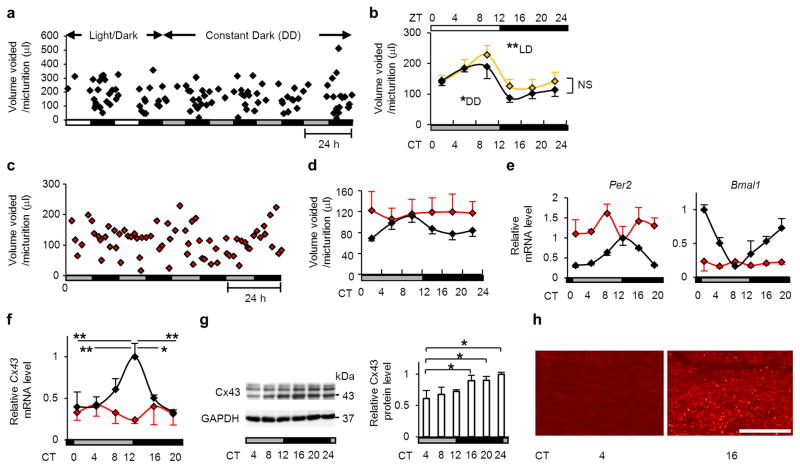
Figure 2. Rhythmicity of micturition, clock genes and Cx43 expression in wild-type mice is disturbed in Cry-null mice.
(a) A representative chart of UVVM of WT C57BL/6 mice under light/dark (LD) conditions followed by constant dark (DD) conditions. (b) Temporal UVVM every 4 hours in WT mice (n=5), for 8 days under LD conditions (940 micturitions) and 5 days under DD conditions (556 micturitions). Diurnal variation of UVVM in LD conditions (F(5[degrees of freedom (DF) for the time factor],20[error DF])=17.28, **P < 0.005 by one-way repeated measures ANOVA) was also observed in DD conditions (F(5,20)=8.23, *P < 0.05), with no significant difference among times in LD vs. DD by two-way repeated measures ANOVA. (c, d) Loss of circadian rhythm of UVVM in Cry-null mice under DD conditions. Age-matched female WT, 1493 micturitions; Cry-null, 1009 micturitions, n=5 each. (c) A representative chart of UVVM of Cry-null mice. (d) Temporal UVVM every 4 hours in Cry-null (red-diamond) and WT (black-diamond) mice. Diurnal variation detected in WT mice (F(5,20)=8.21, P < 0.05 by one-way repeated measures ANOVA) was not observed in Cry-null mice. (e) Temporal Per2, Bmal1 and (f) Cx43 mRNA accumulation in the bladder in WT and Cry-null mice (n=3 for each time point). MaxCorrs of Per2, Bmal1 and Cx43 were (0.96, 0.93 and 0.85) in WT and (0.19, 0.42 and 0.38) in Cry-null mice, respectively. There was no significant difference in temporal Cx43 mRNA levels in Cry-null mice by one-way ANOVA. (g) Immunoblots showing temporal changes in protein levels of Cx43 in WT-mouse bladder (three independent samples for each time point). (h) Immunostaining of the muscle layer in mouse urinary bladder showing a difference in immunoreactivity with a decrease in Cx43 at CT4 compared to CT16. Representative photographs of three replicated experiments with similar results are shown. Bar, 50 μm. *P < 0.05 and **P < 0.01 by one-way ANOVA with Tukey’s post hoc test in f and g. Error bars represent s.e.m. in b and d, and s.d. in e–g. For the relative levels, the maximal values of WT were set as 1 in e and f. F(x,y), x=DF for the time factor; y=error DF in b and c. CT, circadian time.
We next examined whether the molecular machinery of the circadian clock is present in the urinary bladder. We found that the core oscillating machinery was present in the urinary bladder, as core clock genes including Per2 and Bmal1(Fig. 2e), Per1, Cry1, Clock and Dbp (Supplementary Fig. S4) showed characteristic circadian expression profiles by real-time RT-PCR of circadian sampling of the urinary bladder every 4 hours (6 time points of the day) in WT mice. Dysfunction of the bladder circadian clock in Cry-null mice was demonstrated by a disturbed rhythm of Per2 and Bmal1 (Fig. 2e). We performed DNA microarray analysis to investigate the genes showing circadian rhythm in the urinary bladder more extensively. Besides the clock genes, there are thousands of oscillating genes in the bladder, as in other organs32,33. Notably, our target gene, Cx43 (also known as Gja1), was among the 184 genes with clear circadian rhythmicity (defined as greater than the max correlation of 0.85 from the cosine curve with a 1.5-fold amplitude of expression level34,35) (Supplementary Data 1).
Cx43 mRNA showed a clear circadian rhythm with a peak at CT12 and a trough at CT0 by real-time RT-PCR (Fig. 2f). Cx43 protein levels remained low during the sleep phase (CT4–12), began to elevate 4 hours after the peak of mRNA expression, and formed a plateau during the active phase (CT16–24) (Fig. 2g). Immunostaining of Cx43 in the muscle layer of the urinary bladder at CT4 and at CT16 also showed a clear difference in immunoreactivity (Fig. 2h). In rats, in which day-night difference of micturition behaviour has been described22,29, a similar correlation was observed between micturition rhythm (Fig. 3a), temporal variations of clock genes (Per2 and Bmal1) and Cx43 mRNA expressions in the urinary bladder (Fig. 3b), Cx43 protein levels (Fig. 3c) and Cx43 immunoreactivity (Fig. 3d).
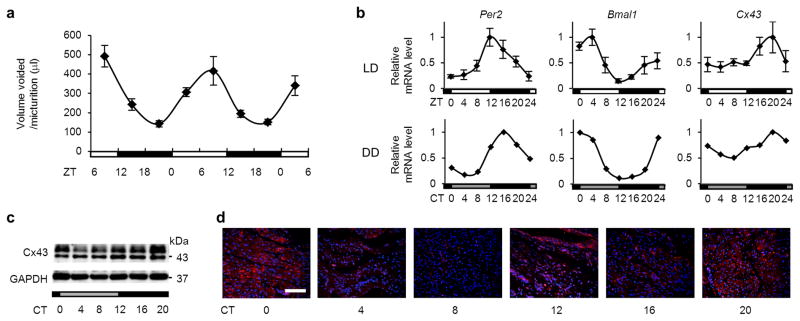
Figure 3. Cx43 and clock-gene expression rhythms in rats and their correlation with micturition rhythm.
(a) Patterns of UVVM in female Sprague-Dawley rats under LD conditions for 2 days (n=15, 1001 micturitions; F(2.7[degrees of freedom [DF] for the time factor],38.3[error DF])=11.9; *P < 0.005 by one-way repeated measures ANOVA with a Greenhouse-Geisser correction). (b) Temporal mRNA accumulation of Per2 Bmal1 and Cx43 in the rat bladder under LD and DD conditions (n=5 and n=2 for each time point, respectively). MaxCorrs were 0.87, 0.90 and 0.84 in LD conditions, respectively, and 0.98, 0.95 and 0.93 in DD conditions, respectively. (c) Temporal Cx43 protein accumulation in the rat bladder under DD conditions as shown by immunoblotting. (d) Immunostaining of Cx43 in the rat bladder under DD conditions (red, Cx43; blue, DAPI). The scale bar indicates 100 μm. Error bars represent s.e.m. in a and s.d. in b. For the relative expression, maximal values were set as 1 in b.
The circadian change of mRNA was reflected in the protein level, and the increase/decrease in Cx43 protein expression correlated with the decrease/increase of UVVM, respectively, in WT mice and rats. Similarly, Cry-null mice tended to show a constantly low level of Cx43 mRNA (Fig. 2f) with a high level of UVVM (Fig. 2d). This inverse expression can be accounted for by altered bladder sensitivity caused by a differential level of gap junction formation by Cx43 because the half-life of connexin proteins is up to 5 hours and expression largely follows transcript levels36,37. These findings suggest that the bladder circadian clock coordinates UVVM via circadian regulation of Cx43 gene expression.
Internal oscillations of bladder clock and Cx43 function
We investigated if the circadian clock oscillates in the bladder without systemic control. Therefore, we investigated its oscillation in ex vivo bladder in culture. We used mice carrying a PER2::LUC fusion protein, which has been engineered to produce bioluminescence when the clock gene Per2 is activated in the presence of luciferin38. The ex vivo slice culture of the bladder from Per2::luc mice demonstrated a robust oscillation of bioluminescence in the muscle layer of the bladder for at least four cycles (Fig. 4a and Supplementary Movie 1). The oscillation continued for approximately 2 months in the medium changed every 5 days. This oscillation was not observed in Per2::luc mice with the Clock-mutation (ClkΔ19/ClkΔ19) (Supplementary Fig. S5). These findings clearly demonstrate that a functional circadian clock exists in the smooth muscle of the urinary bladder.
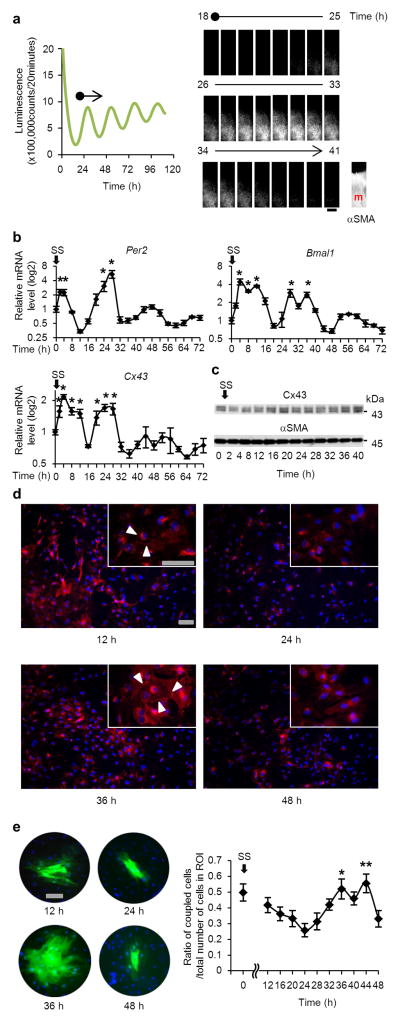
Figure 4. Oscillation of the circadian clock, Cx43 and gap-junction function in bladder muscle cells without systemic control.
(a) Oscillation of luminescence in bladder ex vivo slice culture obtained from mPer2Luciferase knock-in (Per2::luc) mice. The period of oscillation was 24.92 ± 0.56 (mean ± s.d.) (n=10). The muscle layer of the bladder is shown by alpha smooth muscle actin (αSMA) immunostaining. m, muscle. The scale bar indicates 100 μm. The oscillation of luminescence is also shown by a movie in Supplementary Movie 1. (b) Temporal variation of Per2, Bmal1 and Cx43 mRNA levels in serum-shocked rat bladder smooth muscle cells (BSMC). *P < 0.01 against the nadir of each genes’ mRNA levels (time 12 for Per2, time 48 for Bmal1 and time 64 for Cx43) by one-way ANOVA with Dunnett’s post hoc test (n=3–6). SS, serum shock. For relative levels, the values before serum shock were set as 1. (c) Immunoblots showing temporal changes in Cx43 protein levels with αSMA as a loading control in serum-shocked rat BSMC. (d) Immunostaining of Cx43 at times 12, 24, 36 and 48 hours in serum-shocked rat BSMC (red, Cx43; blue, DAPI). Arrow heads indicate typical plaques of gap junctions. Representative images of two replicate experiments with similar results are shown in c and d. (e) Oscillation of gap junction function evaluated by Lucifer yellow (LY) microinjection in serum-shocked rat BSMC. One representative photograph of each time point (green, LY; blue, Hoechst 33342) and overall quantification of the degree of dye-coupling (n=6–9, a total of 71 injections) is shown. *P < 0.05 and **P < 0.01 vs. time 24 hours by one-way ANOVA with Tukey-Kramer’s post hoc test. Similar significant differences were obtained in two independent experiments. Error bars represent s.e.m. Scale bars in d and e indicate 100 μm.
We then examined cultured bladder smooth muscles cells (BSMC) under serum shock, an in vitro model of genetic oscillation39. After serum shock in BSMC, autonomous oscillation of clock genes (Per2 and Bmal1) was observed (Fig. 4b). Concurrently, Cx43 levels also showed autonomous rhythmicity (Fig. 4b), which was followed by a change in protein levels (Fig. 4c,d), as observed in the bladder in vivo (Figs. 2f–h and 3b–d). This change occurred in parallel with a change in cell-cell communication rate as shown by a dye-transfer experiment with lucifer yellow microinjected intracellularly (Fig. 4e). At 24 h after the initiation of rhythm when Cx43 protein levels were close to their nadir, the amount of dye-transferred cells was at its minimum. However, at 36 h when Cx43 protein levels were close to their peak, the amount of dye-transferred cells was considerably increased (more than 2-fold compared with the minimum level at 24 h). These findings clearly demonstrate that bladder muscle cells have an internal rhythm generating system, which elicits oscillation in gap junction function.
Activation of Cx43 promoter by a clock component Rev-erbα
We next investigated the molecular mechanism of Cx43 regulation by the clock. We searched for E-box, D-box and RORE sequences in the 5’ prime region of Cx43, because clock genes are known to regulate clock-controlled genes by binding to these sequences15,40,41. However, we identified no such canonical sequences in a species-conserved manner within 10,000 bases from the transcription start site* (*for RORE, there are 3 atypical RORE sequences detected from −720 to −467, but their transcriptional role for Cx43 was negated as shown in Supplementary Fig. S6 and Supplementary Discussion). Therefore, we suspected that the circadian clock might regulate the Cx43 promoter by a novel mechanism.
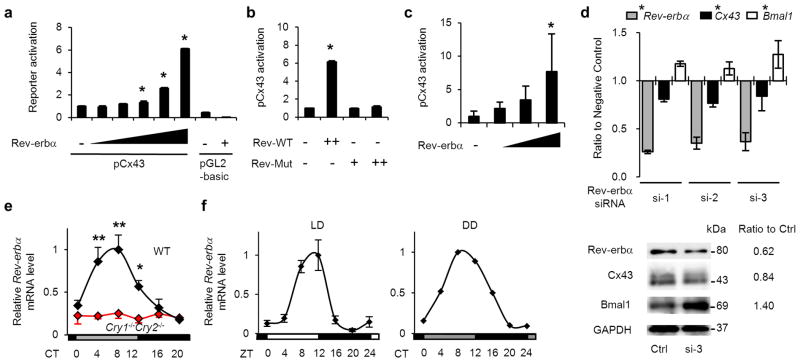
We examined the effect of clock genes on the Cx43 promoter by promoter-reporter assays in HEK293T cells using a pGL-2-mouse Cx43-promoter-reporter construct containing −1686/+16542. There was little effect of Clock/Bmal1 and Cry1 (positive and negative regulators of genes with E-box elements as shown for the Per1 promoter) or of Dbp and E4bp4 (regulators of genes with D-box elements as shown for the Per1 promoter) on the Cx43 promoter (Supplementary Fig. S7a,b). A RORE-binding protein, Rorα, also did not have any effect on Cx43 promoter activity. However, Rev-erbα, which has been reported as a negative competitor of Rorα43 (as shown for the Bmal1 promoter, Supplementary Fig. S7c), markedly increased Cx43 promoter activity (Supplementary Fig. S7a) in a dose-dependent manner (Fig. 5a), while the application of various concentrations of a mutant Rev-erbα (Rev-erbα truncated mutant deleted with 127–206 amino acids) failed to activate the Cx43 promoter (Fig. 5b).
Figure 5. Rev-erbα upregulates Cx43 expression.
(a) Dose-dependent activation of Cx43 transcription by Rev-erbα in HEK293T cells (n=3 for each dose). (b) Impaired activation of Cx43 transcription by a mutant of Rev-erbα without 127–206 amino acids from the N-terminal (Rev-Mut) in HEK293T cells (n=3 for each dose). (c) Activation of Cx43 transcription by Rev-erbα in rat BSMC (n=6 for each dose). *P < 0.01 vs. Rev-erbα (−) by one-way ANOVA with Dunnett’s post hoc test in a–c. Similar data obtained in three independent experiments for a and b, and in two independent experiments for c. (d) Suppression of Cx43 expression by knock-down of endogenous Rev-erbα in BSMC. Three types of Rev-erbα siRNAs, containing high (si-1), middle (si-2) and low (si-3) GC ratios or their controls containing corresponding GC ratios were transfected. (n=4). Messenger RNA and protein expression (data of si-3) was normalized by 18s ribosomal RNA and GAPDH, respectively. Interference of Rev-erbα mRNA significantly decreased mRNA expression of Cx43 and increased Bmal1 compared with their corresponding controls (F(1[DF for the treatment factor],18[error DF])=324 for Rev-erbα, 9.7 for Cx43 and 11.7 for Bmal1. *P < 0.01 by two-way ANOVA). Temporal bladder Rev-erbα mRNA accumulation in WT and Cry-null mice (n=3). (e) and in rats under LD (n=5) and DD (n=2) conditions (f). *P < 0.05 vs. CT8 and **P < 0.01 vs. CT0, 16 and 20 in WT by one-way ANOVA with Tukey’s post hoc test. No significant difference in Cry-null mice. MaxCorrs were WT, 0.98; Cry-null, 0.31; rats in LD, 0.84; in DD, 0.93. The maximal value of WT was set as 1. Error bars represent s.d. in a–f. For relative levels, Rev-erbα (−) was set as 1 in a–c. CT, circadian time; ZT, zeitgeber time.
Additionally, in BSMC, Rev-erbα dose-dependently upregulated Cx43 promoter activity (Fig. 5c). Conversely, inhibition of Rev-erbα by siRNA decreased Cx43 mRNA and protein expression (Fig. 5d), while the same siRNA enhanced expression of Bmal1. These results suggest that Cx43 activation is elicited through an unreported positive transcriptional control by Rev-erbα. Rev-erbα mRNA showed a clear circadian rhythm in mouse and rat urinary bladders with a peak time at CT/ZT 4–12 and a trough at CT/ZT16–24 (Fig. 5e,f). In Cry-null mice, Rev-erbα mRNA stayed arrhythmic at a lower level (Fig. 5e). These two patterns are consistent with the role of Rev-erbα as a positive regulator for Cx43, because the Cx43 expression profile showed a peak at CT/ZT12–20 and a trough at CT/ZT0–8 in WT mice and rats, but stayed arrhythmic at a lower level in Cry-null mice (Figs. 2f and 3b).
Sp1 dependent activation of Cx43 transcription by Rev-erbα
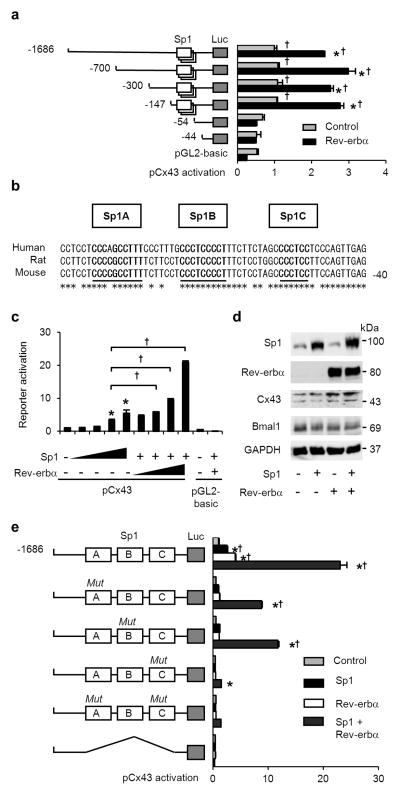
To clarify the novel molecular mechanism of Rev-erbα on promoter activity, we first attempted to identify the site with effects on the Cx43 promoter by a deletion experiment because the 5’ region of Cx43 contains several cis-elements including AP-1, AP-2, Sp1, half ERE, and cAMP-response element44. Truncated −700/+165, −300/+165 and −147/+165 constructs did not affect the activation of the Cx43 promoter by Rev-erbα. In contrast, the transcription activation of Cx43 by Rev-erbα was markedly diminished in the −54/+165 and −44/+165 constructs (Fig. 6a).
Figure 6. Sp1-dependent activation of Cx43 expression by Rev-erbα.
(a) Sequences including Sp1 sites are indispensable for Cx43 promoter activation by Rev-erbα. *P < 0.001 vs. the control of each construct and †P < 0.001 vs. −54 (without Sp1 sequences) construct by two-way ANOVA with Bonferroni’s post hoc test (n=3 for each). (b–e) Rev-erbα and Sp1 activate Cx43 expression using Sp1 sites. (b) Diagram of Cx43 promoter sequences including three Sp1 sites, labelled as Sp1A, B and C. The asterisk indicates corresponding nucleotide sequences among humans, rats and mice. (c) Dose dependent activation of Cx43 transcription by Sp1 and Rev-erbα with Sp1. *P < 0.001 vs. the value -without Sp1 and Rev-erbα, and †P < 0.001 by one-way ANOVA with Tukey’s post hoc test (n=3 for each). (d) Immunoblot analysis of the effect of Sp1 and Rev-erbα on expression of Cx43 and Bmal1 (control of negative regulatory effect by Rev-erbα). (e) Impaired activation of pCx43 with the Sp1 sites mutation by Sp1 and Rev-erbα. *P < 0.001 vs. the controls of each construct, and †P < 0.001 vs. the MutC construct by two-way ANOVA with Bonferroni’s post hoc test (n=3 for each group). Error bars represent s.d. in a, c and e. Cells used were HEK293T in all transfection experiments. The control without Rev-erbα and Sp1 was set as 1 in a, c and e. One representative of two experiments with similar results is shown in a, c, d and e.
Between −147 and −54 of the Cx43 promoter, there are cis-elements evolutionally conserved among humans, rats and mice including three putative GC-rich Sp1-binding sites (Fig. 6b)45,46. Exogenous expression of a transcription factor Sp1 activated the Cx43 promoter in a dose-dependent manner, and its effect was dramatically increased by the addition of exogenous Rev-erbα (Fig. 6c). Protein expression of Cx43 was also upregulated by exogenous Sp1 and Rev-erbα (Fig. 6d). The promoter activation induced by Sp1/Rev-erbα was inhibited by mutations of the three Sp1 cis-elements, in which the proximal was most crucial (Sp1C in Fig. 6e), and was completely abolished by deletion of all Sp1 sites (Fig. 6e). Sp3, another activator bound to Sp1 sites of Cx43 promoter45, also enhanced Cx43 promoter activity in the presence of Rev-erbα (Supplementary Fig. S8), supporting the involvement of Sp1 sites for enhancement of Cx43 transcription by Rev-erbα.
In conclusion, unlike the negative transcriptional role of Rev-erbα using RORE sites, the novel positive transcriptional role of Rev-erbα requires Sp1 cis-elements on the Cx43 promoter.
Rhythmic assembly of Rev-erbα and Sp1 at Cx43 promoter
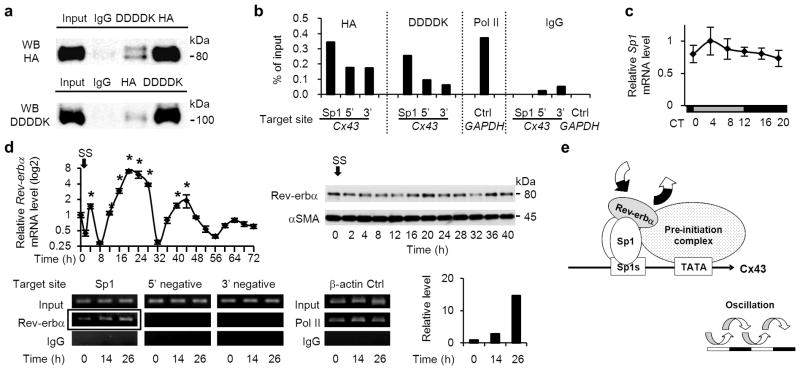
We hypothesized that Rev-erbα acts as a co-factor of Sp1, because Rev-erbα dose-dependently enhanced Cx43 activation by Sp1. To determine whether Rev-erbα interacts with Sp1 at Sp1 sites of the Cx43 promoter, a co-immunoprecipitation assay and chromatin immunoprecipitation assay (ChIP) were performed in HEK293T cells transfected with HA-Rev-erbα and DDDDK-Sp1 (protein expression of these constructs are shown in Supplementary Fig. S9). We observed a DDDDK-Sp1 band in immunoprecipitates with an anti-HA antibody, and HA-Rev-erbα was detected in immunoprecipitates with an anti-DDDDK antibody (Fig. 7a), indicating that Rev-erbα can form a complex with Sp1. The ChIP assay revealed that, in immunoprecipitates of the chromatin fragments using antibodies for HA and DDDDK, specific enrichment was obtained by primers targeted on Sp1 cis-elements of the human Cx43 promoter (Fig. 7b). These findings demonstrate that Rev-erbα interacts with Sp1 atSp1 sites of the Cx43 promoter.
Figure 7. Rhythmic assembly of Rev-erbα and Sp1 at Sp1 sites of the Cx43 promoter.
(a) Co-immunoprecipitation showing a complex formation between HA-tagged Rev-erbα and DDDDK-tagged Sp1 transfected in HEK293T cells, using antibodies for HA and DDDDK. One representative of three experiments with similar results is shown. (b) Chromatin immunoprecipitation (ChIP) assay using antibodies for HA and DDDDK in HEK 293T cells transfected with HA-Rev-erbα and DDDDK-Sp1. Analyses by real-time RT-PCR are shown, targeted against endogenous Sp1 sites of the human Cx43 promoter and its negative control sites, which are approximately 7 kbp up-(5’) and 10 kbp down- (3’) stream from the transcription start site. A ChIP assay using an antibody for RNA polymerase II and primers for human GAPDH promoter was used as a positive control. One representative of two experiments with similar results is shown. (c) Temporal Sp1 mRNA accumulation in the mouse bladder (n=3 for each time point). There were no significant differences among time points by one-way ANOVA. (d) Oscillations of Rev-erbα mRNA (left) and protein expression (right) in serum-shocked rat BSMC (top row). *P < 0.01 vs. the nadir value (time 8) by one-way ANOVA with Dunnett’s post hoc test (n=3–6). SS, serum shock. The ChIP assay, using an antibody for endogenous Rev-erbα, was analysed by RT-PCR targeted against endogenous Sp1 sites of the rat Cx43 promoter and negative control sites, which are approximately 8 kbp up- (5’ negative) and down- (3’ negative) stream (bottom row). β-actin is a positive control. Results of real-time RT-PCR are added; it was targeted against Sp1 sites of the Cx43 promoter, which was immunoprecipitated using an antibody for Rev-erbα (corresponding to the framed bands, bottom). One representative of two experiments with similar results is shown. (e) A mechanistic scheme of Cx43 oscillation, controlled by the Rev-erbα and Sp1 complex binding to Sp1 sites of the Cx43 promoter. Error bars represent s.d. in c and s.e.m. in d. For relative levels, the maximal value was set as 1 in c and the values before serum shock (time 0) was set as 1 in d.
We next examined whether the endogenous Rev-erbα/Sp1 complex is rhythmically formed on Sp1 sites of the Cx43 promoter. It is likely that Rev-erbα, but not Sp1, could contribute to the rhythmic formation of the Rev-erbα/Sp1 complex, because Sp1 mRNA showed no marked rhythm (Fig. 7c), in contrast with the strong rhythm of Rev-erbα mRNA in the bladder (Fig. 5e,f). To verify the rhythmic role of Rev-erbα estimated in vivo, we applied rat BSMC under serum shock in vitro for the ChIP assay on the Cx43 promoter using an anti-Rev-erbα antibody. After confirming the circadian expression of Rev-erbα mRNA and protein (Fig. 7d), we harvested the cells before serum shock and 14 h and 26 h after serum shock. At 26 h the level of Rev-erbα detected on Sp1 sites of the Cx43 promoter was higher than that at 14 h (Fig. 7d), when Cx43 mRNA expression showed a peak and nadir, respectively (Fig. 4b). Therefore, we conclude that this rhythmic formation of the Rev-erbα/Sp1 complex on the Sp1 sites of Cx43 promoter is one mechanism that the clock induces for circadian oscillation of Cx43 expression (Fig. 7e).
DISCUSSION
Day-night micturition rhythm in humans enables a sound sleep during the sleep phase. This rhythm is not simply caused by a higher water intake during the day, because temporal variation in urine production is maintained in subjects under constant routine, when food and drink are taken equally during 24 hours47. In physiological conditions, nocturnal micturition is prevented not only by a decrease in urine production rate from the kidneys, but also by an increase in storage capacity of the urinary bladder7–9. Day-night differences in bladder capacity are experienced in the daily life of humans and their disturbance can be seen in disorders such as nocturnal enuresis and nocturia. The treatment of these patients in clinics is often limited to palliation because the precise mechanism underlying the micturition rhythm is unknown.
In the beginning of the current study, we aimed to use a mouse model with/without genetic modification for investigating this mechanism. A day-night difference in micturition is known to exist in humans and rodents8,22,28,29, but circadian analysis of micturition in mice has been precluded by the difficulty in diachronic measuring of urine volume with high accuracy. To overcome these problems, we designed the aVSOP system, which enabled an accurate record of minute micturition volume as little as 10 μl for several days. This method clearly recorded circadian rhythm of micturition in mice (also avoiding micturition during the sleep phase), suggesting that this rhythm should have a mechanism conserved among species. The aVSOP method also enabled us to use genetically engineered mice for unravelling the molecular events responsible for controlling micturition. In Cx43+/− mice, this method showed the importance of Cx43 for determining functional bladder capacity, and in Cry-null mice, it showed a novel function of the circadian clock: circadian regulation of functional bladder capacity. Oscillation of the internal clock was demonstrated in the urinary bladder muscle layer ex vivo and in cultured BSMC, in parallel with the functional change in gap junctions, compatible with the rhythm of Cx43 levels. Collectively, these findings constitute the key concept of this study: the clock elicits oscillation in Cx43 levels and sensitivity of BSMC, which contribute to altered functional bladder capacity and micturition frequency during the 24 hour cycle.
Micturition during the sleep phase is undesirable for humans, in terms of arousal from sleep, hygiene or maintenance of body temperature, which could also be applicable to rodents. By focusing on Cx43 in the urinary bladder and the circadian clock in the present study, we showed a novel aspect in normal and pathological physiology of the diurnal micturition rhythm. However, we should also note that Cx43 in the bladder is not the only determinant of this rhythm. Enuresis and nocturia are caused not only by decreased functional bladder capacity, but also by impairment of cortical arousal level in the brain, urine production rhythm in the kidneys4, and interaction between each other48. In the bladder, there are also many other candidate molecules with diurnal oscillation, as listed in our microarray data (Supplementary Data 1), such as genes associated with smooth muscle contraction (Cacna1g, Ednrb and ucy1a3) or response to pain (Slc6a2, Ednrb and Grik1). These genes could also be contributing to micturition rhythm. Future studies may elucidate the association of these factors with the circadian clock, and the aVSOP method could serve as an important tool for that purpose.
The molecular mechanism of Cx43 oscillation that may underlie these phenomena is also noteworthy. Rev-erbα protein, known as a constitutive repressor43,49, acts as a transcriptional activator for Cx43 by luciferase-reporter assay. Intriguingly, for Cx43 activation, Rev-erbα does not require a canonical RORE site, but acts indirectly on Sp1 sites by interacting with Sp1 protein. This is similar to previous reports showing the physical interaction of Sp1 and nuclear receptors, such as RAR, RXR, ERs and PPARγ50–52. The identification of a co-activator-like function of Rev-erbα advocates a novel paradigm for controlling circadian gene expression: circadian clock components can modulate the activity of transcription factors coded by non-clock genes by functioning as transcriptional co-factors. Genome-wide screening of putative clock-associated sequences has revealed that many genes showing circadian oscillation do not have binding sequences for clock proteins14,15, and our finding suggests that these genes could possibly be regulated by clock proteins acting as co-factors.
In summary, the circadian clock is associated with the oscillating expression of Cx43 in BSMC via a previously unknown regulatory mechanism, and it contributes to changes in bladder capacity with an increase during the sleep phase and a decrease during the active phase. This study warrants chronobiological approaches for the investigation and treatment of nocturnal enuresis and nocturia.
METHODS
Animals
Female Cx43 heterozygote KO mice (Cx43+/−)53 aged 16–21 weeks, their female WT littermates (Cx43+/+), and female Cry1−/−Cry2−/− mice (Cry-null)11 aged 10 weeks were used. C57BL/6 mice and Sprague-Dawley (SD) female rats were purchased from Japan Lab Animals Co., Ltd and Japan SLC. Animals were treated in accordance with NIH animal care guidelines, and the Kyoto University Animal Experiment Committee approved all animal experiments.
Micturition analysis in mice
Micturition assessment machines for aVSOP were manufactured by Real-designs Co., Ltd (Kyoto, Japan). Rolled laminated filter paper, pre-treated to turn the edge of urine stains deep purple, was wound up at a speed of 10 cm/h under a water-repellent wire lattice. Urine stains were counted and traced to convert micturition volume by the formula of a standard curve, calculated by the correlation of normal saline and the stained area ranging from 10 to 800 μl. Cx43+/− and Cx43+/+ mice were kept in a cage with the dimensions of 110×160×75 mm (height × depth × width), measured under LD conditions for 4 days and sacrificed for RNA extraction. The male WT mice were measured for 8 days under LD and followed by 5 days under DD conditions. The female WT and Cry-null mice were kept in a cage with dimensions of 75×160×75 mm, and measured with a simultaneous actogram under DD conditions for 5 days. Total urine volume per hour was estimated by dividing the volume by the time interval between the given and preceding voiding (filling time) when the filling time was more than 1 hour7.
Micturition analysis in rats
Micturition of SD rats was recorded by an electronic balance system22 under LD conditions for 2 days.
Real-time RT-PCR analysis
Female C57BL/6 mice aged 8 weeks and Cry-null mice aged 10 weeks were sacrificed every 4 hours at six time points during the day under a dim light (n=3 for each time/strain) after acclimation for 2 weeks under LD conditions followed by DD conditions. Female SD rats aged 7 weeks were acclimated for a week under LD conditions and sacrificed at every 4 hours (n=5 for each time), followed by DD conditions, and then sacrificed in the same manner (n=2 for each time). Complementary DNA was synthesized from 1 μg of RNA extracted from the bladder and cultured cells using a Superscript VILO cDNA Synthesis Kit (Invitrogen). Primers used are listed in Supplementary Table S1. Real-time RT-PCR was performed using SYBR Green PCR Master Mix with 7500 Fast Real-Time PCR system (Applied Biosystems)22. Each sample was normalized against an internal 18s ribosomal RNA control (Takara). Maximal correlation coefficients (MaxCorr) were calculated using Mathematica ver. 5.1 according to a modified program35.
Immunoblotting
Whole cell lysates from bladder tissues and cultured cells were lysed with radioimmunoprecipitation assay (RIPA) buffer containing proteinase inhibitors, which were resolved by SDS-PAGE and transferred to an Immobilon-P membrane. The membranes were incubated with antibodies against Cx43 (Zymed, 1:200; Sigma-Aldrich, 1:1000), Rev-erbα (Cell Signaling Technology [CST], 1:500; Abcam, 1:600), Bmal1 (Santa Cruz, 1:200), Sp1 (Millipore, 1:2000), αSMA (Sigma-Aldrich, 1:5000), Sp3 (BioLegend, 1:500), HA (Abcam, 1:8000), DDDDK (MBL, 1:2000) and GAPDH (CST, 1:2000). The immunoreactivities were visualized with enhanced chemiluminescence using HRP-conjugated anti-rabbit or mouse IgG antibody (Pierce)22.
Bioluminescence recording
Slice cultures of bladder were obtained from adult Period2Luciferase knock-in (Per2::luc) mice38 (Jackson Laboratories), and Per2::luc mice with a Clock mutation (ClkΔ19/ClkΔ19) generated by crossing Per2::luc mice to Clock mutant (ClkΔ19/ClkΔ19) mice (Jackson Laboratories). The slice cultures were kept at 36°C with culture medium containing 1 mM luciferin54. Bioluminescence was measured with a highly sensitive cryogenic CCD camera (Spectra Video SV16KV/CT; Pixelvision) equipped with a microscope (Carl Zeiss)55.
Immunostaining of the urinary bladder
Mice bladder tissues and slice cultures were fixed with 4% paraformaldehyde in 0.1M phosphate buffer (0.1MPB, pH 7.4) for 24 hours54. After embedding bladder tissues in paraffin, we cut 5 μ thick sections with a microtome. After being treated with 1% BSA, sections were incubated with Cx43 rabbit polyclonal antibody (Invitrogen, 1:50) or αSMA (Sigma-Aldrich, 1:400). Immunoreactions were visualized by donkey anti-rabbit Alexa 594 (Molecular Probes), and observed by a fluorescent microscope (Carl Zeiss).
Serum shock analysis of BSMC
Primary BSMC, isolated from female SD rats aged 9 weeks22,56, were cultured after two passages until sub-confluent in DMEM with 10% FCS and 1% penicillin streptomycin followed by 72 hours incubation in DMEM with 0.5% FCS. The cells were treated with 50% horse serum (GIBCO-BRL) in DMEM for 2 hours39, and washed twice with DMEM and then maintained in DMEM with 0.5% of FCS for a maximum of 72 hours.
Immunostaining of BSMC
BSMC were serum-shocked and fixed with 4% formaldehyde, permeabilized with 0.4% Triton X-100, and blocked with 10% goat serum (Invitrogen). The cells were incubated with Cx43 rabbit polyclonal antibody (Sigma-Aldrich, 1:500) followed by goat anti-rabbit Alexa 594 (Molecular Probes) and counter stained with DAPI57.
Lucifer yellow microinjections
Nuclei of serum-shocked BSMC were pre-stained by 16 μM of Hoechst 33342 (Invitrogen) for 15 minutes to identify the site of injection. A single cell was impaled with a microelectrode and Lucifer Yellow was injected by an electrometer (model 3100; A-M systems) for 1 minute with a continuous current of 0.1 μA. Images were acquired using a CoolSNAP-HQ2 CCD camera (Photometrics)57.
Promoter-reporter assay
The promoter-reporter constructs used were, mouse pGL-2-Cx43 (pCx43 −1686/+165-luc)42, mouse pGL-3-Per1 (pPer1-luc), pGL-3-Bmal1 (pBmal1-luc), and pGL-2 basic, pRL-TK (Promega) as controls. The expression vectors used were, Sp1 and Sp3 (fully lengthened according to a previous report)58, 59, Clock, Bmal1, Cry1, Dbp, E4bp4, Rev-erbα and Rorα (Open Biosystems). Site-directed mutagenesis, deletion and addition of aimed sequences were performed using a mutagenesis basal kit (Takara). Corrected mutants were all verified by sequencing. For luciferase assays, reporter plasmids (100 ng) with various expression vectors (total 250 ng) were transfected to HEK293T cells in 24-well plates and those (total 100 ng) to BSMC in 96-well plates using Fugene6 (Roche) in DMEM with 10% FCS. Plasmid dosage was kept constant by the EGFP-N1 vector. Lysates were harvested 48 hours post-transfection, and the luciferase activity was measured using a dual luciferase assay reagent (Promega).
RNA interference assay
Three sets of Stealth Select RNAi targeted against rat Rev-erbα (Rat Nr1d1 1330003) and Stealth RNAi Negative Control (low, medium and high GC, Invitrogen) were used. After two passages, BSMC were plated on 6-well plates and kept for 24 hours in DMEM with 10% FCS. This was followed by transfection of siRNA (75 pmol/well) in serum-reduced αMEM for 5 hours using Lipofectamin RNAiMAX (Invitrogen), followed by medium-change to DMEM with 10% FCS. After 48 hours of the transfection, total RNA and cell lysates were extracted.
Co-immunoprecipitation assay
Rev-erbα with N-terminal MYPYDVPDYA-tag (HA-Rev-erbα) and Sp1 with N-terminal MDYKDDDDK-tag (DDDDK-Sp1) were constructed using a mutagenesis basal kit (Takara). Nuclear extracts were prepared from HEK293T cells transfected with expression vectors for 48 hours, using a Nuclear Complex Co-IP kit (Active Motif,). A total of 100 μg of nuclear extracts were incubated with antibodies for 0.27 μg of Rev-erbα (CST), 4 μg of HA (Abcam), DDDDK (MBL) and control rabbit IgG (Zymed), in 500 μl of low IP buffer overnight at 4°C with rotation followed by the addition of 30 μl of Dynabeads Sheep anti-Rabbit IgG (Veritas) for 1 hour. After washing with low IP buffer with/without BSA three times each, the binding protein was eluted in 40 μl of RIPA buffer for immunoblotting. A total of 2 μg of nuclear extracts were used as input.
Chromatin immunoprecipitation
Formaldehyde-cross-linked chromatins were obtained from HEK293T cells and serum-shocked BSMC, which were sheared using Chip-IT Express Enzymatic (Active Motif). Sheared chromatins were immunoprecipitated with antibodies to 0.23 μg of Rev-erbα (Lifespan Biosciences, LS-C37817), 2 μg of HA, DDDDK and control rabbit IgG overnight at 4°C with rotation followed by the addition of 15 μl of Dynabeads Sheep anti-Rabbit IgG for 1 hour. The Chip-IT control kits Rat and Human (Active Motif) were used as an experimental control. The enrichment of Sp1-binding sequences in eluted DNA was de-crosslinked by incubation at 65°C for 4 hours followed by digestion of protein by proteinase K. Purified DNA, using a QIAquick PCR purification kit (Qiagen), was quantified by real-time RT-PCR and normalized by the quantity of input DNA60. Samples of BSMC after real-time RT-PCR were visualized by electrophoresis. Primers used are listed in Supplementary Table S1.
Microarray analysis of the mouse bladder
Whole bladder RNA of female C57BL/6 mice sacrificed at CT 0, 4, 8, 12, 16 and 20 as described above was used (n=2 for each time point). A total of 250 ng of qualified RNA was employed for the synthesis of Cy3 labelled cRNA, which was qualified and hybridized to the Whole Mouse Genome Oligo Microarray (44K; Agilent Technologies) according to the manufacturer’s protocol (entrusted to DNA Chip Research, Inc., Yokohama, Japan). Statistical analysis of microarray data was performed using R statistical software (version 2.8.1, 2008-12-22) with BioConductor (version 2.3). Signal processing was performed by Agilent Feature Extraction (version. 9.5.3). Normalization was performed based on the Agi4x44PreProcess package (version 1.2.0). The mgug4122a.db package (version 2.2.5) was used for annotation of each probe. Selection of circadian-oscillated genes was based on the modified procedure of Yamada et al35 and McDonald et al34. Specifically, we calculated correlation coefficients between each six-point time course and 60 different-phased cosine curves with six time points. For each probe, we selected one cosine curve with MaxCorr from the 60 different-phased cosine curves. The false-positive proportion was estimated on the basis of the null distribution of maximum correlations derived from randomly generated expression profiles.
Statistical analysis
For the micturition experiments, we used one-way ANOVA with Bonferroni’s post hoc test to evaluate differences among time points. Two-way repeated measures ANOVA was used to compare differences between the two groups of mice tested, and time points were compared with Bonferroni’s post hoc test. For the experiments in which three or more test groups were compared, we used one-way ANOVA, and for those including two factors, we used two-way ANOVA.
Supplementary Material
Acknowledgments
We thank D.C. Spray, A. Mello, M.M. Thi, M. Tanaka, R. Matsuoka, Y. Kimura, N. Kawakami, Y. Sugino, T. Kobayashi, Y. Kajita, S.J. Lye, J. Yao, G. Suske, J. Toguchida, M. Yamamoto, S. Karki, J.M. Fustin and A. Negoro. This work was supported by a Grant-in-Aid for Scientific Research (20390425, 21390439, 23659756 and 18002016) from the Japan Society for the Promotion of Science, a grant from the National Institutes of Health (DK 081435), a grant from the Translational Research Centre in Kyoto University, an Asahi Kasei Pharma Urological Academy Grant, the Suzuki Urological Foundation and the Kyoto University Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
H.N. and A.K. designed the experiments, analysed data and prepared the manuscript. H.N. performed most of the experiments. M.D. and H.O. contributed to the study design and manuscript preparation. S.O.S. provided Cx43+/− mice and contributed to the microinjection of Lucifer yellow. M.M. contributed to the analysis of rhythm-associated experiments. M.I., T. Okinami, N.N., K.S., M.T. and S.U. helped with in vitro experiments. T. Oura and S.M. analysed microarray data. E.K. contributed to morphological analysis. T.T. provided Cry-null mice. H.O., Y.T. and O.O. supervised the study.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Accession codes: Microarray data have been deposited in the Gene Expression Omnibus under accession code GSE35795.
References
- 1.Robson WL. Clinical practice. Evaluation and management of enuresis. N Engl J Med. 2009;360:1429–1436. doi: 10.1056/NEJMcp0808009. [DOI] [PubMed] [Google Scholar]
- 2.Neveus T. Diagnosis and management of nocturnal enuresis. Curr Opin Pediatr. 2009;21:199–202. doi: 10.1097/MOP.0b013e3283229b12. [DOI] [PubMed] [Google Scholar]
- 3.Bosch JL, Weiss JP. The prevalence and causes of nocturia. J Urol. 2010;184:440–446. doi: 10.1016/j.juro.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 4.van Kerrebroeck P, et al. The standardisation of terminology in nocturia: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:179–183. doi: 10.1002/nau.10053. [DOI] [PubMed] [Google Scholar]
- 5.Van Hoeck K, et al. Urine output rate and maximum volume voided in school-age children with and without nocturnal enuresis. J Pediatr. 2007;151:575–580. doi: 10.1016/j.jpeds.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Weiss JP, Blaivas JG, Stember DS, Chaikin DC. Evaluation of the etiology of nocturia in men: the nocturia and nocturnal bladder capacity indices. Neurourol Urodyn. 1999;18:559–565. doi: 10.1002/(sici)1520-6777(1999)18:6<559::aid-nau6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Van Hoeck K, Bael A, Lax H, Hirche H, van Gool JD. Circadian variation of voided volume in normal school-age children. Eur J Pediatr. 2007;166:579–584. doi: 10.1007/s00431-006-0286-x. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura S, et al. Circadian changes in urine volume and frequency in elderly men. J Urol. 1996;156:1275–1279. [PubMed] [Google Scholar]
- 9.Witjes WP, Wijkstra H, Debruyne FM, de la Rosette JJ. Quantitative assessment of uroflow: is there a circadian rhythm? Urology. 1997;50:221–228. doi: 10.1016/s0090-4295(97)00190-8. [DOI] [PubMed] [Google Scholar]
- 10.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 11.Doi M, et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16:67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 14.Panda S, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 15.Ueda HR, et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37:187–192. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- 16.Okamura H, Doi M, Fustin JM, Yamaguchi Y, Matsuo M. Mammalian circadian clock system: Molecular mechanisms for pharmaceutical and medical sciences. Adv Drug Deliv Rev. 2010;62:876–884. doi: 10.1016/j.addr.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Birder L, et al. Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourol Urodyn. 2010;29:128–139. doi: 10.1002/nau.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson KE. Antimuscarinic Mechanisms and the Overactive Detrusor: An Update. Eur Urol. 2010;59:377–386. doi: 10.1016/j.eururo.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 19.Imamura M, et al. Basic fibroblast growth factor causes urinary bladder overactivity through gap junction generation in the smooth muscle. Am J Physiol Renal Physiol. 2009;297:F46–F54. doi: 10.1152/ajprenal.90207.2008. [DOI] [PubMed] [Google Scholar]
- 20.Christ GJ, et al. Increased connexin43-mediated intercellular communication in a rat model of bladder overactivity in vivo. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1241–1248. doi: 10.1152/ajpregu.00030.2002. [DOI] [PubMed] [Google Scholar]
- 21.Suadicani SO, Urban-Maldonado M, Tar MT, Melman A, Spray DC. Effects of ageing and streptozotocin-induced diabetes on connexin43 and P2 purinoceptor expression in the rat corpora cavernosa and urinary bladder. BJU Int. 2009;103:1686–1693. doi: 10.1111/j.1464-410X.2008.08337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negoro H, et al. Regulation of connexin 43 by basic fibroblast growth factor in the bladder: transcriptional and behavioral implications. J Urol. 2011;185:2398–2404. doi: 10.1016/j.juro.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Haferkamp A, et al. Increased expression of connexin 43 in the overactive neurogenic detrusor. Eur Urol. 2004;46:799–805. doi: 10.1016/j.eururo.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Sugino Y, et al. Voided stain on paper method for analysis of mouse urination. Neurourol Urodyn. 2008;27:548–552. doi: 10.1002/nau.20552. [DOI] [PubMed] [Google Scholar]
- 25.Wood R, Eichel L, Messing EM, Schwarz E. Automated noninvasive measurement of cyclophosphamide-induced changes in murine voiding frequency and volume. J Urol. 2001;165:653–659. doi: 10.1097/00005392-200102000-00089. [DOI] [PubMed] [Google Scholar]
- 26.Birder LA, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 27.Reaume AG, et al. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 28.Bassuk JA, Grady R, Mitchell M. Review article: The molecular era of bladder research. Transgenic mice as experimental tools in the study of outlet obstruction. J Urol. 2000;164:170–179. [PubMed] [Google Scholar]
- 29.Herrera GM, Meredith AL. Diurnal variation in urodynamics of rat. PLoS One. 2010;5:e12298. doi: 10.1371/journal.pone.0012298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meredith AL, et al. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Horst GT, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 32.Storch KF, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 33.Hoogerwerf WA, et al. Transcriptional profiling of mRNA expression in the mouse distal colon. Gastroenterology. 2008;135:2019–2029. doi: 10.1053/j.gastro.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald MJ, Rosbash M. Microarray analysis and organization of circadian gene expression in Drosophila. Cell. 2001;107:567–578. doi: 10.1016/s0092-8674(01)00545-1. [DOI] [PubMed] [Google Scholar]
- 35.Yamada R, Ueda HR. Microarrays: statistical methods for circadian rhythms. Methods Mol Biol. 2007;362:245–264. doi: 10.1007/978-1-59745-257-1_17. [DOI] [PubMed] [Google Scholar]
- 36.Fallon RF, Goodenough DA. Five-hour half-life of mouse liver gap-junction protein. J Cell Biol. 1981;90:521–526. doi: 10.1083/jcb.90.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett MV, et al. Gap junctions: new tools, new answers, new questions. Neuron. 1991;6:305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- 38.Yoo SH, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 40.Mitsui S, Yamaguchi S, Matsuo T, Ishida Y, Okamura H. Antagonistic role of E4BP4 and PAR proteins in the circadian oscillatory mechanism. Genes Dev. 2001;15:995–1006. doi: 10.1101/gad.873501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preitner N, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 42.Chen ZQ, et al. Identification of two regulatory elements within the promoter region of the mouse connexin 43 gene. J Biol Chem. 1995;270:3863–3868. [PubMed] [Google Scholar]
- 43.Ueda HR, et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- 44.Oyamada M, Oyamada Y, Takamatsu T. Regulation of connexin expression. Biochim Biophys Acta. 2005;1719:6–23. doi: 10.1016/j.bbamem.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Teunissen BE, et al. Analysis of the rat connexin 43 proximal promoter in neonatal cardiomyocytes. Gene. 2003;322:123–136. doi: 10.1016/j.gene.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Echetebu CO, Ali M, Izban MG, MacKay L, Garfield RE. Localization of regulatory protein binding sites in the proximal region of human myometrial connexin 43 gene. Mol Hum Reprod. 1999;5:757–766. doi: 10.1093/molehr/5.8.757. [DOI] [PubMed] [Google Scholar]
- 47.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Sensitivity of the human circadian pacemaker to moderately bright light. J Biol Rhythms. 1994;9:315–331. doi: 10.1177/074873049400900311. [DOI] [PubMed] [Google Scholar]
- 48.Yeung CK, Diao M, Sreedhar B. Cortical arousal in children with severe enuresis. N Engl J Med. 2008;358:2414–2415. doi: 10.1056/NEJMc0706528. [DOI] [PubMed] [Google Scholar]
- 49.Yin L, Lazar MA. The orphan nuclear receptor Rev-erbalpha recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol Endocrinol. 2005;19:1452–1459. doi: 10.1210/me.2005-0057. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki Y, et al. Physical interaction between retinoic acid receptor and Sp1: mechanism for induction of urokinase by retinoic acid. Blood. 1999;93:4264–4276. [PubMed] [Google Scholar]
- 51.Shimada J, et al. Transactivation via RAR/RXR-Sp1 interaction: characterization of binding between Sp1 and GC box motif. Mol Endocrinol. 2001;15:1677–1692. doi: 10.1210/mend.15.10.0707. [DOI] [PubMed] [Google Scholar]
- 52.Sun G, Porter W, Safe S. Estrogen-induced retinoic acid receptor alpha 1 gene expression: role of estrogen receptor-Sp1 complex. Mol Endocrinol. 1998;12:882–890. doi: 10.1210/mend.12.6.0125. [DOI] [PubMed] [Google Scholar]
- 53.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi S, et al. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- 55.Doi M, et al. Circadian regulation of intracellular G-protein signalling mediates intercellular synchrony and rhythmicity in the suprachiasmatic nucleus. Nat Commun. 2011;2:327. doi: 10.1038/ncomms1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang HZ, Brink PR, Christ GJ. Gap junction channel activity in short-term cultured human detrusor myocyte cell pairs: gating and unitary conductances. Am J Physiol Cell Physiol. 2006;291:C1366–C1376. doi: 10.1152/ajpcell.00027.2006. [DOI] [PubMed] [Google Scholar]
- 57.Thi MM, Urban-Maldonado M, Spray DC, Suadicani SO. Characterization of hTERT-immortalized osteoblast cell lines generated from wild-type and connexin43-null mouse calvaria. Am J Physiol Cell Physiol. 2010;299:C994–C1006. doi: 10.1152/ajpcell.00544.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hagen G, Muller S, Beato M, Suske G. Sp1-mediated transcriptional activation is repressed by Sp3. EMBO J. 1994;13:3843–3851. doi: 10.1002/j.1460-2075.1994.tb06695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sapetschnig A, Koch F, Rischitor G, Mennenga T, Suske G. Complexity of translationally controlled transcription factor Sp3 isoform expression. J Biol Chem. 2004;279:42095–42105. doi: 10.1074/jbc.M404989200. [DOI] [PubMed] [Google Scholar]
- 60.Kanematsu A, Ramachandran A, Adam RM. GATA-6 mediates human bladder smooth muscle differentiation: involvement of a novel enhancer element in regulating alpha-smooth muscle actin gene expression. Am J Physiol Cell Physiol. 2007;293:C1093–1102. doi: 10.1152/ajpcell.00225.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.