The present study shows that RNA-binding proteins CUGBP1 and HuR jointly regulate the translation of occludin and play a crucial role in the maintenance of tight junction integrity.
Abstract
RNA-binding proteins CUG-binding protein 1 (CUGBP1) and HuR are highly expressed in epithelial tissues and modulate the stability and translation of target mRNAs. Here we present evidence that CUGBP1 and HuR jointly regulate the translation of occludin and play a crucial role in the maintenance of tight junction (TJ) integrity in the intestinal epithelial cell monolayer. CUGBP1 and HuR competed for association with the same occludin 3′-untranslated region element and regulated occludin translation competitively and in opposite directions. CUGBP1 overexpression decreased HuR binding to occludin mRNA, repressed occludin translation, and compromised the TJ barrier function, whereas HuR overexpression inhibited CUGBP1 association with occludin mRNA and promoted occludin translation, thereby enhancing the barrier integrity. Repression of occludin translation by CUGBP1 was due to the colocalization of CUGBP1 and tagged occludin RNA in processing bodies (P-bodies), and this colocalization was prevented by HuR overexpression. These findings indicate that CUGBP1 represses occludin translation by increasing occludin mRNA recruitment to P-bodies, whereas HuR promotes occludin translation by blocking occludin mRNA translocation to P-bodies via the displacement of CUGBP1.
INTRODUCTION
Epithelial tight junctions (TJs) are highly specialized structures with a complex molecular architecture that determines the cell polarity and prevents the diffusion of toxins, allergens, and pathogens from the lumen into the tissue (Forster, 2008; Turner, 2009). TJs are highly dynamic, and their constituent protein complexes undergo continuous remodeling and turnover under tight regulation by numerous extracellular and intracellular factors (Suzuki et al., 2009; Raleigh et al., 2010; 2011; Ye et al., 2011). Dysfunctional TJs are associated with the pathogenesis of inflammatory diseases (Forster, 2008), tumor metastasis (Wang et al., 2007), and sepsis in critical illnesses (Yu et al., 2011), but the exact mechanisms that control the integrity of TJs are not fully known. The assembly of TJs involves four classes of TJ transmembrane proteins and >30 membrane-associated proteins in mammalian epithelial and endothelial cells (Forster, 2008; Schulzke and Fromm, 2009). Occludin, the first-identified integral TJ protein, has an ∼60-kDa tetraspanning membrane topology with three cytoplasmic domains and two extracellular loops with a key role in occludin localization (Furuse et al., 1993; Chen et al., 1997; Marchiando et al., 2010). An increasing body of evidence indicates that occludin plays an important role in the regulation of TJ integrity and in the maintenance of normal tissue physiology (Saitou et al., 2000; Suzuki et al., 2009; Raleigh et al., 2011; Su et al., 2011; Cummins, 2012). Modulation of occludin expression impacts TJ barrier function in vitro, whereas disrupting occludin by occludin-derived peptides results in TJ barrier dysfunction (Furuse et al., 1993; McCarthy et al., 1996; Wong and Gumbiner, 1997). The findings obtained from both in vitro and in vivo studies have associated occludin endocytosis with pathophysiological and pharmacological TJ barrier loss (Yu et al., 2005; Suzuki et al., 2009; Su et al., 2011). Additionally, occludin associates with many signal transduction molecules (Seth et al., 2007; Cummins, 2012) and regulates the actin organization and directional migration of epithelial cells (Du et al., 2010), suggesting that it is involved in a broader spectrum of biological functions.
CUG-binding protein 1 (CUGBP1, also named CELF1, CUGBP and embryonic lethal abnormal vision-like factor 1) and HuR are RNA-binding proteins (RBPs) that function as potent posttranscriptional regulators of gene expression, particularly by altering mRNA stability and translation (Abdelmohsen et al., 2007; Vlasova and Bohjanen, 2008). Changes in mRNA stability and translation are primarily governed through a process involving the interaction of specific mRNA sequences (cis-elements) with specific trans-acting factors including RBPs and microRNAs (miRNAs; Keene, 2007; Bartel, 2009; Houseley and Tollervey, 2009). AU-/U-rich elements (AREs) and GU-rich elements (GREs) are among the best-characterized cis-acting sequences located at the 3′-untranslated regions (3′-UTRs) from a variety of labile mRNAs. An individual RBP selectively recognizes and binds to a given mRNA bearing various AREs or GREs; the resulting ribonucleoprotein (RNP) association modulates the stability and translation of the target mRNA (Furuse et al., 1993; Gherzi et al., 2004; Keene, 2007; Houseley and Tollervey, 2009; Liu et al., 2009). CUGBP1 generally interacts with mRNAs through GREs (Vlasova and Bohjanen, 2008; Tsuda et al., 2009; Lee JE et al., 2010), and the CUGBP1 association with mRNAs is shown to enhance mRNA decay and repress the translation of several target transcripts (Zhang et al., 2008; Rattenbacher et al., 2010), although in some instances CUGBP1 also promotes mRNA translation (Iakova et al., 2004). Recently we reported that in intestinal epithelial cells (IECs), CUGBP1 represses cyclin-dependent kinase 4 (CDK4) translation in a GRE-dependent manner and that cellular CUGBP1 abundance is regulated by miRNA-503 (Xiao et al., 2007a; Cui et al., 2012), suggesting a regulatory network or “regulon” of CUGBP1 in the intestinal epithelium. However, no studies have yet investigated the mechanism of CUGBP1 in the regulation of TJ occludin expression and epithelial barrier function.
Unlike CUGBP1, HuR binds with high affinity and specificity to target mRNAs through AREs and increases the stability and translation of target transcripts (Abdelmohsen et al., 2007; Liu et al., 2009; Zhang et al., 2009; Wang et al., 2010; Zou et al., 2006, 2010). HuR was recently found to interact with the occludin mRNA via its 3′-UTR AREs and to enhance occludin translation, thus promoting TJ barrier function (Yu et al., 2011). The HuR-mediated occludin translation is crucial for normal TJ barrier function of the intestine under biological conditions, because gut barrier dysfunction in mice exposed to septic stress associates with decreased levels of occludin protein as a result of a reduction in HuR/occludin mRNA association. Given the multiple posttranscriptional functions of CUGBP1 and the fact that occludin mRNA contains several computationally predicted hits of the CUGBP1 motif, we sought to determine whether CUGBP1 is implicated in regulating occludin expression and to further define its functional interaction with HuR in this process. The results represented herein indicate that CUGBP1 interacts with and represses occludin mRNA translation in human IECs. CUGBP1 and HuR competitively bind to the same occludin 3′-UTR element, and the two RBPs regulate occludin translation in opposite directions. Moreover, CUGBP1-induced repression of occludin translation was linked to the colocalization of CUGBP1/occludin mRNA complex within processing bodies (P-bodies), where nontranslating mRNAs assemble. In contrast, HuR promotes occludin translation by competing with CUGBP1 for binding to occludin mRNA, thereby blocking the recruitment of occludin mRNA to P-bodies.
RESULTS
CUGBP1 inhibits occludin expression and disrupts epithelial barrier function
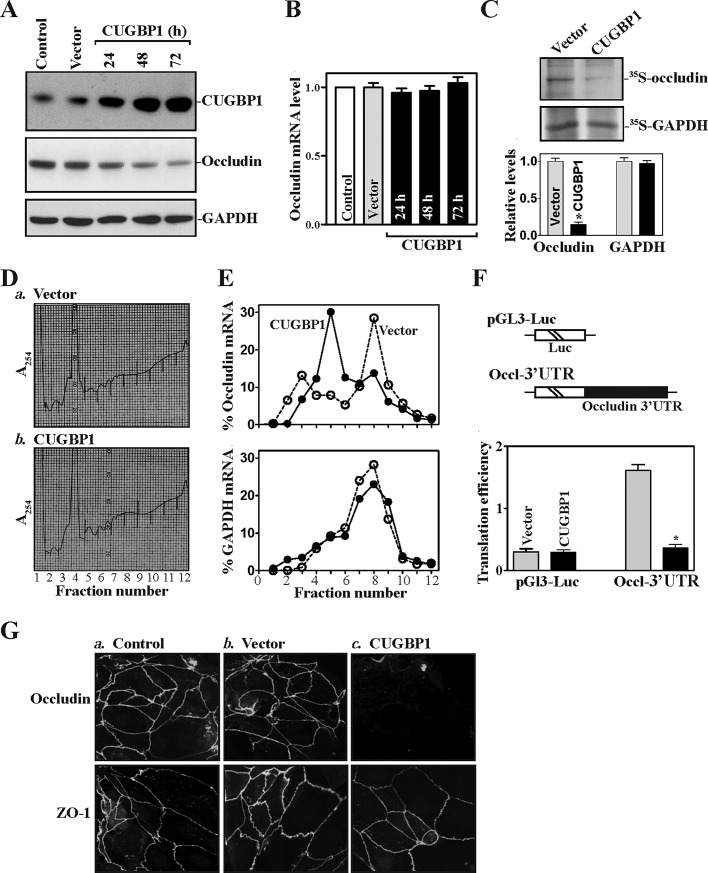
To elucidate the involvement of CUGBP1 in the regulation of intestinal epithelial barrier function, we determined the effect of overexpressing the wild-type CUGBP1 gene on the expression of the TJ proteins in Caco-2 cells, a widely used cell model for epithelial barrier studies (Chen et al., 2008; Suzuki et al.,2009). As shown in Figure 1A, transient transfection with the CUGBP1 expression vector increased CUGBP1 protein expression levels (by approximately sixfold at 24 h and by ∼10-fold at 48 and 72 h after transfection) compared with those observed in control cells and cells transfected with the empty vector. Increased CUGBP1 was associated with a potent inhibition in occludin expression, but not in the levels of ZO-1 and β-catenin (Supplemental Figure 1). This inhibitory effect of CUGBP1 on occludin expression occurs at the translation level: ectopic CUGBP1 overexpression did not decrease the levels of total occludin mRNA (Figure 1B), but it repressed the rate of nascent occludin protein synthesis (Figure 1C). Inhibition of occludin protein synthesis by CUGBP1 was specific: neither the global protein translation (Xiao et al.,2011) nor nascent glyceraldehyde 3-phosphate dehydrogenase (GAPDH) synthesis was altered in cells overexpressing CUGBP1. To further define the role of CUGBP1 in the regulation of occludin translation, we examined the relative distribution of occludin mRNA in individual fractions from polyribosome gradients after CUGBP1 overexpression. Although increasing the levels of CUGBP1 did not affect global polysomal profiles (Figure 1D), the abundance of occludin mRNA associated with actively translating fractions 7–12 decreased dramatically in CUGBP1-transfected cells, with a significant shift of occludin transcripts to low-translating fractions 4–6 (Figure 1E, top). In contrast, housekeeping GAPDH mRNA distributed similarly in both groups (Figure 1E, bottom). To examine whether the translational effect of CUGBP1 on occludin mRNA was exerted through GREs, we used a firefly luciferase reporter gene construct containing the occludin GRE within the 3′-UTR (Occl-3′-UTR) and negative control vector pGL3-Luc (Figure 1F, schematic). A plasmid expressing renilla luciferase was also cotransfected as an internal control for normalization of firefly luciferase. To distinguish translational output from changes in mRNA turnover, the luciferase activities were normalized to luciferase-reporter mRNA levels to assess the translational efficiency. The basal levels of Occl-3′-UTR reporter gene activity were higher than those of control pGL3-Luc (Figure 1F), suggesting that the occludin 3′-UTR may have a translation-enhancing element. Inhibition of occludin translation by CUGBP1 was mediated at least partially through its 3′-UTR: ectopic CUGBP1 overexpression decreased the levels of Luc-Occl-3′-UTR reporter gene activity (Figure 1F, bottom). Consistently, occludin immunostaining decreased dramatically after ectopic CUGBP1 overexpression (Figure 1G, top), although there were no differences in the levels of ZO-1 immunostaining (Figure 1G, bottom) between CUGBP1-transfected cells and control cells or cells transfected with empty vector.
FIGURE 1:
CUGBP1 overexpression inhibits occludin mRNA translation via its 3′-UTR. (A) Representative immunoblots of CUGBP1 and occludin proteins. Cells were transfected with the vector expressing CUGBP1 or control empty vector; protein levels were measured by Western immunoblotting analysis at various times after the transfection by using specific antibody against CUGBP1 or occludin. (B) Levels of occludin mRNA in cells treated as described in panel (A). Total RNA was harvested, and the levels of occludin mRNA were measured by Q-PCR analysis. Data were normalized to GAPDH mRNA levels, and values are shown as the means ± SEM of data from triplicate experiments. (C) Newly translated occludin protein as measured by l-[35S]methionine and l-[35S]cysteine/IP assays in cells transfected with the CUGBP1 expression vector or control vector for 48 h. Top, representative immunoblots of newly synthesized occludin; bottom, quantitative analysis of the immunoblotting signals as measured by densitometry. Values are mean ± SEM of data from three separate experiments. * p < 0.05 compared with cells transfected with control vector. (D) Polysomal profiles in cells described in (C): a) cells transfected with control vector; b) cells transfected with the CUGBP1 expression vector. Nuclei were pelleted, and the resulting supernatants were fractionated through a 10-50% linear sucrose gradient. (E) Distributions of occludin (top) and GAPDH (bottom) mRNAs in each gradient fraction prepared from cells described in panel (D). Total RNA was isolated from the different fractions, and the levels of occludin and GAPDH mRNAs were measured by Q-PCR analysis and plotted as a percentage of the total occludin or GAPDH mRNA levels in that sample. Three independent experiments were performed and showed similar results. (F) Changes in occludin translation efficiency as measured by occludin 3′-UTR-luciferase reporter assays. Top, schematic of plasmids: control (pGL3-Luc); and chimeric firefly luciferase-occludin 3′-UTR (Luc-Occl-3′-UTR). Bottom, levels of occludin translation. The Luc-Occl-3′-UTR or pGL3-Luc (negative control) was cotransfected with a Renilla luciferase reporter. Luciferase values were normalized to luciferase mRNA levels to calculate the translation efficiencies and expressed as means ± SEM of data from three separate experiments. *p < 0.05 compared with cells transfected with control vector. (G) Cellular distribution of occludin and ZO-1 in cells described in (C). After the transfection for 48 h, cells were fixed, permeabilized, incubated with the antibody against occludin or ZO-1, and then with anti-IgG conjugated with FITC. Original magnification, 1000×. Three experiments were performed that showed similar results.
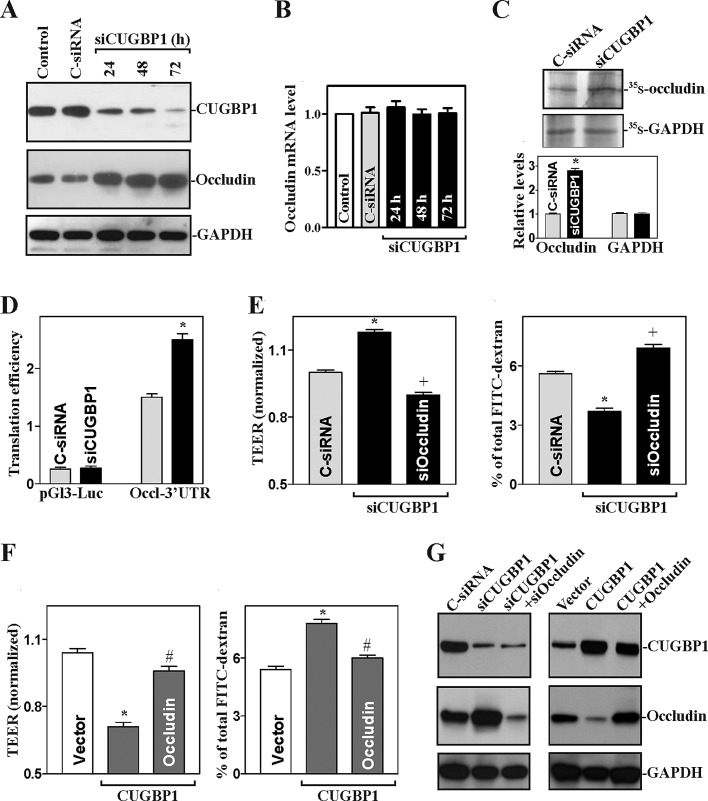
The results in Figure 2A further showed that CUGBP1 silencing by transfection with small interfering RNA (siRNA) targeting the CUGBP1 mRNA (siCUGBP1) increased occludin expression levels. These specific siCUGBP1 nucleotides were designed to reduce CUGBP1 mRNA with high specificity and efficacy and low toxicity (Xiao et al., 2011; Cui et al., 2012). The levels of CUGBP1 protein decreased by ∼65% at 24 h and by >80% at 48 or 72 h after the transfection with siCUGBP1, whereas the levels of occludin protein increased by approximately threefold compared with those in cells transfected with control siRNA (C-siRNA). Decreasing the levels of endogenous CUGBP1 by siRNA induced occludin by enhancing its translation: CUGBP1 silencing did not alter the levels of total occludin mRNA (Figure 2B), but it enhanced nascent synthesis of occludin protein (Figure 2C) and increased the occludin 3′-UTR–luciferase reporter gene activity (Figure 2D). We also examined changes in the levels of claudin-2, claudin-3, and claudin-5 proteins (three major members of the claudin family in IECs) after decreasing or increasing the levels of CUGPB1 and found that neither CUGBP1 silencing nor ectopic CUGBP1 overexpression altered the levels of these claudin family proteins (unpublished data).
FIGURE 2:
CUGBP1 silencing enhances occludin translation and promotes the epithelial barrier function. (A) Representative immunoblots of CUGBP1 and occludin proteins. After cells were transfected with either siRNA targeting the CUGBP1 mRNA CR (siCUGBP1) or C-siRNA for different times, whole-cell lysates were harvested for Western blot analysis. (B) Levels of occludin mRNA as measured by Q-PCR analysis in cells described in panel (A). The data were normalized to GAPDH mRNA levels and shown as the means ± SEM of data from triplicate experiments. (C) Newly translated occludin protein in cells transfected with siCUGBP1 or C-siRNA for 48 h as measured by l-[35S]methionine and l-[35S]cysteine/IP assays. Top, representative immunoblots of newly synthesized occludin; bottom, quantitative analysis of the immunoblotting signals as measured by densitometry. Values are means ± SEM of data from three separate experiments. * p < 0.05 compared with cells transfected with C-siRNA. (D) Changes in occludin translation efficiency as measured by using pGL3-Luc-Occl-3′-UTR reporter assays in cells described in panel (C). Twenty-four hours after cells were transfected with the Luc-Occl-3′-UTR or pGL3-Luc, the levels of luciferase activity were examined and normalized to the mRNA levels to calculate the translation efficiencies. Values were expressed as means ± SEM of data from three separate experiments; * p < 0.05 compared with cells transfected with C-siRNA. (E) Changes in TEER (left panel) and FITC–dextran paracellular permeability (right panel) in cells transfected with siCUGBP1 alone or cotransfected with siCUGBP1 and siRNA targeting occludin (siOccludin). TEER assays were performed on 12-mm Transwell filters 48 h after transfection as described in Materials and Methods; paracellular permeability was assayed by using the membrane-impermeable trace molecule FITC–dextran that was added to the insert medium. Values are the means ± SEM of data from six samples. *,+ p < 0.05 compared with cells transfected with C-siRNA or siCUGBP1, respectively. (F) Changes in TEER and FITC–dextran paracellular permeability in cells transfected with the CUGBP1 expression vector or cotransfected with CUGBP1 and occludin expression vectors. *, # p < 0.05 compared with cells transfected with control vector (vector) or CUGBP1 expression vector alone, respectively. (G) Changes in the levels of CUGBP1 and occludin proteins in cells described in (E) and (F).
Consistent with these findings, the CUGBP1-regulated expression of occludin also modulated the intestinal epithelial barrier function in an in vitro model. Increasing the levels of occludin by silencing CUGBP1 enhanced barrier function, as indicated by an increase in transepithelial electrical resistance (TEER) values (Figure 2E, left) and a decrease in the levels of paracellular flux of fluorescein isothiocyanate (FITC)–dextran (Figure 2E, right). In CUGBP1-silenced cells, transfection with a specific siRNA targeting occludin (siOccludin) not only prevented the induction in occludin levels (Figure 2G, left) but also abolished the enhancement of barrier function. In contrast, lowering occludin levels by CUGBP1 overexpression disrupted barrier function. TEER values decreased in CUGBP1-transfected cells (Figure 2F, left), whereas paracellular permeability increased (Figure 2F, right); these effects were almost completely rescued by overexpression of occludin from a vector lacking the occludin 3′-UTR (and hence resistant to CUGBP1-elicited repression; Figure 2G, right). In addition, the decrease in occludin expression and epithelial barrier function in cells overexpressing CUGBP1 did not result simply from decreased cell growth, because growth inhibition triggered by the removal of serum for 72 h did not alter the levels of occludin expression or paracellular permeability (unpublished data). These results indicate that CUGBP1 represses occludin translation at least partially through a process involving the occludin 3′-UTR, resulting in dysfunction of the epithelial barrier.
CUGBP1 directly interacts with the occludin mRNA via its 3′-UTR
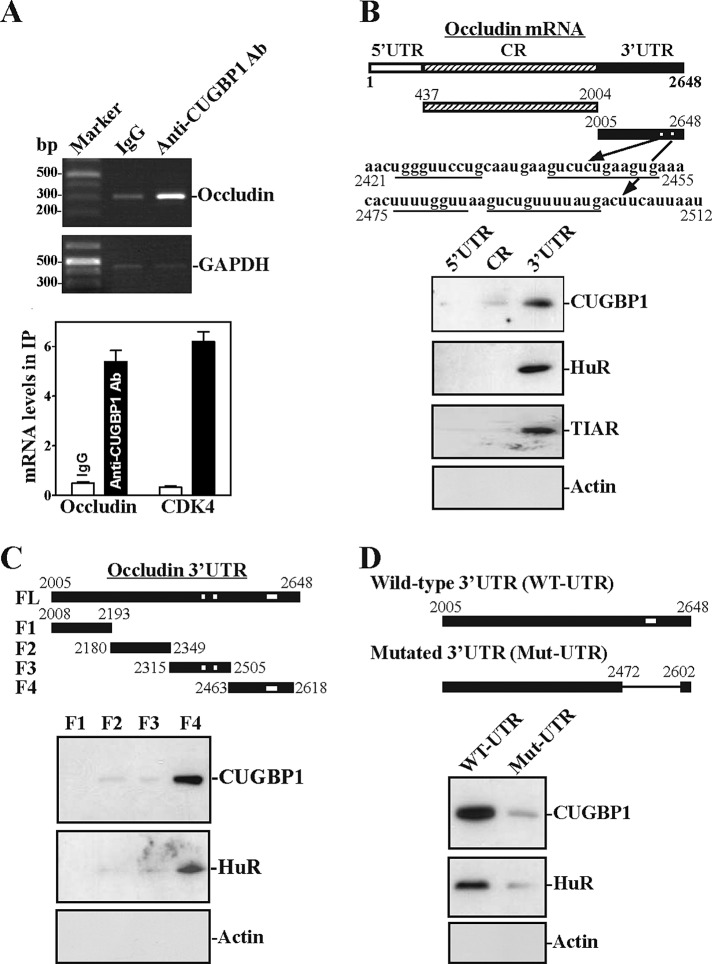
There are several computationally predicted hits of the CUGBP1 motif in the occludin 3′-UTR based on the reported CUGBP1-binding sequences (Tsuda et al.,2009; Rattenbacher et al., 2010), suggesting that the occludin 3′-UTR is a direct target of CUGBP1. To study the interaction of CUGBP1 with occludin mRNA, we first examined whether CUGBP1 bound to the occludin mRNA by performing RNP immunoprecipitation (IP) assays using anti-CUGBP1 antibody under conditions that preserved RNP integrity (Lal et al., 2004; Kawai et al., 2006). As shown in Figure 3A, the occludin PCR products were highly enriched in CUGBP1 samples compared with control immunoglobulin G (IgG) samples. The enrichment of CDK4 PCR product was also examined and served as a positive control, because CDK4 mRNA is a known target of CUGBP1 (Xiao et al.,2011), and the amplification of GAPDH PCR products, to detect GAPDH mRNA, a nonspecific contaminating housekeeping transcript (not a target of CUGBP1), served to monitor the evenness of sample input, as reported previously (Zhang et al., 2009). CUGBP1 was also found to interact with mRNA encoding another TJ protein, JAM-1, but it did not bind to mRNAs encoding ZO-1, ZO-2, claudin-10, and claudin-15 (Supplemental Figure 2). CUGBP1/occludin mRNA associations were further tested by using biotinylated transcripts that spanned the occludin 5′-UTR, coding region (CR) or 3′-UTR (Figure 3B, schematic). Although some occludin mRNAs contain long 3′-UTRs (up to ∼4 kb in length), based on recently updated GenBank information, the occludin mRNA with 3′-UTR spanning positions 2005–2648 is the predominant variant in Caco-2 cells (Supplemental Figure 3). Therefore this study focused on this short occludin 3′-UTR. Following incubation with cytoplasmic lysates, the interaction between the biotinylated occludin transcripts and CUGBP1 was examined by biotin pull-down followed by Western blot analysis (Abdelmohsen et al., 2007; Liu et al., 2009). The occludin 3′-UTR transcripts readily associated with CUGBP1 (Figure 3B, bottom), but CUGBP1 did not interact with the occludin 5′-UTR and CR. Other RBPs such as HuR and TIAR also formed complexes with the occludin 3′-UTR but not with the 5′-UTR and CR. In contrast, none of the occludin partial transcripts (5′-UTR, CR, 3′-UTR) was found to interact with β-actin, included here as a negative control.
FIGURE 3:
CUGBP1 binds the occludin mRNA 3′-UTR via GRE. (A) Association of endogenous CUGBP1 with endogenous occludin mRNA. After IP of RNA-protein complexes from cell lysates using either anti-CUGBP1 antibody (Ab) or control IgG1, RNA was isolated and measured by RT-PCR (top) and Q-PCR (bottom) analyses. Low-level amplification of GAPDH (housekeeping mRNA, which is not a CUGBP1 target) served as negative controls. Values are the means ± SEM from triplicate samples. (B) Representative CUGBP1, HuR, and TIAR immunoblots using the pull-down materials by biotinylated transcripts of the occludin 5′-UTR, CR, or 3′-UTR. Top panel, schematic representation of the biotinylated transcripts used in this study. Cytoplasmic lysates were incubated with 6 μg of biotinylated occludin 5′-UTR, CR, or 3′-UTR, and the resulting RNP complexes were pulled down by using streptavidin-coated beads. The presence of CUGBP1, HuR, or TIAR in the pull-down material was assayed by Western blotting. β-Actin in the pull-down material was also examined and served as a negative control. (C) Binding of CUGBP1 or HuR to different fractions of 3′-UTR of the occludin mRNA. Top panel, schematic representation of the occludin 3′-UTR biotinylated transcripts. After incubation of cytoplasmic lysates with the full-length (FL) or various fractions (F) of the occludin 3′-UTR, the resulting RNP complexes were pulled down, and the abundance of HuR and β-actin proteins in the pull-down material was examined. (D) Changes in binding of occludin 3′-UTR to CUGBP1 and HuR after deletion mutation of the F4. Top panel, schematic representation of the biotinylated transcripts of mutated occludin 3′-UTR used in this study.
To further define the specific CUGBP1 binding regions in the occludin 3′-UTR, various partial biotinylated transcripts spanning the occludin 3′-UTR (spanning positions 2005–2648) were prepared (Figure 3C, schematic). CUGBP1 bound predominantly to the F4 (spanning positions 2463–2618) of the occludin 3′-UTR. There was no detectable binding of CUGBP1 to fragments F1, F2, and F3, although F3 contains two hits of predicted CUGBP1 signature motif. Interestingly, HuR also only interacted with the F4 but not with the F1, F2, and F3. To further define the role of the F4 in CUGBP1/occludin mRNA association, the sequences spanning positions 2472–2602 were internally deleted from the occludin 3′-UTR (Figure 3D, schematic). Internal deletion-mutation of the F4 significantly decreased CUGBP1 binding to the occludin 3′-UTR (Figure 3D, bottom). HuR interaction with the occludin mRNA was also abolished when the F4 sequences were deleted from the occludin 3′-UTR. These results indicate that CUGBP1 interacts with the occludin mRNA via specific RNA segments within the F4 of the 3′-UTR.
To investigate whether CUGBP1 represses occludin translation by interacting specifically with the F4 fragment, we generated reporter constructs that expressed chimeric RNA containing the luciferase and partial transcripts spanning the occludin 3′-UTR (Figure 4A, schematic). Ectopic CUGBP1 overexpression decreased the levels of luciferase reporter gene activity when cells were transfected with the pLucFL (containing full-length occludin 3′-UTR) or pLucF4 (containing the F4) but not with the pLucF1, pLucF2, or pLucF3 in which the GRE-binding sites were deleted. The results presented in Figure 4B further show that decreased CUGBP1/occludin mRNA interaction by CUGBP1 silencing increased the levels of reporter gene activity of pLucFL or pLucF4, but it failed to induce the reporter activity of pLucF1, pLucF2, or pLucF3. To further define the role of the F4 region in regulating occludin mRNA translation, we generated a reporter construct in which the F4 was internally deleted from the occludin 3′-UTR (pLM-UTR; Figure 4C, schematic). The internal deletion-mutation of F4 in the occludin 3′-UTR abrogated the CUGBP1-mediated regulation: neither CUGBP1 overexpression nor CUGBP1 silencing altered the levels of reporter gene activity when cells were transfected with the pLM-UTR. These data suggest that the CUGBP1-binding sites located at the F4 of occludin 3′-UTR are primarily responsible for the regulatory process mediated by CUGBP1.
FIGURE 4:
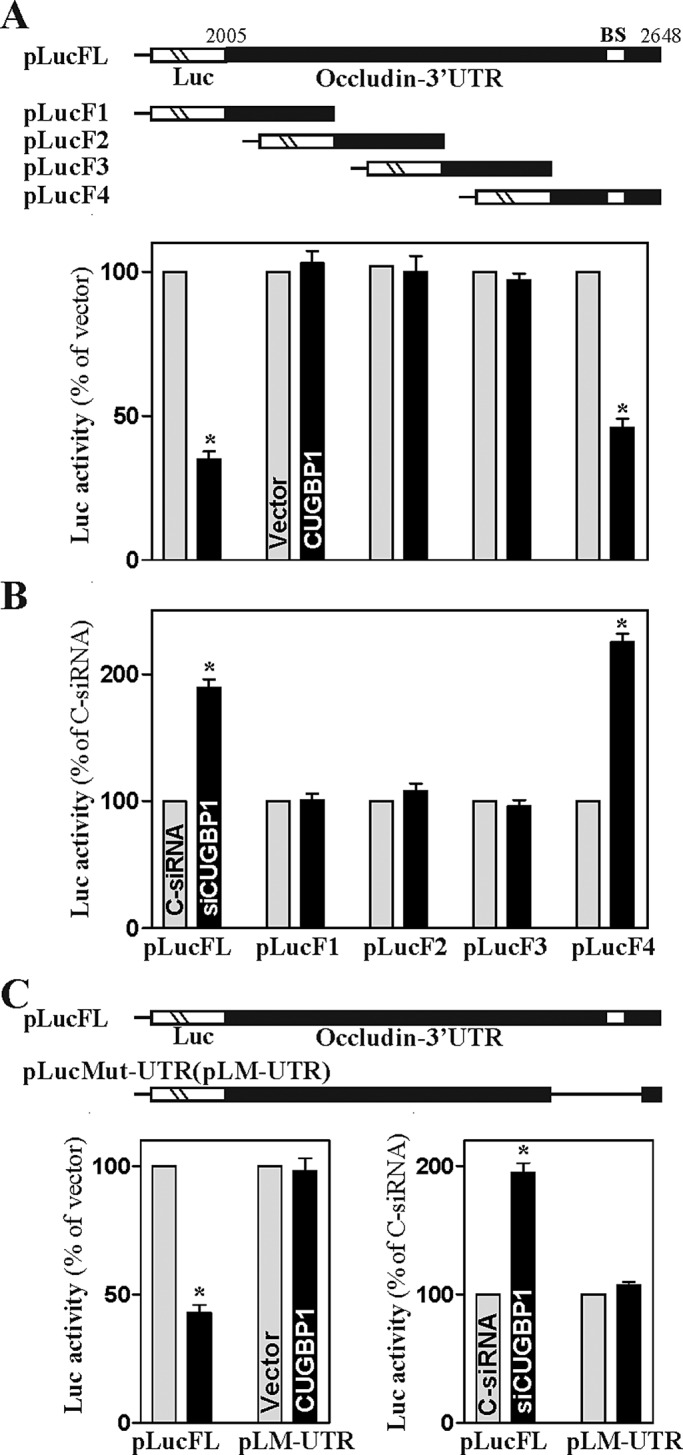
Changes in the levels of occludin 3′-UTR luciferase reporter activity after deletion of CUGBP1-binding sites. (A) Activity of various pLuc-occludin 3′-UTR luciferase reporters with or without CUGBP1-binding site in cells overexpressing CUGBP1. Top panels, schematic of firefly luciferase reporter constructs containing full-length (FL) or different fragments (F) of the occludin 3′-UTR. BS, CUGBP1-binding site. Twenty-four hours after the cells were transfected with either CUGBP1 expression vector or control vector, the cells were further transfected with each of various occludin 3′-UTR luciferase reporter constructs and a Renilla luciferase control reporter. The levels of firefly and Renilla luciferase activities were assayed 24 h later. The results were normalized to the Renilla luciferase activity and are shown as the means ± SEM of data from three separate experiments. * p < 0.05 compared with cells transfected with control vector. (B) Activity of various pLuc-occludin 3′-UTR luciferase reporters after CUGBP1 silencing. Cells were initially transfected with either siCUGBP1 or C-siRNA for 24 h and then with each of various occludin 3′-UTR luciferase reporter constructs in cotransfection with the Renilla luciferase reporter. * p < 0.05 compared with cells transfected with C-siRNA. (C) Effect of deletion of specific CUGBP1-binding site (schematic) in occludin 3′-UTR on luciferase reporter activity after CUGBP1 overexpression or CUGBP1 silencing. * p < 0.05 compared with cells transfected with control vector or C-siRNA.
HuR and CUGBP1 competitively bind to the occludin mRNA
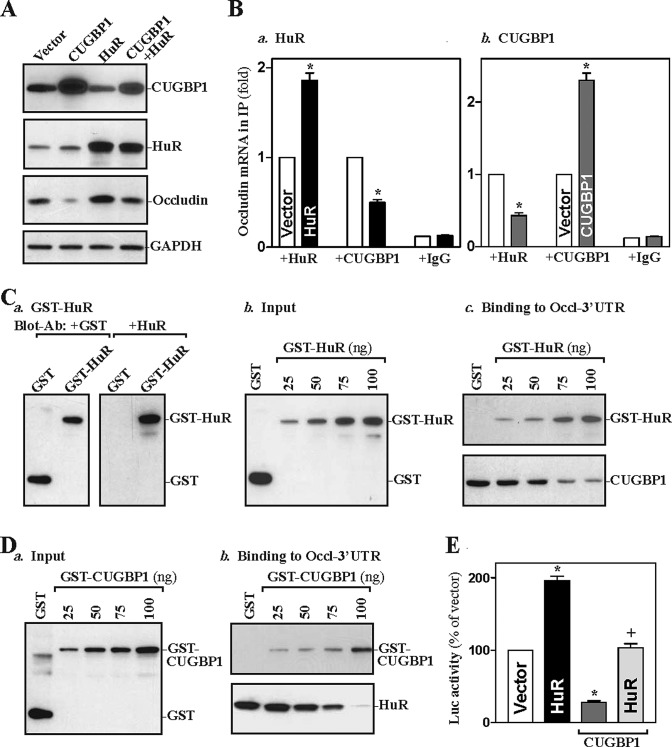
We recently showed that HuR associates with the occludin mRNA and enhances its translation in rat IECs (Yu et al., 2011). As both HuR and CUGBP1 predominantly bound to transcript F4 of the human occludin 3′-UTR (Figure 3), we postulated that HuR and CUGBP1 might compete for interaction with occludin mRNA, thus jointly regulating occludin expression. To test our hypothesis, we examined the effect of ectopic HuR overexpression on CUGBP1 binding to occludin mRNA and its expression. As shown in Figure 5A, transient transfection with the HuR expression vector dramatically increased total HuR protein and also enhanced the expression of occludin protein, although it had no effect on occludin mRNA levels as reported previously (Yu et al., 2011) (unpublished data). HuR overexpression also enhanced the barrier integrity as indicated by a decrease in the levels of paracellular tracer flux as reported previously. Interestingly, cotransfection with HuR and CUGBP1 prevented CUGBP1-induced repression of occludin expression. Increased HuR also induced the levels of HuR/occludin mRNA complex, but it decreased the amount of occludin mRNA associated with CUGBP1 (Figure 5Ba). In contrast, CUGBP1 overexpression not only increased the amount of occludin mRNA associated with CUGBP1, it also decreased the levels of occludin mRNA bound to HuR (Figure 5Bb).
FIGURE 5:
HuR represses occludin mRNA/CUGBP1 association and prevents CUGBP1-induced repression of occludin translation. (A) Representative immunoblots of CUGBP1, HuR, and occludin. After cells were transfected with the CUGBP1 or HuR expression vector alone or cotransfected with both for 48 h, whole-cell lysates were harvested for Western blot analysis. (B) Changes in binding of the occludin mRNA to CUGBP1 and HuR as detected by RNP-IP/Q-PCR analysis: a) cells overexpressing HuR; b) cells overexpressing CUGBP1. Values were means ± SEM from triplicate samples. * p < 0.05 compared with cells transfected with control vector. (C) Effect of GST-HuR added to the binding reaction on association of HuR or CUGBP1 with the occludin 3′-UTR: a) GST-HuR fusion protein identified by anti-GST antibody (left) or recognized by anti-HuR antibody (right); b) protein input in the binding reaction mixture; c) interactions of HuR and CUGBP1 with the occludin 3′-UTR. Various concentrations of GST-HuR were used; the levels of binding complexes were detected by pull-down assays. Three independent experiments were performed showing similar results. (D) Effect of GST-CUGBP1 on association of HuR or CUGBP1 with the occludin 3′-UTR: a) protein input in the binding reaction mixture; b) bindings of HuR and CUGBP1 to the occludin 3′-UTR. (E) Effect of increasing the levels of HuR on occludin translation as measured by occludin 3′-UTR-luciferase reporter assays in cells overexpressing CUGBP1 as described in (A). Values were expressed as means ± SEM of data from three separate experiments. * p < 0.05 compared with cells transfected with control vector.
To further study the competitive binding of HuR and CUGBP1 to the occludin mRNA, we examined the effect of purified glutathione S-transferase (GST)-HuR or GST-CUGBP1 fusion proteins added to the binding reaction mixture on the occludin 3′-UTR association with HuR and CUGBP1. The construct expressing GST-HuR fusion protein was generated, and the expressed GST-HuR fusion protein was harvested, purified, and characterized by immunoblotting analysis using a specific anti-HuR antibody (Figure 5Ca). When increasing concentrations of GST-HuR were added to the binding reaction (Figure 5Cb), the occludin 3′-UTR association with HuR was progressively increased, but its interaction with CUGBP1 was reduced with increasing GST-HuR levels. Neither HuR nor CUGBP1 binding to the occludin 3′-UTR was affected by GST added to the binding reaction. In contrast, increasing the concentrations of GST-CUGBP1 (commercially available from Abnova, Taipei, Taiwan) in the binding reaction mixture dose-dependently increased occludin 3′-UTR association with CUGBP1 but decreased the levels of HuR/occludin 3′-UTR complex (Figure 5D). To study the role of competitive binding of HuR and CUGBP1 in the regulation of occludin translation, results presented in Figure 5E show that ectopic HuR overexpression increased the levels of occludin 3′-UTR luciferase reporter gene activity and also abolished CUGBP1-induced repression of occludin translation. These results indicate that HuR and CUGBP1 competitively bind to the occludin 3′-UTR and that CUGBP1 represses occludin mRNA translation by displacing HuR.
CUGBP1 and HuR modulate occludin mRNA recruitment to P-bodies
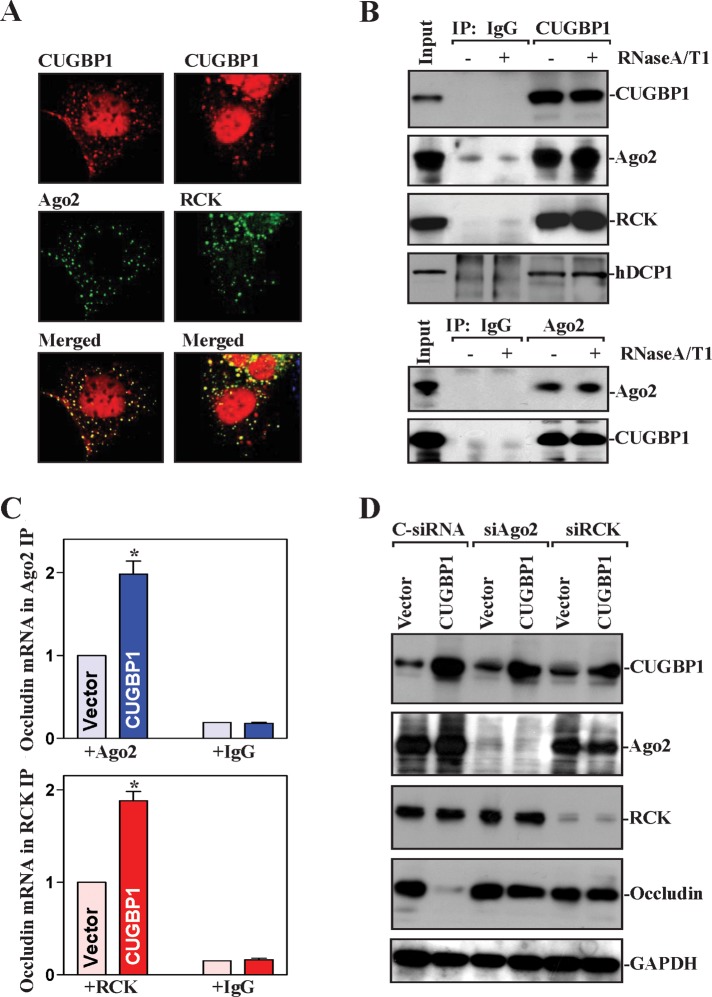
P-bodies are cytoplasmic RNP foci where mRNAs are believed to be sorted for degradation and/or translational repression (Buchan and Parker, 2009; Kulkarni et al., 2010). We hypothesized that CUGBP1 might repress occludin translation by increasing occludin mRNA recruitment to P-bodies, whereas HuR might relieve the translational block by displacing CUGBP1. First, we examined the distribution of CUGBP1 and P-bodies by immunofluorescence and found extensive colocalization of CUGBP1 with the P-body resident proteins Ago2 and RCK (Figure 6A). Results presented in Figure 6B further show that CUGBP1 associated with Ago2, RCK, and hDCP1 (another P-body marker) in an RNA-independent manner, as determined by IP followed by Western blot analysis in the presence of both RNase A and RNase T1. This analysis revealed that, in IECs, CUGBP1 is highly abundant in P-bodies.
FIGURE 6:
CUGBP1 promotes interaction of the occludin mRNA with components of P-bodies. (A) Fluorescence analysis of CUGBP1 colocalization with PB-resident proteins Ago2 and RCK. Red (top), antibody detecting CUGBP1; green (middle), antibodies detecting Ago2 and RCK; yellow (bottom), merging of the two signals. (B) Physical interaction of CUGBP1 with PB component proteins. IP of protein complexes were performed on intact lysates (–) or lysates that had been incubated with both RNase A and RNase T1 (+), using IgG or antibody against CUGBP1 (top) or Ago2 (bottom). The levels of CUGBP1, Ago2, RCK, and hDCP1 were examined by Western blot analysis. (C) Effect of CUGBP1 on occludin mRNA interaction with components of PBs. After cells were transfected with the CUGBP1 expression vector or control vector for 48 h, the association of occludin mRNA with Ago2 or RCK was measured by RNP IP using anti-Ago2 (top) or RCK (bottom) antibodies, which was followed by Q-PCR analysis. Values are means ± SEM of data from three separate experiments. * p < 0.05 compared with cells transfected with control vector. (D) Effect of silencing Ago2 and RCK on occludin expression in cells overexpressing CUGBP1. Cells were transfected with CUGBP1 expression vector or cotransfected with the CUGBP1 and specific siRNA targeting Ago2 (siAgo2) or RCK (siRCK); 48 h later, the levels of CUGBP1, Ago2, RCK, and occludin proteins were assessed by Western blot analysis. Equal loading was monitored by blotting GAPDH.
Second, we determined whether Ago and RCK proteins were functionally linked to the inhibitory effect of CUGBP1 on occludin translation. Increased CUGBP1 enhanced the association of occludin mRNA with P-bodies as indicated by an increase in the levels of occludin mRNA in the Ago2 IP (Figure 6C, top) and RCK IP materials (Figure 6C, bottom) compared with those observed in cells transfected with control vector. As negative control, there were no changes in the levels of occludin mRNA in IgG IP materials between two groups. Furthermore, CUGBP1 did not repress occludin translation in the absence of RCK or Ago2: silencing Ago2 or RCK prevented CUGBP1-induced repression of occludin expression (Figure 6D). In contrast, increased levels of both CUGBP1 and Ago1 or Ago2 by cotransfection with the CUGBP1 vector and vectors expressing hemagglutinin (HA)-tagged Ago1 or HA-Ago2 synergistically inhibited occludin expression (Supplemental Figure 4). In keeping with the suppression of gene expression by Ago proteins, ectopic overexpression of HA-Ago1 or HA-Ago2 alone also modestly repressed occludin expression.
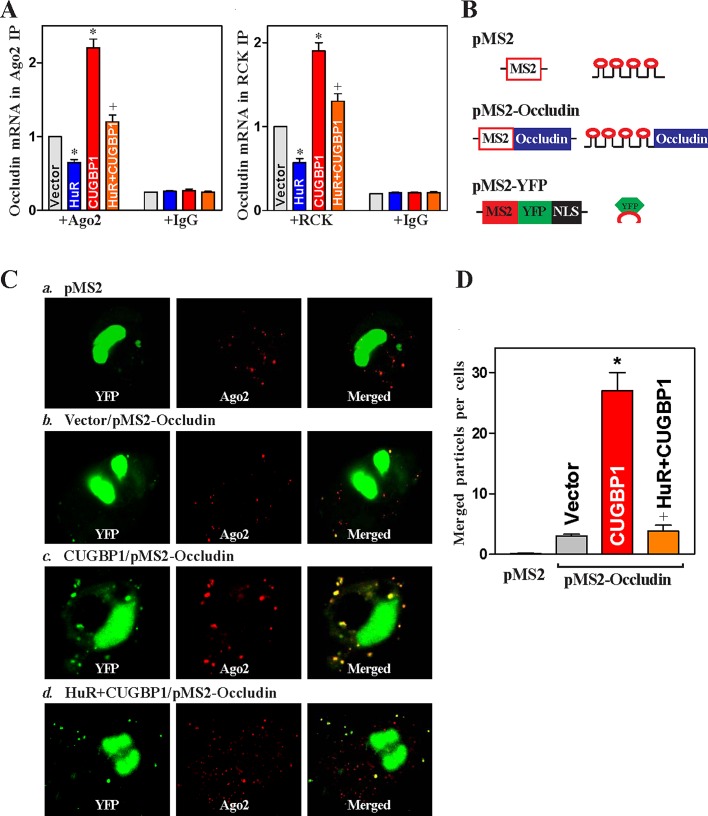
Third, we examined whether increasing the levels of HuR altered CUGBP1-induced association of the occludin mRNA with P-bodies. As shown in Figure 7A, ectopic HuR overexpression decreased the levels of occludin mRNA associated with Ago2 and RCK, suggesting the inhibition of occludin mRNA association with P-bodies by HuR. Consistent with our previous studies (Yu et al., 2011), HuR overexpression did not affect the levels of total occludin mRNA (unpublished data). Furthermore, the increased HuR also prevented the CUGBP1-induced interaction of occludin mRNA with P-bodies. When cells were cotransfected with the HuR and CUGBP1 expression vectors, the levels of occludin mRNA associated with Ago2 or RCK decreased significantly compared with those observed in cells transfected with the CUGBP1 expression vector alone.
FIGURE 7:
HuR inhibits CUGBP1-induced occludin mRNA recruitment to P-bodies. (A) Interaction of occludin mRNA with components of P-bodies in cells overexpressing CUGBP1 or HuR alone, or both. After cells were transfected with the HuR or CUGBP1 expression vector alone or they were cotransfected with both HuR and CUGBP1 vectors for 48 h, the associations of occludin mRNA with Ago2 (left) and RCK (right) were measured by RNP-IP using anti-Ago2 or RCK antibodies, or control IgG followed by Q-PCR analysis. Values are means ± SEM of data from three separate experiments. *,+ p < 0.05 compared with cells transfected with control vector or cells transfected with the CUGBP1 expression vector, respectively. (B) Schematic of the plasmids used for the visualization of occludin mRNA. pMS2 and pMS2-occludin expressed MS2 and MS2-occludin RNAs, each containing 24 tandem MS2 hairpins; pMS2-YFP expressed a fusion fluorescent protein (MS2-YFP) capable of detecting MS2-containing RNA.. (C) Images of occludin mRNA colocalization with P-bodies: a) cells transfected with pMS2 alone; b) cells transfected with control vector and pMS2-occludin; c) cells transfected with CUGBP1 expression vector and pMS2-occludin; d) cells cotransfected with HuR and CUGBP1 expression vectors and with pMS2-occludin. Using confocal microscopy, MS2 and MS2-occludin mRNA were visualized using MS2-YFP (green fluorescence); red, Ago2 (P-body marker) signals; yellow, colocalized red and green signals. Three experiments were performed that showed similar results. (D) Quantification of the number of merged signals indicative of colocalization of MS2-occludin RNA and Ago2 signals in cells described in (C). Values are the means ± SEM of data from six samples. * p < 0.05 compared with cells transfected with control vector; + p < 0.05 compared with cells transfected with the CUGBP1 expression vector.
Finally, we investigated directly whether the localization of occludin mRNA in P-bodies plays an important role in translational repression by CUGBP1 and its regulation through HuR by examining the subcytoplasmic localization of occludin mRNA after different treatments. The reporter construct pMS2-Occludin, which expressed a chimeric RNA (MS2-Occludin) comprising the occludin 3′-UTR and 24 tandem MS2 RNA hairpins (Figure 7B), was prepared as described previously (Lee EK et al., 2010; Cui et al., 2012). Cotransfection of pMS2-Occludin together with plasmid pMS2-YFP, which expressed the chimeric fluorescent protein MS2-yellow fluorescent protein (YFP) with a nuclear localization signal (NLS), allowed us to track the subcellular localization of the chimeric MS2-Occludin RNA (as the complex MS2-YFP/MS2-Occludin) as well as the control MS2 RNA (as the complex MS2-YFP/MS2) by confocal microscopy. Signals of the P-body marker Ago2 were detected in the same cells. As shown, the control MS2 RNA appeared to be exclusively nuclear in cells (Figure 7Ca) due to the presence of the NLS in the chimeric protein MS2-YFP. As shown in Figure 7, Cb and D, some MS2-Occludin RNA was retained in the cytoplasm, colocalizing with some Ago2 signals in cells transfected with control vector. However, CUGBP1 overexpression increased the colocalization of MS2-Occludin RNA and Ago2 signals (colocalization results in yellow signals in the merged image in Figure 7, Cc and D), suggesting that increasing the levels of CUGBP1 enhanced the association of occludin mRNA with P-bodies. Interestingly, the colocalization of MS2-Occludin RNA and Ago2 signals was lost when cells were cotransfected with the HuR and CUGBP1 expression vectors (Figure 7, Cd and D). Together, these data support a novel regulatory model whereby CUGBP1 represses occludin translation at least partially by increasing the recruitment of occludin mRNA to P-bodies, whereas HuR promotes occludin translation by competing for the interaction of CUGBP1 with occludin 3′-UTR, thus preventing the recruitment of occludin mRNA to P-bodies.
DISCUSSION
Occludin plays an important role in the TJ barrier (Yu et al., 2005; Shen and Pili, 2008; Suzuki et al., 2009) and in many other cellular functions (Seth et al., 2007; Du et al., 2010; Cummins, 2012), but the exact mechanisms that control occludin abundance remain largely unknown. Our recent studies demonstrated that HuR associates with the occludin mRNA via its 3′-UTR AREs and enhances occludin translation in vitro as well as in vivo (Yu et al., 2011). The present study further identified occludin mRNA as a target of CUGBP1 and found that occludin translation decreased with CUGBP1 association. Interestingly, altering the interaction of CUGBP1 with occludin mRNA affected the levels of occludin mRNA associated with HuR. As shown, CUGBP1 overexpression increased CUGBP1/occludin mRNA complex, lowered occludin mRNA associated with HuR, and repressed occludin translation, whereas increased HuR promoted its binding to occludin mRNA and inhibited association of occludin transcript with CUGBP1, thus enhancing occludin translation. These findings advance our understanding of the molecular mechanisms underlying the regulation of occludin gene expression and indicate that competitive binding of CUGBP1 and HuR to the occludin mRNA controls its translation and therefore modulates TJ barrier function.
CUGBP1 is a member of the CELF family of RBPs and contains three RNA recognition motifs through which it binds to specific mRNAs bearing GREs in their 5′-UTRs, 3′-UTRs, or CRs (Vlasova and Bohjanen, 2008; Tsuda et al., 2009). Human CUGBP1 and its homologues in chickens, zebrafish, frogs, flies, and worms have been long known to regulate gene expression at posttranscriptional levels and to control important developmental processes and cellular functions (Paillard et al., 1998; Shen and Pili, 2008; Vlasova and Bohjanen, 2008). Knockout of the CUGBP1 homologue ETR1 in Caenorhabditis elegans is lethal and impairs muscle development (Milne and Hodgkin, 1999); CUGBP1 knockout in mice is also potently lethal, although the few mice that are born display severe fertility defects (Kress et al., 2007), suggesting that CUGBP1 coordinates the expression of important developmental genes. Our present results provide evidence showing that CUGBP1 regulates TJ barrier function by interacting with HuR. CUGBP1 competes with HuR for binding to occludin mRNA in the cytoplasm, and the dynamic balance of their binding actions plays an important role in the regulation of occludin translation. It is likely that HuR is essential for promoting occludin expression, whereas CUGBP1 negatively regulates occludin expression; deregulation of this balance might contribute to gut barrier dysfunction in patients with the pathological condition “leaky gut.” In support of this notion, repression of HuR-mediated occludin translation is shown to play an important role in the pathogenesis of gut barrier dysfunction in conditions of septic stress (Yu et al., 2011), although the involvement of CUGBP1 in this process remains unknown. In addition, the occludin mRNA joins a few mRNAs encoding c-Myc (Liao et al., 2007), p21 (Lal et al., 2004), and amyloid precursor protein (APP) (Lee EK et al., 2010), whose translation is determined by competitive interactions between translation-promoting and translation-inhibiting RBPs.
Our results indicate that CUGBP1 associated with the 3′-UTR of occludin but not with the 5′-UTR or CR in cells of human origin (Figure 3). In some instances, CUGBP1 was also shown to bind to the 5′-UTR of target mRNAs (Huichalaf et al., 2010). The occludin 3′-UTR does not contain any high-affinity CUGBP1-binding site such as the canonical GRE (defined as UGUUUGUUUGU), but there are several GU repeats and CUG repeats in the occludin 3′-UTR that were also recently recognized as GREs and CUGBP1-interacting RNA (Tsuda et al., 2009; Rattenbacher et al., 2010). Moreover, only the sequence spanning positions 2463–2618 (F4 fragment) within the occludin 3′-UTR was found to be functional, because both the repression of occludin by CUGBP1 overexpression and the stimulation of the reporter activity by CUGBP1 silencing were completely prevented when this fragment was mutated (Figure 4). HuR also predominantly associated with the same occludin 3′-UTR element (F4 fragment), which also contains several AREs. However, it is still unclear whether CUGBP1 and HuR interact with the occludin 3′-UTR through a distinct nonoverlapping binding site or whether there are common sites for both CUGBP1 and HuR, because in some instances CUGBP1 also can associate with AREs (Tsuda et al., 2009; Rattenbacher et al., 2010). We did not further characterize the specific occludin 3′-UTR nucleotides with which CUGBP1 or HuR interacts, because those experiments would require more specialized biochemical, crystallographic, and molecular methods than those used here.
The most significant findings from the present study are that CUGBP1 and HuR jointly regulate occludin translation by altering the recruitment of occludin mRNA to P-bodies where nontranslating mRNAs accumulate (Buchan and Parker, 2009; Kulkarni et al., 2010). CUGBP1 overexpression increased occludin mRNA levels in P-bodies as measured by RNP IP using an antibody against the P-body resident proteins Ago2 and RCK (Figure 6) and by measuring colocalization of the occludin mRNA in P-bodies (Figure 7). Furthermore, silencing of Ago2 or RCK decreased CUGBP1-mediated repression of occludin translation (Supplemental Figure 3), whereas increased levels of both CUGBP1 and Ago2 or RCK by cotransfection synergistically inhibited occludin expression, suggesting that CUGBP1 represses occludin translation at least partially by recruiting the occludin mRNA to P-bodies. Consistent with the current findings, CUGBP1 is also shown to repress CDK4 translation by increasing CDK4 mRNA translocation to P-bodies (Xiao et al., 2011). In contrast, HuR overexpression decreased the levels of occludin mRNA associated with Ago2 and RCK and reduced the colocalization of occludin mRNA in P-bodies in cells overexpressing CUGBP1, indicating that HuR competes with CUGBP1 for the binding to occludin mRNA, thereby blocking induced occludin mRNA translocation to P-bodies by CUGBP1.
Although the exact mechanism by which P-bodies repress the translation of resident mRNAs remains to be fully investigated, P-bodies contain many proteins that are associated with RNA-induced silencing complex and mRNA decay. These proteins include all four human Ago proteins, GW182 (TNRC6A) together with its two human paralogues (TNRC6B and TNRC6C), two RNA helicases (RCK and MOV10), decapping enzymes (DCP1 and DCP2), mRNA deadenylation factors (such as the CCR4–CAF-1–Not complex), activators of decapping (Dhh1/RCK/p54, Pat1, Scd6/RAP55, Edc3, and the LSm1–7 complex), and exonucleases (such as XRN-1) (Chu and Rana, 2006; Kulkarni et al., 2010; Saito et al., 2011). A compelling example of the transient association of RBP target mRNAs in P-bodies was reported previously (Lee JE et al., 2010), which showed that the endogenous APP mRNA and the RBP fragile X mental retardation protein (FMRP) colocalize to P-bodies, linked to the inhibition of APP translation. The same mRNA is released from P-bodies when its translation is activated by another RBP heterogeneous nuclear RNP (hnRNP) C. Consistently, the endogenous cationic amino acid transporter-1 (Cat-1) mRNA and miR-122 localize to P-bodies in liver cells and are linked to the inhibition of Cat-1 translation (Bhattacharyya et al., 2006). However, the dynamic turnover of CUGBP1 association with the occludin mRNA in P-bodies in the presence or absence of HuR remains unknown and is the focus of our ongoing studies.
In summary, CUGBP1 and HuR are pivotal posttranscriptional regulators of occludin production and modulate occludin translation in opposite directions. CUGBP1 represses occludin mRNA translation through direct interaction with the occludin 3′-UTR rather than its 5′-UTR or CR, whereas HuR competes with CUGBP1 for binding to the occludin 3′-UTR, promoting occludin translation. On the basis of the present findings that the level of occludin mRNA in the P-body was increased by CUGBP1 and that this induction was prevented by HuR, we propose a novel model in which CUGBP1 represses occludin translation by increasing occludin mRNA recruitment to P-bodies, whereas HuR promotes occludin translation by blocking occludin mRNA translocation to P-bodies via displacing CUGBP1. Because occludin is critical for normal function of TJs, the modulation of occludin mRNA translation by CUGBP1 and HuR plays an important role in the regulation of TJ integrity under various pathophysiological conditions.
MATERIALS AND METHODS
Chemicals and cell culture
Tissue culture medium and dialyzed fetal bovine serum were obtained from Invitrogen (Carlsbad, CA), and biochemicals were obtained from Sigma (St. Louis, MO). The antibodies recognizing CUGBP1, HuR, occludin, and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and BD Biosciences (San Jose, CA). The secondary antibody conjugated to horseradish peroxidase was purchased from Sigma. The 12-mm Transwell filters were obtained from Costar (Clayton, MO); l-[35S]methionine and l-[35S]cysteine were obtained from NEN Radiopharmaceutical (Billerica, MA). Caco-2 cells were purchased from the American Type Culture Collection (Manassas, VA) and maintained in standard culture conditions as described (Chen et al., 2008). Stable Cdx2-transfected IECs (IEC-Cdx2L1) were developed from IEC-6 cells and maintained as described previously (Suh and Traber, 1996; Chen et al., 2008). Before experiments, IEC-Cdx2L1 cells were grown in DMEM containing 4 mM isopropylthio-β-galactoside for 16 d to induce cell differentiation as described earlier (Suh and Traber, 1996).
Plasmid construction
CUGBP1 expression vector was purchased from Origene (Rockville, MD), and the HuR expression vector was described previously (Abdelmohsen et al., 2007). The chimeric firefly luciferase reporter construct containing the occludin 3′-UTR was generated as described (Liu et al., 2009; Yu et al., 2011). The full-length 3′-UTR of human occludin mRNA was amplified and subcloned into the pGL3-Luc plasmid (Promega, Madison, WI) at the Xba1 site to generate the chimeric pGL3-Luc-Occludin-3′UTR (Luc-Occl-3′-UTR) reporter construct. The sequence and orientation of the luciferase reporter were verified by DNA sequencing and enzyme digestion. Transient transfections were performed using Lipofectamine Reagent following the manufacturer's recommendations (Invitrogen, Carlsbad, CA). The luciferase reporter constructs were transfected into cells along with phRL-null, a Renilla luciferase control reporter vector from Promega, to monitor transfection efficiencies as described (Liu et al., 2009). Luciferase activity was measured using the Dual Luciferase Assay System (Promega). To measure translational changes (“translational efficiency”), the ratio of Firefly luciferase to Renilla luciferase was further normalized to the levels of Firefly and Renilla mRNAs. Both pcDNA-MS2 and pcDNA-MS2-YFP plasmids were described previously (Lee JE et al., 2010; Cui et al., 2012), and the full-length of human occludin 3′-UTR was inserted into pcDNA-MS2 at the XhoI site. The expression vector containing HA-tagged Ago1 or Ago2 protein was constructed as described previously (Xiao et al., 2011).
RNAi
CUGBP1 was silenced by transfection with specific siRNA as described (Xiao et al., 2011). The siRNAs specifically targeting CUGBP1 mRNA (siCUGBP1) and C-siRNA were purchased from Santa Cruz Biotechnology. For each 60-mm cell culture dish, 15 μl of either the 20 μM stock duplex siCUGBP1 or C-siRNA was used. Forty-eight hours after transfection using Lipofectamine, cells were harvested for analysis.
Reverse transcription and quantitative real-time PCR analyses
Total RNA was isolated by using an RNeasy Mini Kit (Qiagen, Valencia, CA) and used in reverse transcription (RT) and PCR amplifications as described (Xiao et al., 2007b). PCR primers for detecting occludin mRNA were TTTGTGGGACAAGGAACACA (sense) and GCAGGTGCTCTTTTTG AAGG (antisense). The levels of GAPDH PCR product were assessed to monitor the evenness in RNA input in RT-PCR samples. Real-time quantitative PCR (Q-PCR) analysis was performed using 7500-Fast Real-Time PCR Systems with specific primers, probes, and software (Applied Biosystems, Foster City, CA).
Western blotting analysis
Whole-cell lysates were prepared using 2% SDS, sonicated, and centrifuged (12,000 rpm) at 4°C for 15 min. The supernatants were boiled for 5 min and size-fractionated by SDS–PAGE (7.5% acrylamide). After transferring proteins onto nitrocellulose filters, the blots were incubated with primary antibodies recognizing occludin, CUGBP1, HuR, Ago2, or RCK; following incubations with secondary antibodies, immunocomplexes were developed by using chemiluminescence.
Analysis of newly translated protein and polysome analysis
New synthesis of occludin protein was measured by l-[35S]methionine and l-[35S]cysteine incorporation assays as described (Liu et al., 2009). Cells were incubated with 1 mCi (1 Ci = 37 GBq) l-[35S]methionine and l-[35S]cysteine per 60-mm plate for 60 min, whereupon cells were lysed using RIPA buffer. IPs were carried out for 1 h at 4 ºC using either a polyclonal antibody recognizing occludin or IgG1 (BD PharMingen, Franklin Lakes, NJ). Following extensive washes in TNN buffer (50 mM Tris-HCl [pH 7.5], 250 mM NaCl, 5 mM EDTA, 0.5% NP-40), the immunoprecipitated material was resolved by 10% SDS–PAGE, transferred onto polyvinylidene difluoride filters, and visualized with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Polysome analysis was performed as described (Xiao et al., 2011). Briefly, cells were incubated for 15 min in cycloheximide at 0.1 mg/ml and then lifted by scraping in 1 ml of PEB lysis buffer (0.3 M NaCl, 15 mM MgCl2, 15 mM Tris-HCl [pH 7.6], 1% Triton X-100, heparin at 1 mg/ml, and cycloheximide at 0.1 mg/ml) and lysed on ice for 10 min. Nuclei were pelleted (10,000 × g, 10 min), and the resulting supernatant was fractionated through a 10–50% linear sucrose gradient to fractionate cytoplasmic components according to their molecular weights. The eluted fractions were prepared with a fraction collector (Brandel, Gaithersburg, MD), and their quality was monitored at 254 nm using a UV-6 detector (ISCO, Lincoln, NE). After the RNA in each fraction was extracted with 8 M guanidine–HCl, the levels of each individual mRNA were quantified by Q-PCR analysis in each of the fractions, and their abundance represented as a percentage of the total mRNA in the gradient.
Biotin pull-down assays
The synthesis of biotinylated transcripts and analysis of RBPs bound to biotinylated RNA were performed as described (Xiao et al., 2007b; 2011). cDNA from Caco-2 cells was used as a template for PCR amplification of the 5′-UTR, CR, and 3′-UTR of occludin. The 5′ primers contained the T7 RNA polymerase promoter sequence (T7) CCAAGCTTCTAATACGACTCAC TATAGGGAGA. To prepare the occludin 5′-UTR template (spanning positions 1–436), oligonucleotides (T7)CAATCAGCCATGTCATCCAG and TCAAACAACTTGGCATCAGC were used. To prepare the occludin CR template (spanning positions 437–2004), oligonucleotides (T7)CAATCAGCCATGTCATCCAG and TCAAACAACTTGGCATCAGC were used. To prepare the occludin 3′-UTR template (spanning positions 2005–2648), oligonucleotides (T7)GCTGATGCCAAGTTGTTTGA and TTTAAGAAAAACCTCTTTTAACCATTT were used. All sequences of oligonucleotides for preparation of various short RNA probes for mapping the occludin 3′-UTR are described in Supplemental Table S1. PCR-amplified products were used as templates to transcribe biotinylated RNAs by using T7 RNA polymerase in the presence of biotin–cytidine 5′-triphosphate as described (Zhang et al., 2009). Biotinylated transcripts (6 μg) were incubated with 120 μg of cytoplasmic lysates for 30 min at room temperature. Complexes were isolated with paramagnetic streptavidin-conjugated Dynabeads (Dynal, Oslo, Norway) and analyzed by Western blot analysis.
RNP IP assays
To assess the association of endogenous CUGBP1 or HuR with endogenous occludin mRNA, IP of RNP complexes were performed as described (Zhang et al., 2009; Wang et al., 2010). Twenty million cells were collected per sample, and lysates were used for IP for 4 h at room temperature in the presence of excess (30 μg) IP antibody against CUGBP1 or HuR, or IgG (negative control). RNA in IP materials was used in RT followed by PCR and Q-PCR analysis to detect the presence of occludin and GAPDH mRNAs.
Immunofluorescence staining
Immunofluorescence was performed as described (Cui et al., 2012). Cells were fixed using 3.7% formaldehyde, and the rehydrated samples were incubated overnight at 4 ºC with primary antibody recognizing CUGBP1, Ago2, or RCK diluted in blocking buffer, then incubated with secondary antibody conjugated with Alexa Fluor 594 or FITC 488 (Molecular Probes, Eugene, OR) for 2 h at room temperature. Images were processed using an Axio Observer microscope (ZEISS, Oberkochen, Germany) with LSM 510 Meta (ZEISS) image-processing software.
Paracellular tracer flux assay and transepithelial electrical resistance measurement
Flux assays were performed on the 12-mm Transwell as described previously (Guo et al., 2003). FITC–dextran, a membrane-impermeable molecule, served as the paracellular tracer and was added to a final concentration of 0.25 mM to the apical bathing wells that contained 0.5 ml of medium. The basal bathing well had no added tracers and contained 1.5 ml of the same flux assay medium as in the apical compartment. All flux assays were performed at 37°C, and the basal medium was collected 2 h after addition of FITC–dextran. The concentration of the FITC–dextran in the basal medium was determined using a fluorescence plate reader with an excitation wavelength of 490 nm and an emission wavelength of 530 nm. TEER was measured with an epithelial voltometer under open circuit conditions (WPI, Sarasota, FL) as described previously (Chen et al., 2008), and the TEER of all monolayers was normalized to that of control monolayers in the same experiment.
Statistics
Values are means ± SEM from three to six samples. Autoradiographic results and studies of immunofluorescence staining were repeated three times. The significance of the difference between means was determined by analysis of variance. The level of significance was determined by using Duncan's multiple-range test (Harter, 1960).
Supplementary Material
Acknowledgments
We thank J.L. Martindale for experimental support. This work was supported by merit review grants (to J.-Y. Wang and J.N. Rao) from the Department of Veterans Affairs and by National Institutes of Health (NIH) grants DK-57819, DK-61972, and DK-68491 (to J.-Y. Wang). J.-Y. Wang is a Senior Research Career Scientist, Medical Research Service, U.S. Department of Veterans Affairs. M. Gorospe is supported by the National Institute on Aging—Intramural Research Program, NIH.
Abbreviations used:
- 3′-UTR
3′-untranslated region
- APP
amyloid precursor protein
- ARE
AU-/U-rich element
- Cat-1
cationic amino acid transporter-1
- CDK4
cyclin-dependent kinase 4
- C-siRNA
control siRNA
- CUGBP1
CUG-binding protein 1
- FITC
fluorescein isothiocyanate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GRE
GU-rich element
- GST
glutathione S-transferase
- HA
hemagglutinin
- IEC
intestinal epithelial cell
- IgG
immunoglobulin G
- IP
immunoprecipitation
- miRNA
microRNA
- NLS
nuclear localization signal
- P-bodies
processing bodies
- Q-PCR
quantitative PCR
- RBP
RNA-binding protein
- RNP
ribonucleoprotein
- RT
reverse transcription
- siCUGBP1
small interfering RNA targeting the CUGBP1 mRNA
- siRNA
small interfering RNA
- TEER
transepithelial electrical resistance
- TJ
tight junction
- YFP
yellow fluorescent protein
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-07-0531) on November 14, 2012.
REFERENCES
- Abdelmohsen K, et al. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;25:543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya SN, Habermacher R, Martine U, Closs El, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xiao L, Rao JN, Zou T, Liu L, Bellavance E, Gorospe M, Wang JY. JunD represses transcription and translation of the tight junction protein zona occludens-1 modulating intestinal epithelial barrier function. Mol Biol Cell. 2008;19:3701–3712. doi: 10.1091/mbc.E08-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Merzdorf C, Paul DL, Goodenough DA. COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J Cell Biol. 1997;138:891–899. doi: 10.1083/jcb.138.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CY, Rana TM. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 2006;4:e210. doi: 10.1371/journal.pbio.0040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YH, Xiao L, Rao JN, Zou T, Liu L, Chen Y, Turner DJ, Gorospe M, Wang JY. miR-503 represses CUG-binding protein 1 translation by recruiting CUGBP1 mRNA to processing bodies. Mol Biol Cell. 2012;23:151–162. doi: 10.1091/mbc.E11-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins PM. Occludin: one protein, many forms. Mol Cell Biol. 2012;32:242–250. doi: 10.1128/MCB.06029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du D, et al. The tight junction protein, occludin, regulates the directional migration of epithelial cells. Dev Cell. 2010;18:52–63. doi: 10.1016/j.devcel.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherzi R, Lee KY, Briata P, Wegmuller D, Moroni C, Karin M, Chen CY. A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Mol Cell. 2004;14:571–583. doi: 10.1016/j.molcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Guo X, Rao JN, Liu L, Zou T, Turner DJ, Bass BL, Wang JY. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am J Physiol Cell Physiol. 2003;285:C1174–C1187. doi: 10.1152/ajpcell.00015.2003. [DOI] [PubMed] [Google Scholar]
- Harter JL. Critical values for Duncan's new multiple range test. Biometrics. 1960;16:671–685. [Google Scholar]
- Houseley J, Tollervey D. The many pathways of RNA degradation. Cell. 2009;136:763–776. doi: 10.1016/j.cell.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Huichalaf C, et al. Expansion of CUG RNA repeats causes stress and inhibition of translation in myotonic dystrophy 1 (DM1) cells. FASEB J. 2010;24:3706–3719. doi: 10.1096/fj.09-151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iakova P, Wang GL, Timchenko L, Michalak M, Pereira-Smith OM, Smith JR, Timchenko NA. Competition of CUGBP1 and calreticulin for the regulation of p21 translation determines cell fate. EMBO J. 2004;23:406–417. doi: 10.1038/sj.emboj.7600052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Lal A, Yang X, Galban S, Mazan-Mamczarz K, Gorospe M. Translational control of cytochrome c by RNA-binding proteins TIA-1 and HuR. Mol Cell Biol. 2006;26:3295–3307. doi: 10.1128/MCB.26.8.3295-3307.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene JD. RNA regulons: coordination of post-transcriptional events. Nature Rev Genet. 2007;8:533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- Kress C, Gautier-Courteille C, Osborne HB, Babinet C, Paillard L. Inactivation of CUG-BP1/CELF1 causes growth, viability, and spermatogenesis defects in mice. Mol Cell Biol. 2007;27:1146–1157. doi: 10.1128/MCB.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni M., Ozgur S, Stoecklin G. On track with P-bodies. Biochem Soc Trans. 2010;38:242–251. doi: 10.1042/BST0380242. [DOI] [PubMed] [Google Scholar]
- Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J. 2004;23:3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EK, et al. hnRNP C promotes APP translation by competing with FMRP for APP mRNA recruitment to P bodies. Nature Struct Mol Biol. 2010;17:732–739. doi: 10.1038/nsmb.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Lee JY, Wilusz J, Tian B, Wilusz CJ. Systematic analysis of cis-elements in unstable mRNAs demonstrates that CUGBP1 is a key regulator of mRNA decay in muscle cells. PloS One. 2010;5:e11201. doi: 10.1371/journal.pone.0011201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao B, Hu Y, Brewer G. Competitive binding of AUF1 and TIAR to MYC mRNA controls its translation. Nature Struct Mol Biol. 2007;14:511–518. doi: 10.1038/nsmb1249. [DOI] [PubMed] [Google Scholar]
- Liu L, Rao JN, Zou T, Xiao L, Wang PY, Turner DJ, Gorospe M, Wang JY. Polyamines regulate c-Myc translation through Chk2-dependent HuR phosphorylation. Mol Biol Cell. 2009;20:4885–4898. doi: 10.1091/mbc.E09-07-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiando AM, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;1:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- Milne CA, Hodgkin J. ETR-1, a homologue of a protein linked to myotonic dystrophy, is essential for muscle development in Caenorhabditis elegans. Curr Biol. 1999;9:1243–1246. doi: 10.1016/s0960-9822(99)80504-1. [DOI] [PubMed] [Google Scholar]
- Paillard L, Omilli F, Legagneux V, Bassez T, Maniey D, Osborne HB. EDEN and EDEN-BP, a cis element and an associated factor that mediate sequence-specific mRNA deadenylation in Xenopus embryos. EMBO J. 1998;17:278–287. doi: 10.1093/emboj/17.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21:1200–1213. doi: 10.1091/mbc.E09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh DR, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 2011;193:565–582. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattenbacher B, Beisang D, Wiesner DL, Jeschke JC, von Hohenberg M, St Louis-Vlasova IA, Bohjanen PR. Analysis of CUGBP1 targets identifies GU-repeat sequences that mediate rapid mRNA decay. Mol Cell Biol. 2010;30:3970–3980. doi: 10.1128/MCB.00624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Kondo E, Matsushita M. microRNA 130 family regulates the hypoxia response signal through the P-body protein DDX6. Nucleic Acids Res. 2011;39:6086–6099. doi: 10.1093/nar/gkr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulzke JD, Fromm M. Tight junctions: molecular structure meets function. Ann NY Acad Sci. 2009;1165:1–6. doi: 10.1111/j.1749-6632.2009.04925.x. [DOI] [PubMed] [Google Scholar]
- Seth A, Sheth P, Elias BC, Rao RK. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem. 2007;282:11487–11498. doi: 10.1074/jbc.M610597200. [DOI] [PubMed] [Google Scholar]
- Shen L, Pili R. Posttranscription regulation of prostate cancer growth. Cancer J. 2008;14:46–53. doi: 10.1097/PPO.0b013e318162108a. [DOI] [PubMed] [Google Scholar]
- Su L, Mruk DD, Lui WY, Lee WM, Cheng CY. P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK) Proc Natl Acad Sci USA. 2011;108:19623–19628. doi: 10.1073/pnas.1111414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol. 1996;16:619–625. doi: 10.1128/mcb.16.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKCη regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci USA. 2009;106:61–66. doi: 10.1073/pnas.0802741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, et al. Structural basis for the seqeunce-specific RNA-recognition mechanism of human CUGB-BP1 RRM3. Nucleic Acids Res. 2009;37:5151–5166. doi: 10.1093/nar/gkp546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR. Intestinal mucosal barrier function in health and disease. Nature Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Vlasova IA, Bohjanen PR. Posttranscriptional regulation of gene networks by GU-rich elements and CELF proteins. RNA Biol. 2008;5:201–207. doi: 10.4161/rna.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PY, Rao JN, Zou T, Liu L, Xiao L, Yu TX, Turner DJ, Gorospe M, Wang JY. Post-transcriptional regulation of MEK-1 by polyamines through the RNA-binding protein HuR modulating intestinal epithelial apoptosis. Biochem J. 2010;426:293–306. doi: 10.1042/BJ20091459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wade P, Mandell KJ, Akyildiz A, Parkos CA, Mrsny RJ, Nusrat A. Raf 1 represses expression of the tight junction protein occludin via activation of the zinc-finger transcription factor slug. Oncogene. 2007;26:1222–1230. doi: 10.1038/sj.onc.1209902. [DOI] [PubMed] [Google Scholar]
- Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Cui YH, Rao JN, Zou T, Liu L, Smith A, Turner DJ, Gorospe M, Wang JY. Regulation of cyclin-dependent kinase 4 translation through CUG-binding protein 1 and microRNA-222 by polyamines. Mol Biol Cell. 2011;22:3055–3069. doi: 10.1091/mbc.E11-01-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Rao JN, Zou T, Liu L, Marasa BS, Chen J, Turner DJ, Passaniti A, Wang JY. Induced JunD in intestinal epithelial cells represses CDK4 transcription through its proximal promoter region following polyamine depletion. Biochem J. 2007a;403:573–581. doi: 10.1042/BJ20061436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Rao JN, Zou T, Liu L, Marasa BS, Chen J, Turner DJ, Zhou H, Gorospe H, Wang JY. Polyamines regulate the stability of activating transcription factor-2 mRNA through RNA-binding protein HuR in intestinal epithelial cells. Mol Biol Cell. 2007b;18:4579–4590. doi: 10.1091/mbc.E07-07-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323–1333. doi: 10.1053/j.gastro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231–C1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- Yu TX, Wang PY, Rao JN, Zou T, Liu L, Xiao L, Gorospe M, Wang JY. Chk2-dependent HuR phosphorylation regulates occludin mRNA translation and epithelial barrier function. Nucleic Acids Res. 2011;39:8472–8487. doi: 10.1093/nar/gkr567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lee JE, Wilusz J, Wilusz CJ. The RNA-binding protein CUGBP1 regulates stability of tumor necrosis factor mRNA in muscle cells: implications for myotonic dystrophy. J Biol Chem. 2008;283:22457–22463. doi: 10.1074/jbc.M802803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zou T, Rao JN, Liu L, Xiao L, Wang PY, Cui YH, Gorospe M, Wang JY. Stabilization of XIAP mRNA through the RNA binding protein HuR regulated by cellular polyamines. Nucleic Acids Res. 2009;37:7623–7637. doi: 10.1093/nar/gkp755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou T, Mazan-Mamczarz K, Rao JN, Liu L, Marasa BS, Zhang AH, Xiao L, Pullmann R, Gorospe M, Wang JY. Polyamine depletion increases cytoplasmic levels of RNA-binding protein HuR leading to stabilization of nucleophosmin and p53 mRNAs. J Biol Chem. 2006;281:19387–19394. doi: 10.1074/jbc.M602344200. [DOI] [PubMed] [Google Scholar]
- Zou T, Rao JN, Liu L, Xiao L, Yu TX, Jiang P, Gorospe M, Wang JY. Polyamines regulate the stability of JunD mRNA by modulating the competitive binding of its 3′ untranslated region to HuR and AUF1. Mol Cell Biol. 2010;30:5021–5032. doi: 10.1128/MCB.00807-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.