Abstract
Background
DJ-1 can induce the tumor cell proliferation and invasion via down-regulating PTEN in many malignant tumors, and correlated to prognostic significance. However, the tumorigenesis role and clinical significance of DJ-1 in supraglottic squamous cell carcinoma (SSCC) is unclear. We aimed to evaluate the DJ-1 the relationship between DJ-1 and clinicopathological data including patient survival.
Methods
The expression of DJ-1 and PTEN in SSCCs (52) and adjacent non-cancerous tissues (42) was assessed by immunohistochemistry (IHC), and the relationship between DJ-1 and clinicopathological data was analyzed.
Results
DJ-1 was detected mainly in SSCCs (88.5%) and less frequently in adjacent non-cancerous tissues (21.0%). PTEN expression was detected in 46.2% of SSCCs and in 90.5% of adjacent non-cancerous tissues. DJ-1 expression was linked to nodal status (P = 0.009), a highly significant association of DJ-1 expression with shortened patient overall survival (5-year survival rate 88.0% versus 53.9%; P = 0.007; log rank test) was demonstrated.
Conclusions
Our data suggested that DJ-1 over-expression was linked to nodal status, and might be an independent prognostic marker for patients with SSCC.
Keywords: DJ-1, PTEN, Tumorigenesis, Supraglottic squamous cell carcinoma, Overall survival
Background
Laryngeal squamous cell carcinoma (LSCC), one of the most common malignancies of the head and neck region, accounts for approximately 2.4% of new malignancies worldwide every year [1,2]. Supraglottic squamous cell carcinoma (SSCC), one advanced type of LSCC, is often accompanied by lymph node metastasis or even systemic metastasis, and usually results in substantial annual morbidity and mortality. Hence, to predict the biology of the tumor and the course of the disease in individual patient is importance for appropriate therapy and patient surveillance. The evaluation of a SSCC patient’s prognosis and predictive markers is primarily based on the clinical tumor-node-metastasis (TNM) staging [3]. However, patients with SSCC with similar clinical stage classifications usually have different clinical outcomes, suggesting that TNM staging is not sufficient for precisely determining a SSCC prognosis. Therefore, identifying specific biomarkers which have diagnostic and prognostic value for SSCC remains a priority.
DJ-1, a mitogendependent oncogene, was firstly reported by Nagakubo in 1997 [4]. Recent studies indicated that DJ-1 is closely related to the proliferation, metastasis, occurrence, and prognosis of the malignant tumors [2,5-13]. In our recent study of glottic squamous cell carcinoma [2], DJ-1 was shown as an independent molecular marker for poor prognosis, and was correlated with pT status and tumor grading. In other LSCC studies [2], DJ-1was also identified as an activator of cell proliferation, and was related to T stage and poor prognosis [14,15]. However, the relationship between DJ-1 and lymph node metastasis of LSCC have not been revealed both in our and others’ studies.
Phosphatase and tensin homologue (PTEN) is a dual-specific phosphatase that plays an important role in tumorigenesis and reduced PTEN expression is associated with cell survival, proliferation, tumor invasion, and tumor-node-metastasis (TNM) stage [14-20]. Furthermore, LSCC studies showed that reduced PTEN expression is also related to cell proliferation, tumor invasion, lymphatic metastasis, and TNM stage [21-23]. Recent studies have showed that PTEN might be regulated by DJ-1 in several cancers, such as renal cell carcinoma, breast cancer, bladder carcinoma, and ovarian carcinoma [8,24-26]. Kim RH [8] found that DJ-1 could activate cell proliferation and transformation by negatively regulating PTEN expression in breast cancer cells. The above evidence suggests that the DJ-1-induced PTEN down-regulation may be involved in LSCC progression and act as activator of the invasion process in LSCC.
To date, the relationship between DJ-1 and clinicopathological data including patient survival in SSCC have not been revealed. The aim of this study was to investigate the relationship between DJ-1 and clinicopathological data including patient survival.
Material and methods
Patients
A total of fifty seven SSCC patients were eligible for this study. 2 and 3 patients were excluded because of insufficient tissue samples and incomplete follow-up data, respectively. 52 subjects with SSCCs and 42 subjects with adjacent non-cancerous tissues were thus examined. These patients underwent surgery in our department from January 1996 to September 2006, and clinical follow-up data were completed. The average observation time for overall survival was 62 months for patients still alive at the time of analysis, and ranged from 7 to 122 months. Twenty-eight patients (53.8%) died during follow-up. Tumor tissues from the resected specimens and adjacent non-cancerous tissues were used as normal control (tumor and adjacent non-cancerous tissues were confirmed by pathologic examination). The tissues used for immunohistochemistry were fixed in 4% polyformaldehyde and embedded in paraffin. All specimens and clinical data in this study were procured, handled, and maintained according to the protocols approved by Institutional Review Board (IRB), and all of the patients who participated in the study provided informed consent.
The principal inclusion criteria were primary squamous cell carcinoma of the supraglottis type only, no history of previous malignant disease, and no history of previous radio- or chemotherapy. The main clinical and pathologic characteristics of the patients are presented in Table 1: 49 (94.2%) were male and with a median age was 59.0 years (ranging from 39–81 years of age). Clinical staging and the anatomic site of the tumors were assessed according to the 6th edition of the Union Internationale Contre Cancer (UICC) tumor-node-metastasis classification of malignant tumors.
Table 1.
Clinicopathological parameters of the tumor set
| Number of cases | % | ||
|---|---|---|---|
| Gender |
Male |
49 |
94.2 |
| |
Female |
3 |
5.8 |
| Age(y) |
≤ 61 |
25 |
48.1 |
| |
> 61 |
27 |
51.9 |
| pT status |
Tis |
3 |
5.8 |
| |
T1 |
1 |
1.9 |
| |
T2 |
11 |
21.2 |
| |
T3 |
24 |
46.1 |
| |
T4a |
12 |
23.1 |
| |
T4b |
1 |
1.9 |
| pN status |
N0 |
24 |
46.2 |
| |
N1 |
16 |
30.7 |
| |
N2 |
12 |
23.1 |
| Stage (UICC) |
0 |
3 |
5.8 |
| |
I |
1 |
1.9 |
| |
II |
6 |
11.6 |
| |
III |
24 |
46.2 |
| |
IVA |
17 |
32.6 |
| |
IVB |
1 |
1.9 |
| Tumor grade |
G1 |
17 |
32.6 |
| |
G2 |
21 |
40.5 |
| G3 | 14 | 26.9 | |
Immunohistochemical staining
Immunohistochemistry staining was carried out as previously described [2]. DJ-1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, California, USA) and PTEN monoclonal antibody (Cell Signaling Technology, Denver). DJ-1 staining was graded according to the intensity and extent of staining of the epithelium as previously described, and immunostaining of all slides were evaluated in a blinded manner [2].
Fluorescent immunohistochemistry
To better confirm the cellular location and the relationship between DJ-1 and PTEN in SSCC tissues, fluorescent immunohistochemistry was performed as described previously [27].
Statistical analysis
Statistical analysis was performed with the SPSS software (SPSS Standard version 13.0, SPSS). The association of DJ-1 protein expression with SSCC patient’s clinico-pathological features and the recorrelation between molecular features detected with each other were by the χ2 test or Fischer’s exact test. For survival analysis, we analyzed all SSCC patients by Kaplan-Meier analysis. Log-rank test was used to compare different survival curves. Multivariate survival analysis was performed on all parameters the Cox regression model. P < 0.05 was considered to be statistically significant.
Results
DJ-1 and PTEN expression in SSCCs and adjacent non-cancerous tissues
DJ-1 was detected mainly in SSCCs and less frequently in adjacent non-cancerous tissues. In comparison, PTEN staining of adjacent non-cancerous tissues was stronger and more common than that of SSCCs (Figure 1A). To better study the cellular location and the relationship between DJ-1 and PTEN in SSCCs, fluorescent immunohistochemistry was performed, and the results showed that strong expression of DJ-1 is found in cytoplasm of SSCC tumor cells, while poor staining of PTEN was observed in cytoplasm of SSCC tumor cells, and that strong expression of PTEN is found in cytoplasm of adjacent non-cancerous cells, while poor staining of DJ-1 was observed in cytoplasm of adjacent non-cancerous cells (Figure 1B). A summary of DJ-1 and PTEN expression in normal and SSCC tissues is given in Table 2. DJ-1 expression was detected in 88.5% of SSCCs and in 21.0% of adjacent non-cancerous tissues examined, whereas PTEN expression was detected in 46.2% of SSCCs and in 90.5% of adjacent non-cancerous tissues. Moreover, 65.4% of SSCCs were assessed as high grade DJ-1 staining, whereas 78.6% of adjacent non-cancerous tissue had either no or low-grade DJ-1 staining. A significant difference in grade of DJ-1 expression was demonstrated between SSCCs and adjacent non-cancerous tissues (P < 0.001). Further more, we find that DJ-1 expression was linked to lymph nodal status (P = 0.042), pT status (P = 0.037), and UICC stage (P = 0.027), and there was no significant association of overall DJ-1 staining intensity with patient age and tumor grading (Table 3).
Figure 1.
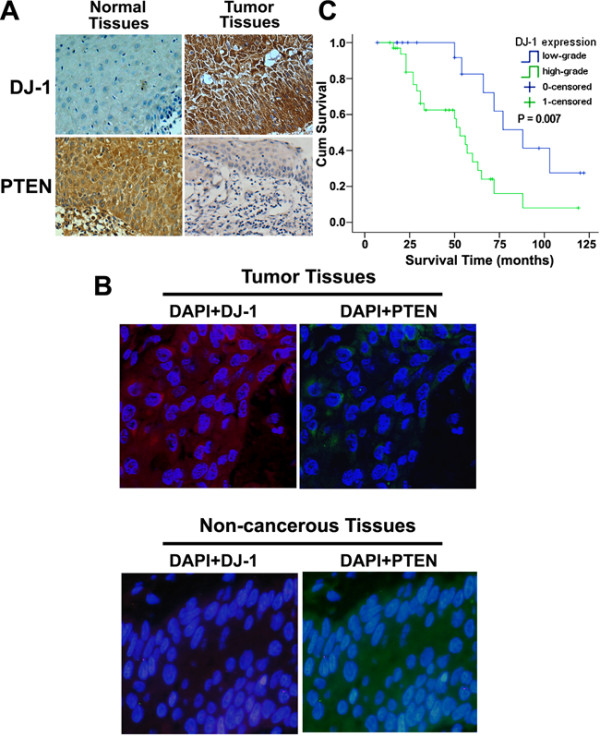
Expression of DJ-1 in SSCC clinical samples and univariate survival analysis. A. DJ-1 staining showed low expression of DJ-1 in adjacent non-cancerous tissues, and high expression of DJ-1 was found in cytoplasm of SSCC tumor tissues (IHC, 400X). In comparison, PTEN staining of adjacent non-cancerous tissues was stronger and more common than that of SSCCs (IHC, 400X). B. Fluorescent-IHC clearly demonstrates that strong expression of DJ-1 is found in cytoplasm of SSCC tumor cells, while poor staining of PTEN was observed in cytoplasm of SSCC tumor cells, and that strong expression of PTEN is found in cytoplasm of adjacent non-cancerous cells, while poor staining of DJ-1 was observed in cytoplasm of adjacent non-cancerous cells (IHC, 400X). C. Kaplan-Meier curves with univariate analyses (log-rank) comparing tumors with low- grade DJ-1 expression with those with high-grade DJ-1 expression. Patients with low-grade DJ1 expression had a cumulative 5-year survival rate of 88.0% compared with 53.9% for patients with high-grade DJ-1 expression.
Table 2.
DJ-1 and PTEN expression in adjacent non-cancerous tissues and SSCCs
|
DJ-1 expression,n (%) |
PTEN expression,n (%) |
Total | |||||
|---|---|---|---|---|---|---|---|
| Absent | Low | High | Absent | Low | High | ||
| SSCC |
6 (11.5%) |
12 (23.1%) |
34 (65.4%) |
28 (53.8%) |
16 (30.8%) |
8 (15.4%) |
52 |
| Normal | 22 (52.4) | 11 (26.2%) | 9 (21.4%) | 4 (9.5%) | 10 (23.8%) | 28 (66.7%) | 42 |
DJ-1: χ2 = 22.917; df = 2; P = 0.000. SSCC, supraglottic squamous cell carcinoma.
PTEN: χ2 = 29.769; df = 2; P = 0.000.
Table 3.
Relationship between DJ-1 expression and various clinicopathological factors
| Characteristic | All cases |
DJ-1 Low-grade |
DJ-1 High-grade |
P |
|---|---|---|---|---|
| All carcinomas |
52 |
18 |
34 |
|
| Age |
|
|
|
1.000 |
| ≤ 61 |
25 |
9 |
16 |
|
| > 61 |
27 |
9 |
18 |
|
| pT status |
|
|
|
0.003 |
| Tis-2 |
15 |
10 |
5 |
|
| T3-4 |
37 |
8 |
29 |
|
| pN status |
|
|
|
0.009 |
| N0 |
24 |
13 |
11 |
|
| N1-3 |
28 |
5 |
23 |
|
| UICC stage |
|
|
|
0.022 |
| 0-II |
10 |
7 |
3 |
|
| III-IV |
42 |
11 |
31 |
|
| Histological grade |
|
|
|
0.758 |
| G1 |
17 |
5 |
12 |
|
| G2-3 | 35 | 13 | 22 |
DJ-1 is a prognostic marker for SSCC
In univariate survival analysis, cumulative survival curves were calculated according to the Kaplan-Meier method (Table 4). Differences in survival were assessed with the long-rank test. The conventional prognostic parameters pT status, lymph node status, and disease stage according to UICC reached significance for overall survival. DJ-1 positivity was associated with overall survival (P = 0.007). Figure 1C illustrates the impact of DJ-1 expression on survival times.
Table 4.
Univariate survival analyses (Kaplan-Meier): survival time of all patients with SSCC according to clinicopathological factors and DJ-1 expresion
|
Overall survial | ||||
|---|---|---|---|---|
| Characteristic | No.of cases | No.of events | 5-year survival Rate ( ± SE) | P |
| DJ-1 expression |
|
|
|
0.007 |
| Low-grade |
18 |
7 |
88.0 ± 8.0 |
|
| High-grade |
34 |
21 |
53.9 ± 5.7 |
|
| Age |
|
|
|
0.244 |
| ≤61 |
25 |
11 |
72.2 ± 7.9 |
|
| > 61 |
27 |
17 |
58.5 ± 7.0 |
|
| pT status |
|
|
|
0.037 |
| Tis-2 |
15 |
5 |
87.0 ± 10.3 |
|
| T3-4 |
37 |
23 |
57.5 ± 5.5 |
|
| pN status |
|
|
|
0.042 |
| N0 |
24 |
12 |
76.0 ± 7.7 |
|
| N1-3 |
28 |
16 |
52.8 ± 5.6 |
|
| UICC stage |
|
|
|
0.027 |
| 0-II |
10 |
3 |
99.5 ± 8.4 |
|
| III-IV |
42 |
25 |
58.5 ± 5.4 |
|
| Histological grade |
|
|
|
0.597 |
| G1 |
17 |
9 |
68.9 ± 9.4 |
|
| G2-3 | 35 | 19 | 62.8 ± 6.4 | |
The multivariate analysis was based on the Cox regression model to test the influence of each parameter on overall survival. We tested the impact of DJ-1 expression on overall survival. The results showed that the overall survival time was significantly dependent on DJ-1 expression, pT status, and UICC stage.
Discussion
The current TNM staging and histopathological grading systems are useful prognostic indicators for SSCC [3]. However, they have limitations with regard to providing critical information regarding patient prognosis. Patients with the same clinical stage and/or pathological grade of SSCC often display considerable variability in disease recurrence and survival [1,28]. Therefore, new objective measures and biomarkers are necessary to effectively differentiating patients with favorable outcomes from those with less favorable outcomes. Molecular biomarkers in conjunction with standard TNM and histopathological strategies have the potential to predict prognoses more effectively.
DJ-1 protein is coded by exons 27, contains 189 amino acids, and weights about 20 kD, and was firstly defined as an oncogene candidate in 1997 [4]. Recent studies showed that DJ-1 is expressed highly in many types of human malignancies [2,5-15]. Lines of evidence have also suggested that the over-expression of DJ-1 is correlated with more aggressive clinical behaviors of pancreatic, esophageal and lung cancers [10-13]. However, in our recent glottic squamous cell carcinoma study [2], DJ-1 has only been identified as a prognostic marker and activator of cell proliferation, and the expression of DJ-1 was not correlated to clinical lymph node metastasis. This non-invasive role of DJ-1 in glottic squamous cell carcinoma which is contradictory to the invasive role of DJ-1 in other malignancies may be attributed to the clinical and biological behavior of glottic squamous cell carcinoma, as this type of LSCC was poorly invaded in clinic. So, in order to identify whether DJ-1 also play the invasive role in LSCC, SSCC, the more aggressive type of LSCC, was selected in the present study.
Recently, several studies showed that PTEN in human malignancies is associated with cell proliferation, tumor invasion, and TNM stage, and can be down-regulated by DJ-1 in several cancers, such as renal cancer, breast cancer, bladder cancer, and ovarian cancer [8,24-26]. In 2005, Kim RH [8] found that DJ-1 could activate cell proliferation and transformation by negatively regulating PTEN expression in breast cancer cells. In 2012, Lee H [25] showed that over-expression of DJ-1 and loss of PTEN are associated with invasive urothelial carcinoma of urinary bladder. Taken together, we hypothesized that DJ-1 would promote migration and invasion of SSCC via down-regulating the expression of PTEN, and may associated with clinical lymph node status in SSCC.
In the immunohistochemistry-based study, we examined the expression of both DJ-1 and PTEN in SSCC tissue versus adjacent non-cancerous tissue. Our results indicate that the expression of DJ-1 was mainly in SSCCs and less frequently in adjacent non-cancerous tissues, whereas PTEN staining of adjacent non-cancerous tissues was stronger and more common than that of SSCCs (Figure 1A, B). Furthermore, an significant difference in grade of DJ-1 expression was demonstrated between SSCCs and adjacent non-cancerous tissues (P < 0.001), and pT status (P = 0.003) and nodal status (P = 0.009) were linked to DJ-1 expression. This scenario is similar to that observed in other type of human cancers [5-13], and the relationship between nodal status and DJ-1 expression in SSCC revealed that DJ-1 may play an invasive role in SSCC. In both univariate and multivariate survival analysis, our study suggests that DJ-1, a prognostic marker for GSCC in our previous study [2], is also a prognostic marker in SSCC (Figure 1C). Thus, expression of DJ-1 appears to have the potential to predict SSCC patients’ outcome.
Conclusions
In conclusion, to the authors’ knowledge, the current study is the first to demonstrate the relationship between DJ-1 and clinicopathological data including lymph node status in SSCC. Furthermore, survival analysis showed that DJ-1 is an independent prognostic maker for reduced patient survival in SSCC. Collectively, the present findings would provide important information into the future design of individualized therapeutic strategies for SSCC with different DJ-1 expression levels.
Competing interests
All the authors have no competing interests.
Authors’ contributions
XLZ performed the experiments and analyzed the data. ZFW and WBL participated in the experiments. HWZ contributed to the acquisition of the data, WJH and YHW has made substantial contribution to collected tissue samples, XLZ and WPW wrote the manuscript, WPW conceived and designed the experiment. All authors have read and approved the final manuscript.
Contributor Information
Xiao-Lin Zhu, Email: lxz.0508@yahoo.com.cn.
Zhang-Feng Wang, Email: yiren_wzf@hotmail.com.
Wen-Bin Lei, Email: leiwb2003@yahoo.com.cn.
Hui-Wen Zhuang, Email: zhwgz@hotmail.com.
Wei-Jian Hou, Email: houwj75@tom.com.
Yi-Hui Wen, Email: yihui-wen@hotmail.com.
Wei-Ping Wen, Email: wwp1901@yahoo.com.cn.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 81072224), the Natural Science Foundation of Guangdong Province (Grant no. S2011040000263), and the Guangdong Medical Science and Technology Research Fund (Grant no. A2011167).
References
- Marioni G, Marchese-Ragona R, Cartei G, Marchese F, Staffieri A. Current opinion in diagnosis and treatment of laryngeal carcinoma. Cancer Treat Rev. 2006;32:504–515. doi: 10.1016/j.ctrv.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhu XL, Wang ZF, Lei WB, Zhuang HW, Jiang HY, Wen WP. DJ-1: a novel independent prognostic marker for survival in glottic squamous cell carcinoma. Cancer Sci. 2010;101:1320–1325. doi: 10.1111/j.1349-7006.2010.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsasser O. Revision of classification of laryngeal cancer; is it long overdue? (Proposals for an improved TN-classification) J Laryngol Otol. 1992;106:197–204. doi: 10.1017/S0022215100119073. [DOI] [PubMed] [Google Scholar]
- Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- Le Naour F, Misek DE, Krause MC, Deneux L, Giordano TJ, Scholl S, Hanash SM. Proteomics-based identification of RS/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res. 2001;7:3328–3335. [PubMed] [Google Scholar]
- MacKeigan JP, Clements CM, Lich JD, Pope RM, Hod Y, Ting JP. Proteomic profiling drug-induced apoptosis in non-small cell lung carcinoma: identification of RS/DJ-1 and RhoGDIalpha and rhogdialpha. Cancer Res. 2003;63:6928–6934. [PubMed] [Google Scholar]
- Shi W, Zhang X, Pintilie M, Ma N, Miller N, Banerjee D, Tsao MS, Mak T, Fyles A, Liu FF. Dysregulated PTEN-PKB and negative receptor status in human breast cancer. Int J Cancer. 2003;104:195–203. doi: 10.1002/ijc.10909. [DOI] [PubMed] [Google Scholar]
- Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, DeLuca C, Liepa J, Zhou L, Snow B, Binari RC, Manoukian AS, Bray MR, Liu FF, Tsao MS, Mak TW. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- González-Polo R, Niso-Santano M, Morán JM, Ortiz-Ortiz MA, Bravo-San Pedro JM, Soler G, Fuentes JM. Silencing DJ-1 reveals its contribution in paraquat-induced autophagy. J Neurochem. 2009;109:889–898. doi: 10.1111/j.1471-4159.2009.06020.x. [DOI] [PubMed] [Google Scholar]
- He X, Zheng Z, Li J, Ben Q, Liu J, Zhang J, Ji J, Yu B, Chen X, Su L, Zhou L, Liu B, Yuan Y. DJ-1 promotes invasion and metastasis of pancreatic cancer cells by activating SRC/ERK/uPA. Carcinogenesis. 2012;33:555–562. doi: 10.1093/carcin/bgs002. [DOI] [PubMed] [Google Scholar]
- Bai J, Guo C, Sun W, Li M, Meng X, Yu Y, Jin Y, Tong D, Geng J, Huang Q, Qi J, Fu S. DJ-1 may contribute to metastasis of non-small cell lung cancer. Mol Biol Rep. 2012;39:2697–2703. doi: 10.1007/s11033-011-1024-7. [DOI] [PubMed] [Google Scholar]
- He XY, Liu BY, Yao WY, Zhao XJ, Zheng Z, Li JF, Yu BQ, Yuan YZ. Serum DJ-1 as a diagnostic marker and prognostic factor for pancreatic cancer. J Dig Dis. 2011;12:131–137. doi: 10.1111/j.1751-2980.2011.00488.x. [DOI] [PubMed] [Google Scholar]
- Yuen HF, Chan YP, Law S, Srivastava G, El-Tanani M, Mak TW, Chan KW. DJ-1 could predict worse prognosis in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:3593–3602. doi: 10.1158/1055-9965.EPI-08-0214. [DOI] [PubMed] [Google Scholar]
- Shen Z, Ren Y, Ye D, Guo J, Kang C, Ding H. Significance and relationship between DJ-1 gene and surviving gene expression in laryngeal carcinoma. Eur J Histochem. 2011;55:e9. doi: 10.4081/ejh.2011.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Jiang Z, Ye D, Xiao B, Zhang X, Guo J. Growth inhibitory effects of DJ-1-small interfering RNA on laryngeal carcinoma Hep-2 cells. Med Oncol. 2011;28:601–607. doi: 10.1007/s12032-010-9474-7. [DOI] [PubMed] [Google Scholar]
- Hou P, Ji M, Xing M. Association of PTEN gene methylation with genetic alterations in the phosphatidylinositol 3-kinase/AKT signaling pathway in thyroid tumors. Cancer. 2008;113:2440–2447. doi: 10.1002/cncr.23869. [DOI] [PubMed] [Google Scholar]
- Bedolla R, Prihoda TJ, Kreisberg JI, Malik SN, Krishnegowda NK, Troyer DA, Ghosh PM. Determining risk of biochemical recurrence in prostate cancer by immunohistochemical detection of PTEN expression and Akt activation. Clin Cancer Res. 2007;13:3860–3867. doi: 10.1158/1078-0432.CCR-07-0091. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Cunha IW, Coudry RA, Fonseca FP, Torres CH, Soares FA, Squire JA. FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br J Cancer. 2007;97:678–685. doi: 10.1038/sj.bjc.6603924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhail M, Velazquez E, Shapiro R, Berman R, Pavlick A, Sorhaindo L, Spira J, Mir C, Panageas KS, Polsky D, Osman I. PTEN expression in melanoma: relationship with patient survival, Bcl-2 expression, and proliferation. Clin Cancer Res. 2005;11:5153–5157. doi: 10.1158/1078-0432.CCR-05-0397. [DOI] [PubMed] [Google Scholar]
- Pérez-Tenorio G, Alkhori L, Olsson B, Waltersson MA, Nordenskjöld B, Rutqvist LE, Skoog L, Stål O. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13:3577–3584. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]
- Li X, Wang HL, Peng X, Zhou HF, Wang X. miR-1297 mediates PTEN expression and contributes to cell progression in LSCC. Biochem Biophys Res Commun. 2012;427:254–260. doi: 10.1016/j.bbrc.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Bai W, Wang L, Ji W, Gao H. Expression profiling of supraglottic carcinoma: PTEN and thrombospondin 2 are associated with inhibition of lymphatic metastasis. Acta Otolaryngol. 2009;129:569–574. doi: 10.1080/00016480802294351. [DOI] [PubMed] [Google Scholar]
- Guney K, Ozbilim G, Derin AT, Cetin S. Expression of PTEN protein in patients with laryngeal squamous cell carcinoma. Auris Nasus Larynx. 2007;34:481–486. doi: 10.1016/j.anl.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Sitaram RT, Cairney CJ, Grabowski P, Keith WN, Hallberg B, Ljungberg B, Roos G. The PTEN regulator DJ-1 is associated with hTERT expression in clear cell renal cell carcinoma. Int J Cancer. 2009;125:783–790. doi: 10.1002/ijc.24335. [DOI] [PubMed] [Google Scholar]
- Lee H, Choi SK, Ro JY. Overexpression of DJ-1 and HSP90α, and loss of PTEN associated with invasive urothelial carcinoma of urinary bladder: Possible prognostic markers. Oncol Lett. 2012;3:507–512. doi: 10.3892/ol.2011.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B, Hadar R, Schlossberg A, Sternlicht T, Slipicevic A, Skrede M, Risberg B, Flørenes VA, Kopolovic J, Reich R. Expression and clinical role of DJ-1, a negative regulator of PTEN, in ovarian carcinoma. Hum Pathol. 2008;39:87–95. doi: 10.1016/j.humpath.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Sun W, Guo MM, Han P, Lin JZ, Liang FY, Tan GM, Li HB, Zeng M, Huang XM. Id-1 and the p65 subunit of NF-κB promote migration of nasopharyngeal carcinoma cells and are correlated with poor prognosis. Carcinogenesis. 2012;33:810–817. doi: 10.1093/carcin/bgs027. [DOI] [PubMed] [Google Scholar]
- Rafferty MA, Fenton JE, Jones AS. The history, aetiology and epidemiology of laryngeal carcinoma. Clin Otolaryngol Allied Sci. 2001;26:442–446. doi: 10.1046/j.1365-2273.2001.00507.x. [DOI] [PubMed] [Google Scholar]


