Abstract
Participation of the intervascular transport system within the rice stem during cadmium (Cd) partitioning was investigated by characterizing 109Cd behaviour in the shoot. In addition, 45Ca, 32P, and 35S partitioning patterns were analysed for comparison with that of 109Cd. Each tracer was applied to the seedling roots for 15min, and the shoots were harvested either at 15min (i.e. immediately after tracer application) or at 48h. Distribution patterns of each element at 15min were studied to identify the primary transport pathway before remobilization was initiated. 32P was preferentially transported to completely expanded leaf blades having the highest transpiration rate. The newest leaf received minimal amounts of 32P. In contrast, the amount of 35S transported to the newest leaf was similar to that transported to the other mature leaf blades. Preferential movement towards the newest leaf was evident for 109Cd and 45Ca. These results directly indicate that elemental transport is differentially regulated in the vegetative stem as early as 15min before the elements are transported to leaves. Cd behaviour in the stem was investigated in detail by obtaining serial section images from the bottom part of shoots after 109Cd was applied to a single crown root. At 30min, the maximum amount of 109Cd was distributed in the peripheral cylinder of the longitudinal vascular bundles (PV) and, interestingly, some amount of 109Cd was transported downwards along the PV. This transport manner of 109Cd provides evidence that Cd can be loaded on the phloem at the stem immediately after Cd is transported from the root.
Key words: Autoradiography, cadmium, calcium, phosphate, radioisotope, rice, sulphate, tracer, transport, xylem-to-phloem
Introduction
Minerals taken up by the plant roots are transported to the shoot and distributed to each leaf and the meristem to maintain proper growth. Primary long-distance transport from the roots to the shoot is assumed to be driven by transpiration flow and root pressure within xylem vessels (Marschner, 1995). After translocation to the leaf, minerals are loaded into the phloem and exported from the old tissue to the developing young tissue at a low transpiration rate. This step known as remobilization occurs depending on the kind of solute. In addition to these transport steps, intervascular transport systems in the stem tissue, such as xylem-to-phloem transfer, have been suggested to be of particular importance for elemental partitioning among shoot tissues (Marschner, 1995). The nutrient circulation model coordinating these transport processes within a whole plant has been described particularly for N and K+/Na+ based on an analysis of the xylem sap and phloem exudate (Wolf et al., 1991; Pate and Jeschke, 1995; Jeschke and Hartung, 2000).
Cadmium (Cd) is a toxic metal that causes serious problems for plants and animals, including humans (Yanagisawa et al., 1984). Decreasing Cd accumulation in edible parts is important to reduce Cd intake. Cd moves in both the xylem and phloem (Reid et al., 2003; Riesen and Feller, 2005; Hart et al., 2006; Tanaka et al., 2007; Lu et al., 2008; Mori et al., 2009; Uraguchi et al., 2009). In rice, Cd transport from the root to the shoot is xylem dependent (Uraguchi et al., 2009), while most Cd in the grain is assumed to have been transported by the phloem (Tanaka et al., 2007). Given that Cd could be transported from the roots to the grain through the vascular system in the stem without going through mature leaves (Kashiwagi et al., 2009; Fujimaki et al., 2010; Rodda et al., 2011), it could be supposed that Cd transfer from the xylem to the phloem in the stem could play a role in Cd accumulation in the grain. Transfer cells present in the nodes (Zee, 1972; Kawahara et al., 1975) may play a role in Cd loading of the phloem (Fujimaki et al., 2010). However, the significance of the stem vascular systems for Cd partitioning has not been fully clarified. Indeed, little evidence is available about the actual Cd transport pathway within the stem.
Many recent studies describing the elemental transport mechanism have focused on characterizing ion transporters using ion specificity, transport kinetics, and elemental accumulation patterns. The Cd-efflux transporter OsLCT1 has been suggested to participate in Cd remobilization in the leaf and during intervascular transfer of Cd in rice (Uraguchi et al., 2011). This is based on the result that OsLCT1 expression levels are high in the flag leaf blade and the uppermost node of the culm and that the Cd content in the grains and phloem exudate from the leaf is reduced in RNAi plants (Uraguchi et al., 2011). The potential importance of the uppermost node in the culm for the intervascular Cd transfer has also been suggested by the Cd accumulation pattern in this tissue (Yamaoka et al., 2010; Yamaguchi et al., 2012). Similarly, molecular biological evidence demonstrating the significance of the stem for boron partitioning in Arabidopsis, and silicon and iron partitioning in rice has been reported (Tanaka et al., 2008; Yamaji and Ma, 2009; Kakei et al., 2012). Based on previous studies, consideration has been given to the intervascular transport systems within the stem during the primary ion transport process before they reach leaves.
The elemental transport pathway must be identified to understand the mechanisms determining ion partitioning within the stem. Subsequently, the elemental transport pathway should be described by tracking actual elemental movement as direct evidence instead of using the accumulation pattern or the solute composition in the xylem and phloem. Applying radioisotope-labelled-tracer experiments is an effective approach for this purpose particularly because it enables the discrimination between elements that are already present in the plant and those that are newly absorbed by the roots. Furthermore, remobilization flow and primary elemental transport must be distinguished to specify the characteristics of the intervascular transport systems within the stem. Thus, in this study, tissue partitioning of a labelled tracer was determined 15–30min after absorption from the roots and before contribution of the remobilization flow on the tracer partitioning pattern increased. The partitioning patterns of 32P, 35S, 45Ca, and 109Cd were determined to identify the features of the Cd vascular transport system. Then, the preferential transport towards the developing sink leaf and the immediate transport directed downwards were demonstrated as a feature of Cd transport, suggesting that phloem transport of Cd occurred during the primary transport process in the vegetative rice stem.
Materials and methods
Plant materials and growth conditions
Seeds of rice (Oryza sativa L. ‘Nipponbare’) were washed with deionized water, submerged for 18h, and transferred to 0.5mM CaCl2 solution. After 2 days, seedlings were transferred to half-strength Kimura B nutrient solution (pH 5.6) Tanoi et al., 2011 and grown in a growth chamber under a 16/8 light/dark cycle at 28 °C with 70% humidity. Seedlings with an expanding fifth leaf were pre-cultured in a nutrient solution containing 0.1 µM CdCl2 for 2 days. Next, seedlings in which the seventh leaf was emerging were treated with a radioactive tracer as described below.
Seedlings with an emerging eighth leaf were transferred to a greenhouse under a light/dark cycle at 30/25 °C with natural light and grown in half-strength Kimura B nutrient solution (pH 5.6) for 40 days for sequential section analysis. Mature plants were used for analysis 1 week after addition of 0.1 µM CdCl2 to the nutrient solution.
Measurement of the transpiration rate
The intact leaf transpiration rate in seedlings in which the seventh leaf was emerging was measured in the growth chamber during the light period. For measurement, a portable photosynthesis system (LI-6400, LI-COR, Nebraska) with an attached leaf chamber whose top side was covered with a transparency film to enable the transpiration measurement under the same light condition as the growth chamber (6400–11, LI-COR) was used. The concentration of carbon dioxide in the air in the leaf chamber was fixed at 0.4%. The sample size of the leaves was four for each L4, L5, and L6 blade.
Plant tracer treatment
The roots of young seedlings on which the sixth leaf was emerging were treated for 15min with 0.05 × Kimura B nutrient solution containing 0.01 µM CdCl2 and radioisotope tracer in a bright growth chamber at 30 °C. A carrier-free solution of H3 32PO4 (100 MBq l−1), H2 35SO4 (300 MBq l−1), 45CaCl2 (500 MBq l−1), or 109CdCl2 (200 MBq l−1) was added to the nutrient solution. To analyse the first stages of transport, the aerial parts of three seedlings were harvested immediately at the end of tracer treatment (15min). The labelled seedlings were transplanted into half-strength Kimura B nutrient solution (pH 5.6) containing 0.1 µM CdCl2 and further incubated for 48h for translocation analysis. Seedlings were also harvested at 1 and 3h after initiation of treatment for 109Cd analysis. Each experiment was performed at least twice.
A mature plant at the end of the vegetative stage was used for sequential section analysis. A single crown root emerging from the middle of the stem was treated for 30min with a 109Cd-containing solution (prepared as described above) by placing the root and solution into a straw-shaped plastic bag in a bright growth chamber. After treatment, the bottom 2cm of the aboveground shoot part and the labelled crown root on the surface were sampled and sectioned as described below.
Analysis of tracer distribution by autoradiography
The aerial parts of the seedlings treated with the tracers were separated into leaf blades, leaf sheaths, and bottom parts of the shoot including the stem. The leaves were named L2–L8, in order of development from the oldest to the youngest leaf. Although rice plants have two other small leaves, the coleoptile and the first incomplete leaf (L1), these were excluded from analysis. L8 was only visible in seedlings sampled at 48h. L2 and L7 were not separated into sheaths and blades. The entire L2 leaf was designated as the L2 sheath. L7, which was just emerging from the top of the L6 sheath at the time of sampling, was designated the L7 blade because the sheath was too short to be harvested separately. The partitioning of tracers between individual leaf parts, excluding the coleoptile and L1, were placed in contact with the imaging plates (BAS IP MS, GE Healthcare UK, Buckinghamshire, UK) at 4 °C. The image on the imaging plate was scanned with an FLA5000 image analyser (Fujifilm, Tokyo, Japan) at a resolution of 100 µm. The net photostimulated luminescence intensities in each leaf part were calculated using image analysis software (Image Gauge version 4.0, Fujifilm). The amount of tracer contained in each leaf part was reported relative to the photostimulated luminescence intensity in the L3 sheath.
Measurement of 109Cd radioactivity
The leaves of seedlings labelled with 109Cd for 15min were air dried and shredded. Samples were then placed in test tubes, and 109Cd activity in each leaf was quantified using a well-type NaI(Tl) scintillation counter (ARC-300, Aloka, Tokyo, Japan). The counts per minute per leaf were calculated. Blades and sheaths were combined for this measurement.
Preparation of serial sections and autoradiography
Seedlings and a mature plant were labelled as described above before preparation of the serial sections. After the aerial part was harvested and the root was cut off, the bottom part of the aboveground shoot was immediately frozen by submerging in liquid nitrogen. Sliced sections were prepared according to a previously described method (Kawamoto, 2003). Each shoot sample was embedded in embedding medium, covered with adhesive film (Cryo-Film transfer kit, Finetec, Tokyo, Japan), and sliced with a cryostat into serial sections of 30-µm thickness. A BAS IP TR imaging plate (GE Healthcare) was stuck to the frozen section on the side directly opposite the attached film. The imaging plate (IP) with the samples was kept in the freezer at −80 °C for either 5 days (for samples from young seedlings) or 2 weeks (for samples from mature plants). IP was scanned with an FLA5000 image analyser at a resolution of 10 µm to obtain an image of the tracer distribution pattern. The sectioning experiments were performed either in two replications for seedlings or once for mature plants.
Results
Transpiration rate of each leaf
The transpiration rates in L5 and L6 were higher than that in L4 (Table 1). At this growth stage, the tip of the L7 blade was just emerging from the L6 sheath in the shape of a scroll. Therefore, the transpiration rate in the L7 blade could not be analysed although it had been developed almost completely in size.
Table 1.
Transpiration rate of L4, L5, and L6 blades in rice seedling L6 is the youngest expanded leaf. Data are means ± SD of measurements from four leaves.
| Blade | Transpiration rate (mmol H2O m–2 s–1) |
|---|---|
| L6 | 3.19±0.718 |
| L5 | 2.99±0.859 |
| L4 | 1.90±0.237 |
Distribution patterns of 32P, 35S, and 45Ca in the leaf sheaths and blades after 15min and 48 h
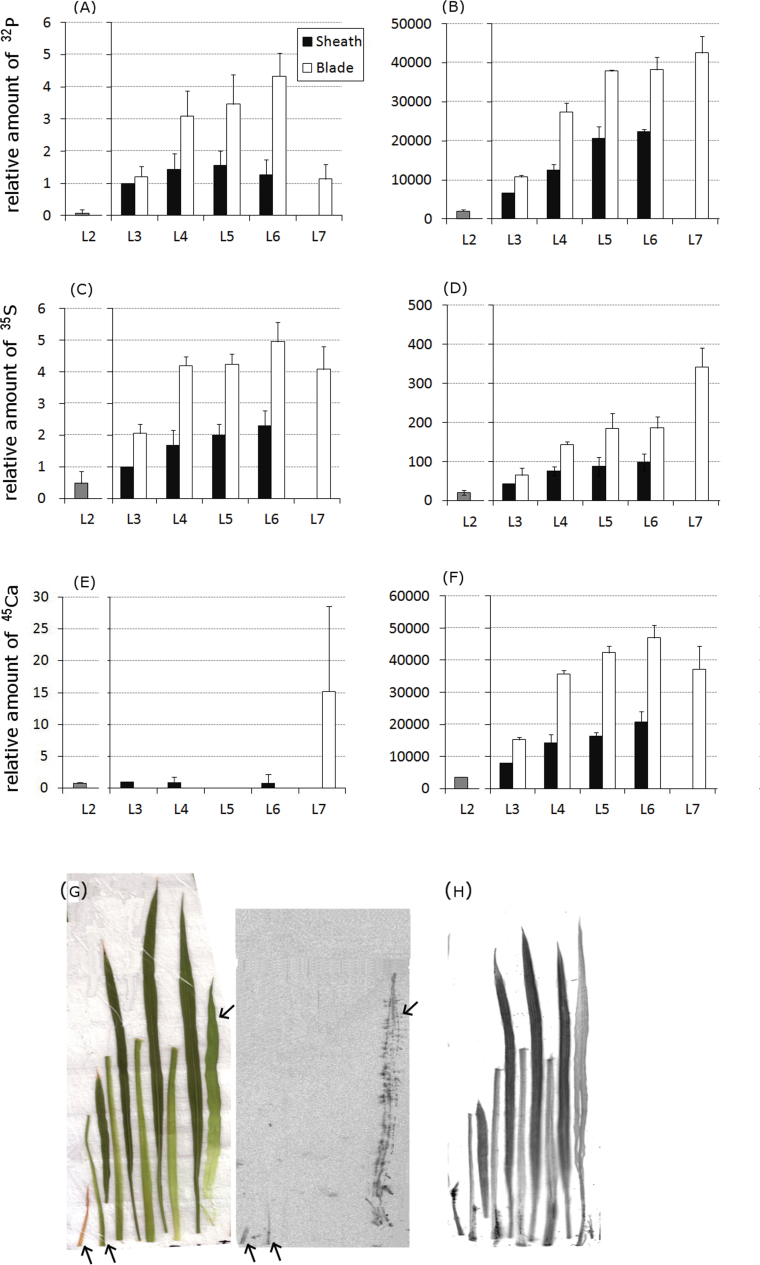
Fifteen minutes after the initiation of incubation, the amount of 32P found in the L4, L5, and L6 blades was higher than that transported to other parts (Fig. 1A). Among these three blades, the L6 blade showed the highest accumulation of 32P (Fig. 1A). The amount of 32P distributed in the L7 blade within 15min was less than one-third of that distributed in the L5 blade (Fig. 1A). At 48h, the amount of 32P transferred to the shoot was 10,000-times higher than that transferred at 15min (Fig. 1A, B). The ratio of the L7 to L3 sheaths particularly increased to approximately the same as that of the L6 blade (Fig. 1B). The distribution pattern of 32P among the other leaves at 48h was similar to that at 15min (Fig. 1A, B). The blade/sheath ratio of 32P amount varied from 1.2 to approximately 4 (Fig. 1A, B).
Fig. 1.
Distribution patterns of 32P, 35S, and 45Ca in each organ 15min and 48h after the initiation of treatment. (A–F) 32P (A, B), 35S (C, D), and 45Ca (E, F) signals were analysed with an imaging plate (IP) at 15min (A, C, E) and 48h (B, D, F) after the initiation of treatment. The signal intensity for each organ was based on the photostimulated luminescence value, which was normalized to the value for the L3 sheath of each plant, and was quantified considering the amount of each radionuclide in the L3 sheath at 15min as a standard. Error bars correspond to standard deviation from three biological repeats. (G, H) Distribution pattern of 45Ca in each organ at 15min (G) and 48h (H) are shown in representative IP images. The leaf samples in (G) are shown in order from L2 (far left) to L7 (far right). L3, L4, L5, and L6 were separated into the corresponding sheath and blade (left and right, respectively, in each pair of organs). The photograph of the analysed plant tissues (G, left) shows the positions of the samples in the detected 45Ca image (G, right). Arrows are appended to assist the recognition of 45Ca signals. The leaf samples in (H) were positioned as in (G), except that an L8 image could be detected and is shown at the far right. Experiments were performed at least twice, providing similar results (this figure is available in colour at JXB online).
Fifteen minutes after the initiation of incubation, the amount of 35S transported to the L7 blade was the same as that transported to the L4 and L5 blades. The amount of 35S transported to L6 was 18% higher than that transported to L4, L5, and L7. After 48h, the maximum amount of 35S was observed in L7, which contained almost twice the amount detected in the L5 and L6 blades (Fig 1D). The total amount of 35S transported to the shoot at 48h was approximately 50-times higher than that transported at 15min (Fig. 1C, D).
The distribution pattern of 45Ca 15min after the initiation of treatment was very different from that of 32P and 35S. Nearly all 45Ca was found in L7 (Fig. 1E). No 45Ca was detected in the L3, L4, L5, or L6 blades (Fig. 1E). However, the distribution pattern of 45Ca was more similar to that of 32P and 35S after 48h (Fig. 1B, D, F). The amount of 45Ca distributed in the L3–L6 blades was twice as high as that distributed in the sheaths (Fig. 1F). L7 contained approximately the same amount of 45Ca as the L4, L5, and L6 blades (Fig. 1F). The total amount of 45Ca transported to the shoot at 48h was approximately 11,000-times higher than that transported at 15min (Fig. 1E, F).
At the end of 15min, the distribution patterns of 45Ca in the L7 and L3–L6 sheaths were completely different (Fig. 1G). In the case of L7, 45Ca was distributed throughout the leaf (Fig. 1G). In contrast, the amount of 45Ca in the sheaths was very low and mainly distributed at the bottom parts of the sheaths, gradually lessening in intensity towards the tip (Fig. 1G). The distribution pattern of 45Ca observed at 15min was not observed after 48h, as 45Ca was distributed uniformly throughout the organs (Fig. 1H).
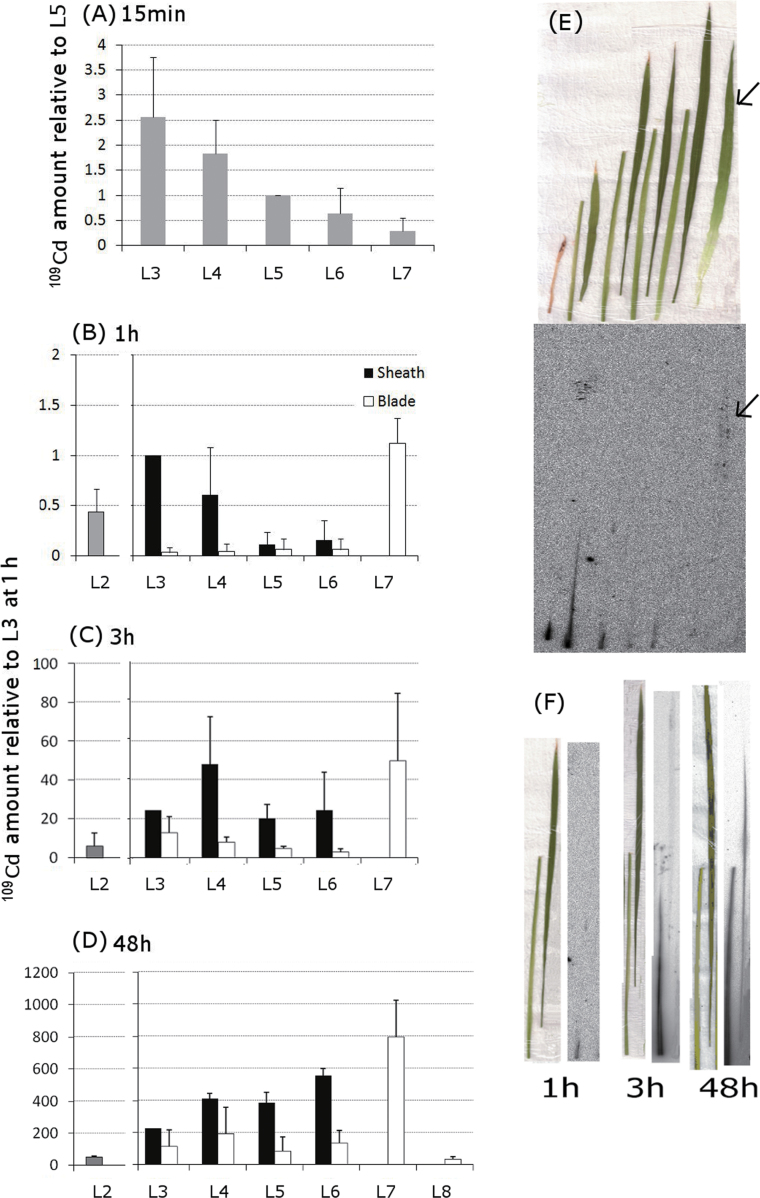
Distribution of 109Cd in the leaf sheath and blade over time
No visible images in 109Cd distribution could be obtained from IP plates 15min after the initiation of treatment: therefore, 109Cd was measured using a scintillation counter. The maximum amount of 109Cd was detected in L3 and L4 at 15min (Fig. 2A). L7 contained very low amount of 109Cd, approximately one-eighth of that in L3 (Fig. 2A). IP could capture 109Cd distribution images 1h after the initiation of treatment when 109Cd in L7 was particularly high, although the distribution pattern in other leaves was similar to that observed at 15min (Fig. 2A, B). The distribution pattern at 1h was confirmed by scintillation counting (data not shown). The amount of 109Cd in the L5 and L6 sheaths within 3h had increased to approximately the same amount as that transported to the L3 sheath (Fig. 2C). The total amount of 109Cd distributed in the shoot at 3h was 55-times higher than that distributed at 1h. After 48h, the total amount of 109Cd distributed in the shoot increased to 820-times the amount distributed at 1h (Fig. 2D). The maximum amount of 109Cd was distributed in L7, which was more than 3-times the amount distributed in the L3 sheath (Fig. 2D). The amount of 109Cd in the L4, L5, and L6 sheaths at 48h was higher than in the L3 sheath. At 48h, 109Cd had accumulated at higher amounts in the L3–L6 sheaths than in the blades, which was different from the distribution patterns of 32P, 35S, and 45Ca (Fig. 1B, D, F and Fig. 2D).
Fig. 2.
Time-dependent changes in the distribution pattern of 109Cd in each organ. (A) The amount of 109Cd in the leaves at 15min was measured using a gamma counter and normalized to that of L5. (B–D) 109Cd signals in the sheath and blade of each leaf were analysed at 1 (B), 3 (C), and 48h (D) after the initiation of treatment. Signal intensities, based on the photostimulated luminescence values for each organ, were normalized to those of the L3 sheath within each plant. Amount of 109Cd relative to that in the L3 sheath at 1h was presented. Error bars correspond to standard deviation from three biological repeats. Experiments were performed at least twice, providing similar results. (E) Typical distribution pattern of 109Cd detected by an imaging plate at 1h (bottom panel) is shown together with a photograph of the organs analysed (top panel). Samples were positioned in order from L2 (far left) to L7 (far right). L3, L4, L5, and L6 were separated into sheaths (left) and blades (right). Arrows are appended to assist the recognition of 109Cd signals in L7. (F) Images of the distribution pattern of 109Cd in L4 at 1, 3, and 48h after the initiation of treatment. For each time point in (F), the photograph on the left corresponds to the image on the right. Within each panel, the organs are arranged with the sheaths on the left and the blades on the right. Experiments were performed at least twice, providing similar results (this figure is available in colour at JXB online).
One hour after the initiation of treatment, 109Cd was concentrated in the bottom part of L2, L3, and L4 sheaths, whereas 109Cd signals in L7 were spread throughout the leaf (Fig. 2E). The distribution patterns of 109Cd in the L4 sheath at 1, 3, and 48h are shown in Fig. 2F. The amount of 109Cd in the L4 organs increased over time, beginning at the bottom of the sheath and gradually spreading upwards (Fig. 2F). 109Cd signals were also found in the L4 blade after 48h (Fig. 2F).
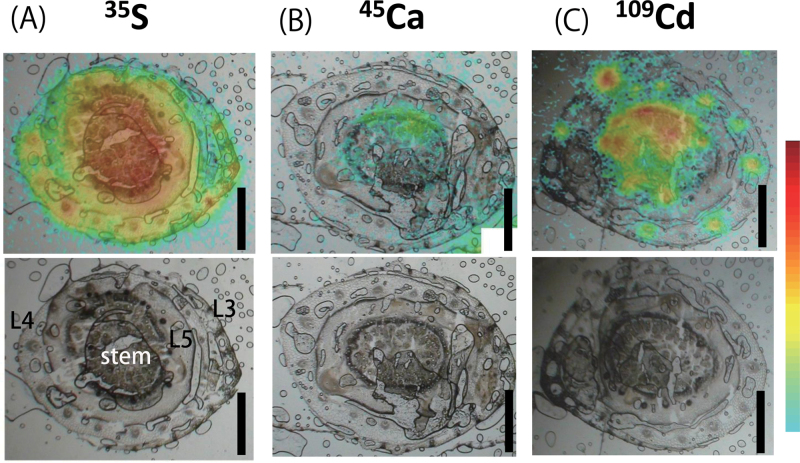
Distribution of 35S, 45Ca, and 109Cd in sections from the base of the plant at 15 min
The outermost leaves were L3 and L4 in the images of sections taken from the very bottom of the aboveground part of the plant. The stem was located at the centre of each section, which is the base part of L6 and L7, surrounded by L5 (Fig. 3). 35S signals were detected in all leaves and the stem at 15min, with the maximum radioactivity being detected in the L3 and L4 stem area and vascular tissues (Fig. 3A). The different distribution pattern of 35S among organs, which was accumulated in the vascular tissues in L3 and L4, but not in L5 and the stem, was notable (Fig. 3A). 45Ca signals were detected only in the stem and were absent in L3 and L4 (Fig. 3B). Intense 109Cd signals were detected in vascular tissues of L3, L4, and the stem but not in L5 (Fig. 3C). Unlike 35S, no 109Cd signals were found in the L3 and L4 mesophyll tissues (Fig. 3A, C).
Fig. 3.
Distribution patterns of 35S (A), 45Ca (B), and 109Cd (C) in tissues of the bottom part of the shoot 15min after the initiation of treatment. Signals from tracers detected by the imaging plate are illustrated by a heat map superimposed on the corresponding bright-field image of the section (top row). The bottom row shows the original bright-field images. Bars, 1mm.
Despite the high amount of 109Cd distributed to the base of L6 and L7 (Fig. 3C), the amount of 109Cd transported to L7 was very low at 15min, as measured by the gamma counter (Fig. 2A). This contradiction could be accounted for in terms of sample preparation. Indeed, the L7 sample analysed by the gamma counter was cut away from the bottom part of the shoot and did not contain any stem tissue in which 109Cd could accumulate at 15min. Hence, 15min was so short that a large amount of 109Cd in the bottom part of the shoot could not enter the L7 blade although it was on track to reach L7.
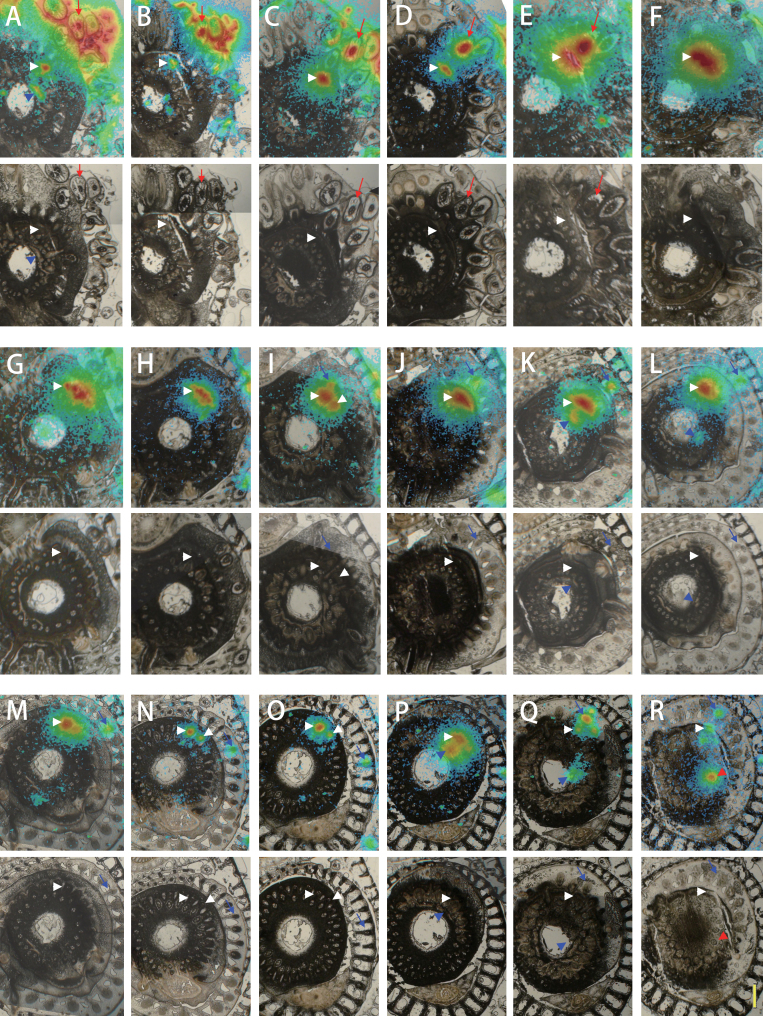
109Cd distribution in sequential sections of the mature plant shoot
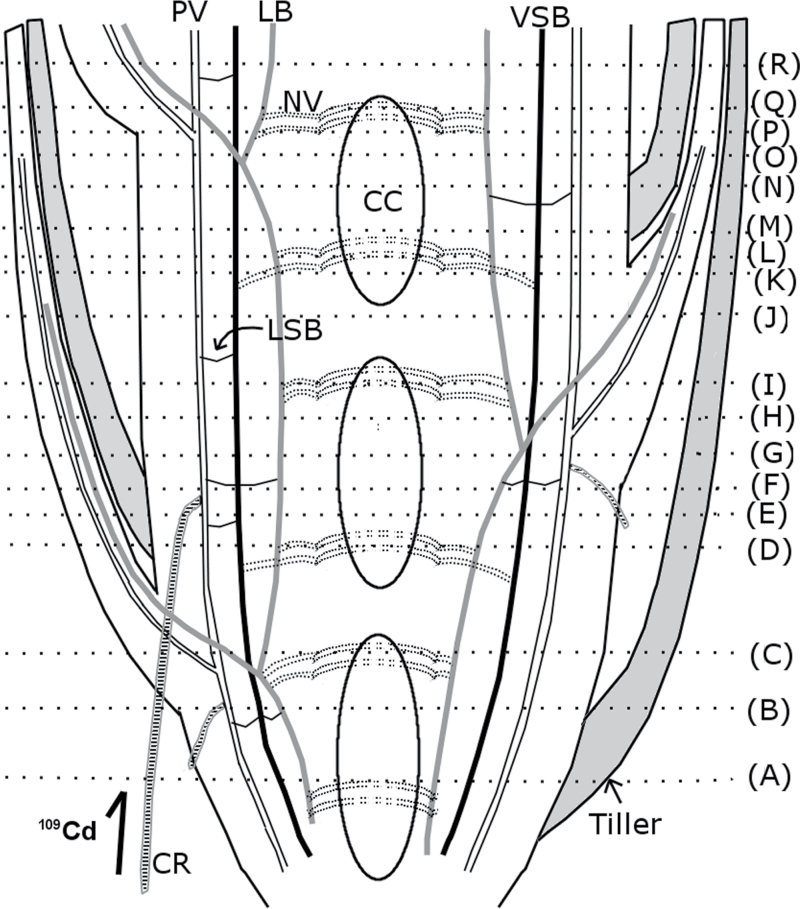
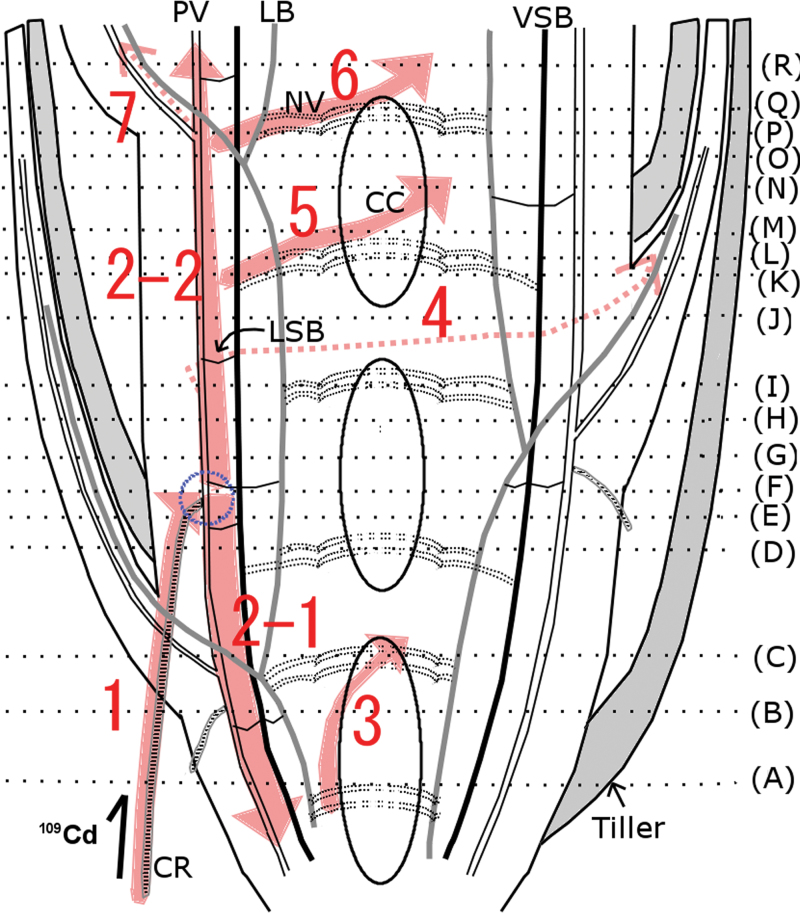
A series of sections corresponding to the positions illustrated in Fig. 4 are shown in Fig. 5, from the lowest to the uppermost section. 109Cd was detected in and around the treated crown root, with the maximum amount in the centre (Fig. 5A). 109Cd signals were also detected in one vascular bundle inside the stem and nodal vascular anastomoses (NV) at the centre of the stem (Fig. 5A). NV are connected to the large vascular bundles (LB) and the vertically running small vascular bundles (VSB), and NV ascend upwards around the central cavity (Fig. 4). Both LB and VSB are linked to the PV via the laterally running small vascular bundles (Fig. 4). In the second section (Fig. 5B), the 109Cd-treated crown root is visible beneath the surface of the shoot tissue. 109Cd signals were detected around the treated crown root and in tissues inside the stem, similar to those seen in Fig. 5A (Fig. 5A, B). The 109Cd-treated crown root was further inwards in the stem in sections from higher positions on the plant (Fig. 5C, D). Intense 109Cd signals were found in the crown root and the tissue around the PV (Fig. 5C, D). In the next section (Fig. 5E), 109Cd was concentrated in the crown root and the PV, which were now located close together inside the stem. In the section shown in Fig. 5F, the shape of the 109Cd-treated crown root can no longer be seen, and intense 109Cd signals were detected along the PV. In the following two sections (Fig. 5G, 5H), 109Cd was maintained in some tissues along the PV. In the next section (Fig. 5I), a small number of 109Cd signals was visible towards the outside of the stem. These signals were located on a small vascular bundle leading into the base of a leaf (Fig. 5I), which was clearly distinguished in the next section (Fig. 5J), where no central cavity was observed at the centre of the stem, indicating the presence of a node. 109Cd was localized mostly in the stem, with some 109Cd detected in the leaf vascular bundle (Fig. 5J). In the next section (Fig. 5K), the leaf base containing a small amount of 109Cd was completely separated from the stem. Some amounts of 109Cd that remained in the stem were also distributed in NV (Fig. 5K). In the next two images (Fig. 5L, M), 109Cd signals were localized in the small leaf vascular bundle and NV around the central cavity. The stem tissue, in which most of 109Cd was localized, was divided into two vascular bundles as shown in Fig. 5M, and even more clearly in the following two sections (Fig. 5N, O). In the next section (Fig. 5P), 109Cd signals were detected within the stem tissue and near the centre of the stem. In the section shown in Fig. 5Q, 109Cd was localized as a small amount in the base of the leaf, the vascular tissues along the PV and NV at the centre of the stem (Fig. 5Q). The last section (Fig. 5R) contained a node (i.e. no central cavity). A new leaf was clearly visible, and the 109Cd signal was detected in a vascular bundle (Fig. 5R). 109Cd was localized within several vascular bundles as well as LB inside the stem (Fig. 5R).
Fig. 4.
Schematic illustration of the longitudinal section of a mature rice stem and crown root. 109Cd was supplied to a mature rice plant through one crown root for 30min (shown at lower left). The shoot was then sliced into sections at the incision lines (A–R) to trace the 109Cd transport pathway inside the stem. The areas between the central cavities are the nodes. CC, central cavity; CR, crown root; LB, large vascular bundles; LSB, laterally running small vascular bundles; NV, nodal vascular anastomoses; PV, peripheral cylinder of longitudinal vascular bundles; VSB, vertically running small vascular bundles.
Fig. 5.
Serial section images of a mature rice stem after applying 109Cd to one crown root for 30min. 109Cd signals are illustrated by heat maps superimposed on bright-field images (top panels). The original bright-field images are shown in the bottom panels. The positions of the sections illustrated in (A–R) correspond to the incision lines indicated in Fig. 4. 109Cd signals found in the centre of the treated crown root (red arrows), peripheral cylinder of longitudinal vascular bundles in the stem (white arrowheads), nodal vascular anastomoses (blue arrowheads), the small vascular bundle heading to the leaf (blue arrows), and the large vascular bundle (red arrowheads) are marked as noted. Bar = 1mm.
Discussion
In the present study, a 109Cd tracer experiment was performed to detect the primary transport pathway of Cd from the root to the shoot, before secondary translocation began. To achieve early detection of 109Cd signals, a higher level of 109Cd radioactivity (200 MBq l−1) was applied compared with that applied in some previous studies, e.g., 0.6 MBq l−1 for 24h in Arabidopsis (Dauthieu et al., 2009), 0.325 MBq l−1 for 2h in soybean (Ohya et al., 2008), and 36 MBq l−1 for 4h in tobacco (Bovet et al., 2006).
According to the transpiration rates in the mature leaves (Table 1) and the observation that L7 was folded in the L6 sheath and was just emerging, L5 and L6 were suggested to be the main target organs for the transpiration stream during this experiment. This suggestion could be supported by the previous observation that completely expanded L5 and L6 leaves have the highest photosynthetic activity and the greatest number of stomata among seedlings with an emerging L7 (Hirasawa et al., 1983). In addition, the consideration that a leaf folded in an older leaf sheath usually has quite a low transpiration rate and is regarded as a sink organ (Matsuo et al., 1995; Tsukamoto et al., 2009) also supports this idea. However, the in situ transpiration amount may differ from the transpiration data measured in the measurement device considering the difference in the environment (e.g. the light enters only from the upper side of the device, and the relative humidity in the device was between 45 and 50%).
The 32P distribution pattern at 15min indicated that phosphorus is initially transported preferentially to tissues with high transpiration rates (Table 1 and Fig. 1A), as was previously reported (Matsuo et al., 1995). The increased proportion of the total amount of 32P found in the L7 blade at 48h (Fig. 1B) could mostly be a consequence of phosphorus remobilization both in organic and inorganic forms (Hayashi and Chino, 1985).
The ions tested were distributed in a different pattern among the leaves at 15min during the primary stage of transport (Fig. 1A, C, E and Fig. 3). This variety in distribution pattern cannot be achieved by the transpiration stream alone. Thus, the intervascular transport system in the vegetative stem is clearly involved in the primary elemental transport in an element-specific manner.
The total amount of each tracer in the shoot considerably increased from 15min to 48h (Fig. 1A–F). This increase may have been due to the root reserving some of the tracer during the 15-min tracer treatment. The root usually initially reserved an extremely high percentage of the tracer and then gradually released it to the shoot over hours (data not shown).
Considering that L5 and L6 are the target organs for the transpiration stream, the rapid primary transport of 45Ca to the L7 blade at 15min (Fig. 1E, G and Fig. 2B) demonstrated that 45Ca can be removed from the transpiration stream during passage through the intervascular transport system and redirected rapidly towards the developing young leaf without passing through mature expanded leaves. In contrast, the transpiration flow was assumed to be significant for the Ca distribution pattern in the long term (Fig. 1F, H). Thus, the velocity of Ca transport could be less during transpiration flow than in flow directed to the sink leaf, while a larger amount of Ca was transported by the transpiration stream. The considerable difference between the 45Ca and 109Cd distribution patterns was based on whether they accumulated in the leaf blades or not at 48h. Once Ca reaches the blade, it is unloaded from the xylem, widely spread throughout the leaf, and gradually accumulated in the blade. In contrast, most Cd is stored in the sheath even at 48h (Fig. 1F and Fig. 2D). 45Ca was widely spread throughout the leaf, whereas most 109Cd was stored in the sheath, even at 48h. This characteristic 109Cd distribution pattern of ‘sheath > blade’ was also found in Arabidopsis thaliana in which 109Cd showed the distribution pattern of ‘petiole > lamina’ within 24h after 109Cd treatment (Dauthieu et al., 2009). The reason may be that the tissue between the blade and sheath/petiole has ion selectivity that is impervious to Cd and/or that a large difference in complexation ability exists between the sheath/petiole and blade.
Cd is coordinated with O and S in the phloem, whereas most of the Cd is coordinated with S in the xylem (Yamaguchi et al., 2012). Cd binds with low-molecular-weight –SH compounds in the phloem sap (Kato et al., 2010). The different forms of Cd in different tissues may be related to the Cd transport mechanism in the stem. In addition, the mechanisms for lateral elemental transport around vascular tissue, such as the xylem unloading mechanism and subsequent transcellular movement, are different for different elements (Metzner et al., 2010). In the present study, sulphur was actively unloaded from the xylem, whereas Cd did not leave the vascular tissue of mature leaf sheaths (Fig. 3). Based on the section analysis (Fig. 3C) and quantitative analysis at 15min (Fig. 2A), it was estimated that the xylem solution supplying in L5 within 15min contained much less 109Cd than that reaching L3 or L4. Thus, the nodes or internodes allowed Cd to be redirected towards the developing new leaf soon after it reached the stem. In barley seedlings, preferential transport of Fe towards the youngest developing leaf, mostly through the phloem, has been reported as early as 1h after absorption by the root (Tsukamoto et al., 2009). Recently, the rice iron transporter OsYSL16 has been suggested to be responsible for the phloem loading of iron at the unelongated nodes (Kakei et al., 2012). Although it was not determined whether xylem or phloem is the main route of Cd import in L7 in this study, the Cd-specific intervascular transport system in the stem is suggested to have a considerable effect on Cd partitioning in vegetative Gramineae plants. The possibility that xylem-to-phloem transfer occurred during Cd passage through the vegetative stem was examined by tracing the 109Cd transport pathway in detail. A mature rice plant was used for this examination, because it has a much larger stem than that of a young seedling. However, considering that the mature rice stem has many nodes and internodes stacked within a few centimetres and that the outgrowth of crown roots from the stem internodes is regularly induced, applying 109Cd to the entire root system would allow it to enter the stem tissue from any internode, making it difficult to follow the tracer route. Thus, 109Cd was applied to a single crown root to restrict entrance of the tracer into the stem; this approach resulted in clear images showing the downward Cd transport, which was observed as 109Cd distribution in PV tissue below the connection point of the treated crown root (Fig. 5A–E and Fig. 6). Considering that the PV in which the crown root was formed was connected to the completely expanded upper leaves (Fig. 4), the xylem flow inside was directed upwards. Therefore, downward Cd transport indicated phloem transport of Cd following xylem-to-phloem transfer. Furthermore, the 109Cd distribution pattern around the PV was clearly restricted to a few vascular bundles (Fig. 5C–E) originating from the crown root connection point (Fig. 5F). Based on this 109Cd distribution pattern, it was concluded that 109Cd around the PV was transferred from the xylem to the phloem near the connection point of the crown root (Fig. 6).
Fig. 6.
Schematic presentation of the route of 109Cd in the stem based on an interpretation of the heat maps in Fig. 5. The connection between the crown root (CR) and the main stem is indicated with a blue circle. 109Cd was absorbed by the treated CR (1), then transported both downwards (2–1) and upwards (2–2) inside the stem. Some of the 109Cd that was first transported downwards was further transported to nodal vascular anastomoses (NV) and then upwards along the central cavity (CC) (3). A small amount of 109Cd was transported to the leaves (4, 7), whereas most of the 109Cd remained inside the stem and was distributed both in peripheral cylinder of longitudinal vascular bundles (PV) and NV (5, 6) (this figure is available in colour at JXB online).
Cd transport to the aboveground part is driven by the transpiration stream. The decrease in the Cd transport after abscisic acid treatment is evidence demonstrating this idea (Salt et al., 1995; Zhao et al., 2006; Uraguchi et al., 2009). In the present experiment, 109Cd accumulated in leaves other than the L7 blade at 48h was shown to be L3 < L4 = L5 < L6 (Fig. 2D), which was consistent with the transpiration amount (Table 1). This 109Cd distribution pattern is consistent with the observation that the main driving force for Cd transport to the shoot is the xylem transpiration stream.
Because the resolution of the images in this study was not sufficiently high to distinguish between the xylem and phloem, it is still unknown whether upward movement of 109Cd in the stem occurs only through the xylem or through both the xylem and phloem. Improvements in imaging techniques will be needed to obtain images with higher resolution to identify the main route for the primary transport of Cd.
In conclusion, the autoradiography technique described here, in which only a brief (15-min) pulse treatment of 32P, 35S, 45Ca, or 109Cd was applied to the rice root, provides direct evidence that the intervascular transport system within the vegetative rice stem participates in specific elemental partitioning. The intervascular transport system adjusts the primary transport of Ca and Cd to flow into the sink leaf that was not expanded, whereas the major driving force for these elements from root-to-shoot was transpiration in the mature expanded leaves. The series of frozen section radioisotope images provided evidence that Cd transfer from xylem to phloem can be occurred as soon as Cd was transported from the root to the PV inside the bottom part of the stem. The finding that Cd immediately moves towards the root as well as towards the sink leaf will be the key to revealing the unique features of the Cd transport pathway inside the stem.
Acknowledgements
This work was supported in part by grants from the Japanese Society for the Promotion of Science to N.I.K. (Research Fellow 22-40171) and to T.M.N. (Funding Program for Next Generation World-Leading Researchers), and from the Ministry of Agriculture, Forestry, and Fisheries of Japan to K.T. (Genomics for Agricultural Innovation grant IPG-0009).
References
- Bovet L, Rossi L, Lugon-Moulin N. 2006. Cadmium partitioning and gene expression studies in Nicotiana tabacum and Nicotiana rustica . Physiologia Plantarum 128, 466–475 [Google Scholar]
- Dauthieu X, Denaix L, Nguyen C, Panfili F, Perrot F, Potin-Gautier M. 2009. Cadmium uptake and distribution in Arabidopsis thaliana exposed to low chronic concentrations depends on plant growth. Plant and Soil 322, 239–249 [Google Scholar]
- Fujimaki S, Suzui N, Ishioka NS, Kawachi N, Ito S, Chino M, Nakamura S. 2010. Tracing cadmium from culture to spikelet: noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiology 152, 1796–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JJ, Welch RM, Norvell WA, Kochian LV. 2006. Characterization of cadmium uptake, translocation and storage in near-isogenic lines of durum wheat that differ in grain cadmium concentration. New Phytologist 172, 261–271 [DOI] [PubMed] [Google Scholar]
- Hayashi H, Chino M. 1985. Nitrate and other anions in the rice phloem sap. Plant and Cell Physiology 26, 325–330 [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Araki T, Matsuda E, Ishihara K. 1983. On exudation rate from the base of the leaf blade in rice plants. Japanese Journal of Crop Science 52, 574–581 [Google Scholar]
- Jeschke WD, Hartung W. 2000. Root-shoot interactions in mineral nutrition. Plant and Soil 226, 57–69 [Google Scholar]
- Kakei Y, Ishimaru Y, Kobayashi T, Yamakawa T, Nakanishi H, Nishizawa NK. 2012. OsYSL16 plays a role in the allocation of iron. Plant Molecular Biology 79, 583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi T, Shindoh K, Hirotsu N, Ishimaru K. 2009. Evidence for separate translocation pathways in determining cadmium accumulation in grain and aerial plant parts in rice. BMC Plant Biology 9, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Ishikawa S, Igarashi K, Chiba K, Hayashi H, Yanagisawa S, Yoneyama T. 2010. Possible chemical forms of cadmium and varietal differences in cadmium concentrations in the phloem sap of rice plants (Oryza sativa L.). Soil Science and Plant Nutrition 56, 839–847 [Google Scholar]
- Kawahara H, Chonan N, Matsuda T. 1975. Studies on morphogenesis in rice plants 8. The morphology of vascular bundles in the dwarf part of stem. Proceedings of the Crop Science Society of Japan 44, 61–67 (in Japanese) [Google Scholar]
- Kawamoto T. 2003. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Archives of Histology and Cytology 66, 123–143 [DOI] [PubMed] [Google Scholar]
- Lu LL, Tian SK, Yang XE, Wang XC, Brown P, Li TQ, He ZL. 2008. Enhanced root-to-shoot translocation of cadmium in the hyperaccumulating ecotype of Sedum alfreddii . Journal of Experimental Botany 59, 3203–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. 1995. Mineral nutrition of higher plants 2ndedn. London, UK: Academic Press; [Google Scholar]
- Matsuo T, Kumazawa K, Ishii R, Ishihara K, Hirata H. 1995. Science of the rice plant. Volume 2: Physiology. Tokyo: Nobunkyo; [Google Scholar]
- Metzner R, Thorpe MR, Breuer U, Blumler P, Schurr U, Schneider HU, Schroeder WH. 2010. Contrasting dynamics of water and mineral nutrients in stems shown by stable isotope tracers and cryo-SIMS. Plant, Cell and Environment 33, 1393–1407 [DOI] [PubMed] [Google Scholar]
- Mori S, Uraguchi S, Ishikawa S, Arao T. 2009. Xylem loading process is a critical factor in determining Cd accumulation in the shoots of Solanum melongena and Solanum torvum . Environmental and Experimental Botany 67, 127–132 [Google Scholar]
- Ohya T, Iikura H, Tanoi K, Nishiyama H, Nakanishi TM. 2008. Cd-109 uptake and translocation in a soybean plant under different pH conditions. Journal of Radioanalytical and Nuclear Chemistry 264, 303–306 [Google Scholar]
- Pate JS, Jeschke WD. 1995. Role of stems in transport, storage, and circulation of ions and metabolites by the whole plant. In: Gardner BL, editor, Plant stems, physiology and functional morphology. San Diego: Academic Press; pp 177–204 [Google Scholar]
- Reid RJ, Dunbar KR, McLaughlin MJ. 2003. Cadmium loading into potato tubers: the roles of the periderm, xylem and phloem. Plant, Cell and Environment 26, 201–206 [Google Scholar]
- Riesen O, Feller U. 2005. Redistribution of nickel, cobalt, manganese, zinc, and cadmium via the phloem in young and maturing wheat. Journal of Plant Nutrition 28, 421–430 [Google Scholar]
- Rodda MS, Li G, Reid RJ. 2011. The timing of grain Cd accumulation in rice plants: the relative importance of remobilisation within the plant and root Cd uptake post-flowering. Plant and Soil 347, 105−–114 [Google Scholar]
- Salt DE, Prince RC, Pickering IJ, Raskin I. 1995. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiology 109, 1427–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Fujimaki S, Fujiwara T, Yoneyama T, Hayashi H. 2007. Quantitative estimation of the contribution of the phloem in cadmium transport to grains in rice plants (Oryza sativa L.). Soil Science and Plant Nutrition 53, 72–77 [Google Scholar]
- Tanaka M, Wallace IS, Takano J, Roberts DM, Fujiwara T. 2008. NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis . The Plant Cell 20, 2860–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoi K, Saito T, Iwata N, Kobayashi NI, Nakanishi TM. 2011. The analysis of magnesium transport system from external solution to xylem in rice root. Soil Science andPlant Nutrition 53, 265–271 [Google Scholar]
- Tsukamoto T, Nakanishi H, Uchida H, Watanabe S, Matsuhashi S, Mori S, Nishizawa NK. 2009. 52Fe translocation in barley as monitored by a positron-emitting tracer imaging system (PETIS): evidence for the direct translocation of Fe from roots to young leaves via phloem. Plant and Cell Physiology 50, 48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uraguchi S, Kamiya T, Sakamoto T, Kasai K, Sato Y, Nagamura Y, Yoshida A, Kyozuka J, Ishikawa S, Fujiwara T. 2011. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proceedings of the National Academy of Sciences, USA 108, 20959–20964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S. 2009. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. Journal of Experimental Botany 60, 2677–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf O, Munns R, Tonnet ML, Jeschke WD. 1991. The role of the stem in the partitioning of Na+ and K+ in salt-treated barley. Journal of Experimental Botany 42, 697−–704 [Google Scholar]
- Yamaguchi N, Ishikawa S, Abe T, Baba K, Arao T, Terada Y. 2012. Role of the node in controlling traffic of cadmium, zinc, and manganese in rice. Journal of Experimental Botany 63, 2729–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Ma JF. 2009. A transporter at the node responsible for intervascular transfer of silicon in rice. The Plant Cell 21, 2878–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka W, Takada S, Takehisa H, Hayashi Y, Hokura A, Terada Y, Abe T, Nakai I. 2010. Study on accumulation mechanism of cadmium in rice (Oriza sativa L.) by micro-XRF imaging and X-ray absorption fine structure analysis utilizing synchrotron radiation. Bunseki Kagaku 59, 463–475 (in Japanese) [Google Scholar]
- Yanagisawa M, Niimura Y, Yamada N, Segawa A, Kida K. 1984. Heavy metal pollution and soil replacement in Jinzu River basin. Bulletin of the Toyama Agricultural Experiment Station 15, 1–110 (in Japanese) [Google Scholar]
- Zee SY. 1972. Transfer cells and vascular tissue distribution in the vegetative nodes of rice. Australian Journal of Botany 20, 41–48 [Google Scholar]
- Zhao FJ, Jiang RF, Dunham SJ, McGrath SP. 2006. Cadmium uptake, translocation and tolerance in the hyperaccumulator Arabidopsis halleri . New Phytologist 172, 646–654 [DOI] [PubMed] [Google Scholar]