Abstract
Miscanthus sacchariflorus is a fast-growing C4 perennial grass that can naturally hybridize with M. sinensis to produce interspecific hybrids, such as the sterile triploid M.× giganteus. The creation of such hybrids is essential for the rapid domestication of this novel bioenergy crop. However, progress has been hindered by poor understanding of the environmental cues promoting floral transition in M. sacchariflorus, which flowers less readily than M. sinensis. The purpose of this work was to identify the flowering requirements of M. sacchariflorus genotypes in order to expedite the introduction of new germplasm optimized to different environments. Six M. sacchariflorus accessions collected from a range of latitudes were grown under controlled photoperiod and temperature conditions, and flowering, biomass, and morphological phenotypic data were captured. Results indicated that M. sacchariflorus, irrespective of origin, is a quantitative short-day plant. Flowering under static long days (15.3h daylength), compared with shorter photoperiods, was delayed by an average 61 d, with an average associated increase of 52% of above-ground biomass (DM plant–1). Timing of floral initiation occurred between photoperiods of 14.2h and 12.1h, and accumulated temperatures of 553–1157 °C above a base temperature of 10 °C. Miscanthus sacchariflorus flowering phenology closely resembles that of Sorghum and Saccharum, indicating potentially similar floral pathways and suggesting that determination of the underlying genetic mechanisms will be facilitated by the syntenic relationships existing between these important C4 grasses.
Key words: Bioenergy, biomass, degree days, flowering time, heading date, photoperiod, thermal time.
Introduction
Miscanthus is a fast-growing perennial C4 grass with a natural distribution over a broad range of latitudes in many parts of Asia and the South Pacific (Hodkinson et al., 1997). Commercially grown Miscanthus consists almost exclusively of the sterile triploid hybrid M.×giganteus. Because of its sterility, M.×giganteus must be propagated via rhizome splitting, which elevates establishment costs above those of seed propagation. The use of such limited germplasm in M.×giganteus also presents a disease risk when grown on a large scale and, again, because of sterility, fails to provide a base for the development of varieties tailored to differing environments and end-uses, such as liquid biofuels for transport and combustion for heat and power. The expansion and development of the Miscanthus industry requires novel hybrid production through the creation of intra- and interspecific hybrids. However, flowering in M. sacchariflorus occurs less readily in European field conditions (Clifton-Brown et al., 2001; Jensen et al., 2011a ) compared with M. sinensis, and has also proven difficult to manipulate in controlled environments (Deuter, 2000). Factors promoting floral transition in M. sacchariflorus are poorly understood, but are essential, both for the rapid domestication of Miscanthus and for the delivery of high-biomass lignocellulosic energy for biofuels and biopower (Rooney et al., 2007).
Various environmental factors are known to impose delays on flowering, until a specific signal has been perceived and conditions are optimal (Koornneef et al., 1998). In Angiosperms, one such signal is photoperiod, more specifically the length of the dark period (Ellis et al., 1997; Blazquez et al., 2003; Balasubramanian et al., 2006). Sorghum and Saccharum (sugar cane), which are closely related to Miscanthus, have been identified as quantitative short-day (SD) plants, which flower when photoperiods fall below a ‘critical’ daylength, for example 12h. Floral initiation in quantitative, as opposed to qualitative, SD plants is promoted by SDs but is not necessarily dependent on them. The actual critical daylength for flowering varies between species and geographical origin, and between different cultivars of Sorghum can vary by as much as 4h (Caddel and Weibel, 1971). Within the Miscanthus genus, M. sacchariflorus flower less readily than M. sinensis, both in North European (Clifton-Brown et al., 2001; Jensen et al., 2011a ) and in diverse Chinese (Yan et al., 2011) field conditions, and reports have indicated that some, particularly thick-stemmed, M. sacchariflorus require SD treatments for floral induction (Deuter, 2000). Field observations in Aberystwyth (Wales, UK) have shown that photoperiod alone does not explain flowering time in a number of M. sacchariflorus lines (Jensen et al., 2011a ). Temperature is also known to have a role in determining maturity and can interact with photoperiod to regulate flowering, including in Sorghum (Hammer et al., 1989; Ellis et al., 1997; Craufurd and Qi, 2001). Flowering time prediction is further complicated by water availability. For example, in Sorghum (Craufurd et al., 1993), panicle initiation and flowering are delayed in response to drought, and a similar effect has been observed in Miscanthus, where water deficits appear to impose a delay on flowering (Jensen et al., 2011b ).
Flowering terminates the production of leaves at the stem apex. This has the potential to reduce the length of the growing season, thereby reducing the time over which radiation is intercepted and hence reducing potential biomass accumulation. Flowering is likely to be at least partially coupled to senescence (Wingler et al., 2009), with associated impact on nutrient remobilization to the underground rhizome, thereby promoting crop sustainability and improving combustion quality by removing undesirable elements (i.e. N, S, and Cl) from the harvested biomass.
Previously a relationship was identified between flowering time, under field-grown conditions, and location of origin for 106 Miscanthus lines (Jensen et al., 2011a ). However, in that study most (~70%, n=36) M. sacchariflorus lines failed to flower under UK conditions. In the current study, the aim was to determine whether M. sacchariflorus accessions from a range of latitudes and grown under controlled environment conditions exhibited a photoperiod response. More specifically the following hypotheses were tested: (i) M. sacchariflorus lines are quantitative SD plants that will not flower when daylengths exceed 12.5h; (ii) the delay in flowering imposed by photoperiods exceeding 12.5h is associated with increased biomass; (iii) flowering is accelerated under warm temperatures; and (iv) flowering time shows adaptation with respect to geographical origin.
Two experiments were used to test the hypotheses. The first was designed to determine the approximate critical daylength required to elicit floral transition in two M. sacchariflorus lines and determine the duration from floral initiation to flag leaf emergence. It is impossible to observe floral initiation directly in Miscanthus because the shoot apical meristem is surrounded by a sheath of developing leaves. Observation of the floral primordia necessitates destruction of the apex. Confirmation of floral transition in this experiment was used to predict approximate timings of floral transition in similar stems following the emergence of flag leaves. Another aim was to evaluate any effect of sloped, stepped, and static photoperiods on floral transition because static photoperiods in Saccharum were reported to delay flowering (Julien, 1968). Having identified approximate critical daylengths using two M. sacchariflorus lines, a second experiment comparing flowering times in two photoperiod treatments and using two temperature regimes was used to determine the effect on biomass accumulation of delayed flowering in the same two, and an additional four, M. sacchariflorus lines from a range of latitudes and morphological types.
Materials and methods
Germplasm
The experimental germplasm included three M. sacchariflorus accessions collected as individual rhizomes from Asia in 2006 (Table 1, lines 1–3), and three M. sacchariflorus accessions obtained as individual rhizomes from the UK national Miscanthus collection at Arthur Rickwood (DEFRA project NF0446), ADAS (Wolverhampton, UK) in 2004 (Table 1, lines 4–6). These lines were selected because they came from known locations representing a range of latitudes and were morphologically diverse, both in leaf and stem architecture, and with respect to the degree of difficulty experienced in promoting flowering. The actual age of rhizomes as originally established from seed was not known.
Table 1.
Source information and description of Miscanthus sacchariflorus lines.
| Line | Morphological type | Origin | Used in | ||||
|---|---|---|---|---|---|---|---|
| Stem (mm) | Leaf width (mm) | Latitude (°N) | Altitude (m) | Country of origin | Experiment 1 | Experiment 2 | |
| 1 | 9–11 | 25 | 28.8 | 33 | China | Yes | Yes |
| 2 | 9–11 | 23 | 29.4 | 27 | China | Yes | Yes |
| 3 | 5–8 | 29 | 34.1 | 22 | Japan | No | Yes |
| 4 | 6–7 | 2.3 | 34.5 | 50 | S. Korea | No | Yes |
| 5 | 5–6 | 2.6 | 38.4 | 1000 | Japan | No | Yes |
| 6 | 3–4 | 1.6 | 39.3 | 30 | China | No | Yes |
Experiment 1: determination of photoperiod treatments critical for floral initiation
Experimental set-up
Lines 1 and 2 (Table 1) were cloned by rhizome splitting in spring 2008. Each clone was placed in 10 inch pots in John Innes No. 3 compost and grown in an unheated and unlit glasshouse in Aberystwyth from April to January to establish strong plants with mature rhizomes. Plants were fed monthly with NPK 3.0.1. (Vitafeed, Vitax Ltd, Coalville, UK). In late January, plants were cut back to soil level and placed in a randomized block design in a glasshouse with a day/night regime of 28 °C/15 °C and 16h daylength. NPK 1.1.1. was applied monthly until the photoperiod treatment was started. During the photoperiod treatment, NPK 1.1.1 was applied every 2 weeks. Five replicates of each line were randomly selected and placed in one of three photoperiod treatments:
Treatment A=sloped photoperiod: 14.7h daylength reducing by 0.028h each day for 90 d, 28 °C/15 °C day/night in a glasshouse.
Treatment B=stepped photoperiod: 14.2h reduced by 0.5h every 18 d (to coincide with the sloped treatment), 28 °C/15 °C day/night in a glasshouse.
Treatment C=static photoperiod: constant 12.5h, 28 °C/15 °C day/night in a controlled environment room.
The sloped and stepped treatments were conducted in customized glasshouse compartments equipped with black-out screens and lit by SON-T lights at 80–200 µmol m–2 s–1 and tungsten filaments (30min at the start and end of the photoperiod to simulate dawn and dusk) (~10 µmol m–2 s–1). The static treatment in the controlled environment was conducted at light intensity levels set at 600 µmol m–2 s–1. Capillary irrigation was used to ensure plants were well watered throughout. Photosynthetically active radiation, humidity, and temperature were recorded using CR1000 data loggers (Campbell Scientific®, Leicester, UK).
Investigation of floral initiation by sectioning
At the end of each 18 d period (determined by the ‘step’ length in Treatment B), the most mature stem on each plant was removed at the base. The stem length (to the uppermost ligule) and, if present, the emergence of a flag leaf, were recorded. The upper stem was dissected to identify whether floral transition had occurred in the shoot apical meristem, which is enclosed in a sheath of leaves. Shoot apical meristems were removed under a dissecting microscope and placed in a fixative of 3% glutaraldehyde (Agar Scientific Ltd, Cambridge, UK) in a 0.1M sodium cacodylate (Agar Scientific) buffer, pH 7.4 at 4 °C overnight. After fixing and rinsing twice for 30min in a wash buffer consisting of 0.1M sodium cacodylate pH 7.4 at 4 °C, samples were placed in double-distilled H2O for 1h. Samples were then dehydrated through a graded ethanol series: 30, 50, 70, and 95% for at least 1h at room temperature, then finally 100% ethanol overnight at 4 °C. Following two changes of 100% ethanol, the samples were transferred to a 1:2 mixture of white-hard grade resin (London Resin Company Ltd, Reading, UK) and ethanol, then 2:1, and finally 100% resin. Samples were then placed in gelatine moulds and polymerized overnight at 60 °C. Resin blocks were cut to shape with glass knives to expose the tissue, and 2 µm sections were taken in a longitudinal presentation until the centre of the tissue had been sectioned. The sections were collected with a cat’s whisker and dried down on a drop of 1% isopropyl alcohol onto polysine-coated slides (VWR International, Lutterworth, UK) on a hotplate at 60 °C. When dry, the sections were stained with AMB stain [1:1 mixture of 1% Azur II and 1% methylene blue (both Agar Scientific) in 1% trisodium tetraborate buffer (Sigma-Aldrich, Gillingham, Dorset, UK)]. Sections were washed in water to remove excess stain and dried down on a hotplate, then covered in Eukitt mounting medium (O. Kindler GmbH & Co., Freiburg, Germany) and a cover slip. The resulting slides were imaged on a Leica DM6000B microscope (Leica Geosystems Ltd, Milton Keynes, UK).
Experiment 2: dissection of photoperiod and temperature requirements for floral initiation
Experimental set-up
A total of 21 clonal replicates of each of six M. sacchariflorus lines (Table 1, lines 1–6) were made by rhizome splitting and then potted into compost and grown in an unheated, naturally lit glasshouse at Aberystwyth for one season (April–October, 2009). Plants were then moved outside and allowed to senesce until December, when all above-ground biomass was removed. Pots were then placed in a poly tunnel and covered with a frost protection fleece. In January 2010, plants were taken from metabolic inactivity, gently re-potted with fresh compost, and randomly allocated to one of three treatments, each of which had two phases.
Warm, SD:
Phase 1: 21 d at 15.3h photoperiod and 28/24 °C day/night.
Phase 2: 119 d at reducing photoperiod (to simulate that of 34.1°N) and 28/24 °C day/night.
Cool, SD:
Phase 1: 21 d at 15.3h photoperiod and 20/16 °C day/night.
Phase 2: 119 d at reducing photoperiod (to simulate that of 34.1°N) and 28/24 °C day/night.
Warm, LD:
Phase 1: 21 d at 15.3h photoperiod and 28/24 °C day/night.
Phase 2: 119 d at 15.3h photoperiod and 28/24 °C day/night.
Phase 2 of the experiment began after the accumulation of 410 °Cd (degree days) in the warm SD and LD treatments and 221 °Cd in the cool SD treatment. The reducing photoperiod was programmed to mimic the declining daylength of the mean latitude of origin for the six experimental lines (i.e. 34.1°N).
As in Experiment 1 (Treatments A and B), Experiment 2 was conducted in customized glasshouse compartments with photoperiod and temperature controlled by a CR1000 (Campbell Scientific®, Leicester, UK). No fertilizer was applied. Five thermocouple wires were placed in different locations in each compartment to monitor temperature consistency, and fans were used to circulate air.
Plants were randomized into treatment groups containing seven replicates of each line.
Data capture
Flowering observations were recorded three times per week in whole plants. Four flowering stages (FS) were scored in accordance with Jensen et al. (2011a): FS1, flag leaf emergence; FS2, the exertion of a >1cm panicle on one stem; FS3, the exertion of a >1cm panicle on >50% stems; FS4, the exertion of a >1cm panicle on >80% stems. In addition, the first stem on each plant to exhibit a shortening of internodes (as noted during Experiment 1) was labelled, and flowering development was subsequently monitored on that stem as follows: internode shortening prior to flag leaf emergence; FS1; FS2; anthesis; seed set.
Days to flowering were defined as the day FS1 was first observed on the single most advanced stem. Data from Experiment 1 indicated that floral transition preceded flag leaf emergence by ~18 d. To provide an approximate day to flag leaf emergence in the warm LD treatment, the 18 d value was added to the date on which developing floral primordia were observed in stems harvested from the warm LD treatment where culm dissection revealed the presence of developing floral primordia.
Total biomass at 5cm and above the soil level was harvested on day 118. Leaf number was counted on the labelled stem of each plant. Total stem number (any stem >5cm) was counted on each plant. The whole plant was dried to constant weight at 60 °C. Dry weight values were determined from the whole sample.
Statistical analysis
Mean values, where used, are quoted with ±SE. All statistical tests were carried out in GENSTAT release 13.2 (VSN International Ltd, Hemel Hempstead, UK). General two-way analysis of variance (ANOVA) with randomized blocks was used to test relationships between warm and cool SD treatments, and between warm SD and LD treatments.
Results
Experiment 1: floral initiation from the most advanced stem under sloped, stepped, and static photoperiods
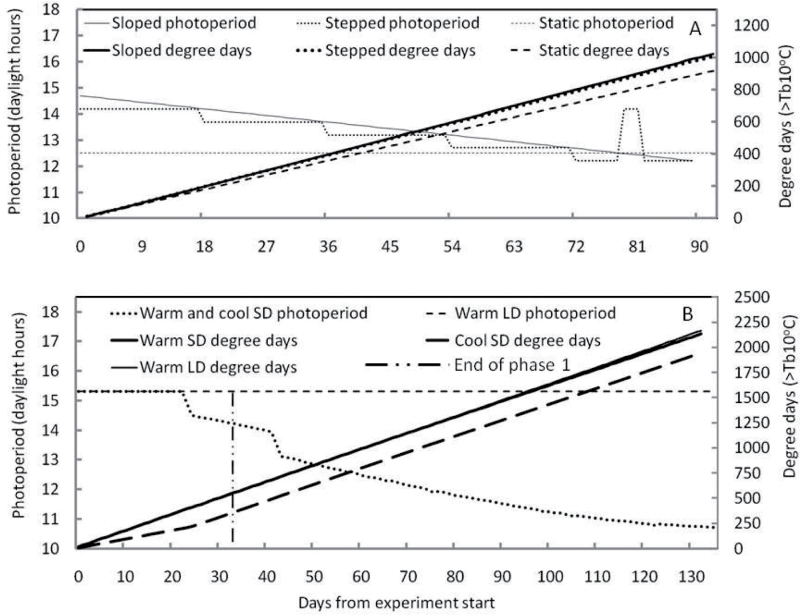
Sloped and stepped photoperiod treatments were achieved for the duration of the experiment, with the exception of a short period in the stepped treatment near the end of the experiment (Fig. 1A). Thermal accumulation in terms of °Cd in the sloped and stepped photoperiods were matched; the °Cd in the static photoperiod treatment in the controlled environment were 10% lower (Fig. 1A).
Fig. 1.
Glasshouse photoperiod and degree day (>Tb10°C) control in Experiment 1 (A) and Experiment 2 (B) at Aberystwyth, Wales.
Under warm glasshouse conditions, shoots from overwintering rhizomes grew rapidly, reaching a maximum of 10 leaves after 18 d, 14 leaves after 36 d, 18 leaves after 54 d, 24 leaves after 72 d, and 25 leaves after 90 d, in harvested stems. This equates to an overall phyllochron of 64 °Cd ±4.20. Neither leaf number nor stem length differed significantly between lines or treatments (Supplementary Table S1 and Supplementary Fig. S1 available at JXB online) and there was no significant interaction between treatment and line for either leaf number or stem length.
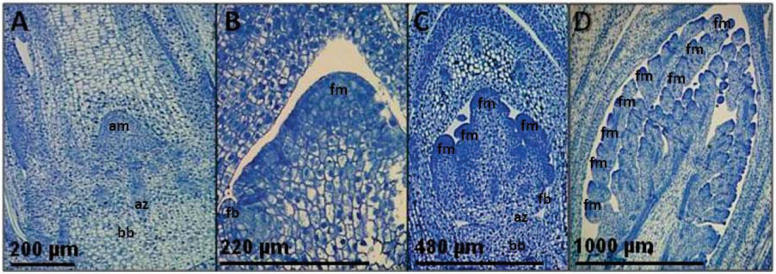
A stage before flag leaf emergence was observed at both 72 d and 90 d, where the uppermost three or four internodes exhibited delayed elongation, with the result that leaves were bunched closely together. This delay in internode elongation was associated with floral initiation, as longitudinal sections of stems revealed tiny panicles.
Flag leaf emergence in both lines was first observed after 72 d in both the sloped and stepped photoperiod treatments (Supplementary Fig. S1A, B at JXB online), at ~800 °Cd above 10 °C and <12.7h daylength (Fig. 1A). In treatment B, this photoperiod had been constant for the previous 18 d, but in Treatment A had been ramping down from 13.3h over the 18 d (Fig. 1A). This coincided with photoperiods of 12.7h, and degree days of 1008–1089 °Cd (Fig. 1A). In stems where the flag leaves had not emerged, longitudinal sections through the growth zone confirmed floral initiation in some stems, where developing panicles were visible with the naked eye. Sections through the growth zone were used to determine whether floral initiation had occurred in the remaining stems, and in many cases early floral primordia (e.g. Fig. 2B–D) were clearly distinguishable from vegetative primordia (Fig. 2A). After a further 18 d, panicle exertion was observed in all three treatments and in both lines (Supplementary Fig. S1A–C).
Fig. 2.
Primordia development during floral transition in Miscanthus sacchariflorus during glasshouse photoperiod Experiment 1, at Aberystwyth. Vegetative stage primordia of ~0.2mm (A), and developing panicles of 0.22mm (B), 0.48mm (C), and 2mm (D), visible in methylene blue-stained longitudinal sections and labelled with apical meristem (am), branch base (bb), abscission zone (az), floral bract (fb), and floral meristem (fm). (This figure is available in colour at JXB online.)
Experiment 2: flowering trends and yield traits under warm SD, cool SD, and warm LD treatments
Data logging confirmed that the plants grown in these compartments were exposed to target photoperiod and temperature treatments (Fig. 1B). The photoperiod programme was modified at day 41 due to glasshouse height limitations. Photoperiod was reduced more rapidly over the course of 3 d from 13.9h to 13.5h and 13.1h, on days 41, 42, and 43, respectively (Fig. 1B), in order to accelerate the floral transition.
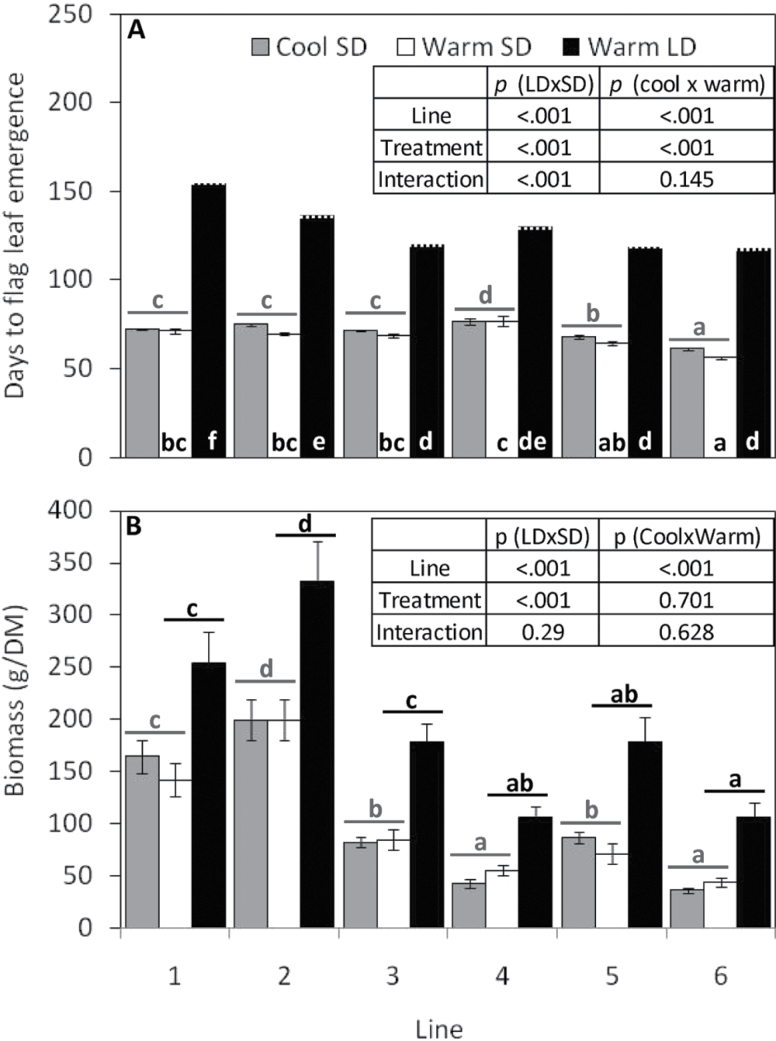
All lines exhibited a highly significant (P < 0.001) delay in flag leaf emergence under LDs (Fig. 3A; Supplementary Table S2 at JXB online). However, every line had undergone the floral transition by day 136, since growing panicles at various stages of development were observed inside the excised stems of each line under the LD treatment (Fig. 3A; panicles which had not yet emerged are represented by a dotted line). The delay in flowering was associated with a highly significant (P < 0.001) increase in biomass (Fig. 3A, B; Supplementary Table S2). Days to flag leaf emergence was also significantly delayed by cool, compared with warm, SDs (Fig. 3A; Supplementary Table S3), although there was no correspondingly significant increase in yield (Fig. 3B; Supplementary Table S3); however, the °Cd required to reach flag leaf emergence was greater in the warm SD treatment, at 1240±106.5, compared with the cool SD treatment, at 1100±115.7.
Fig. 3.
Impacts of short day (SD), long day (LD), and temperature (warm/cool) (see Fig. 1) on days to flowering (A) and biomass (g DM–1) (B) in six Miscanthus sacchariflorus lines grown in a controlled glasshouse experiment ±SE (n=7). No SE is shown for warm, LD bars in (A), where days to flowering were predicted from floral primordia found in dissected stems at final harvest (indicated by a dotted line). Where ANOVA indicated that treatment, line, or the interaction thereof were significant (inset table), groupings from multiple range tests are indicated as lower case letters either inside bars (where interactions were present), or spanning cool/warm (grey) or SD/LD (black) treatments (where interactions were not present).
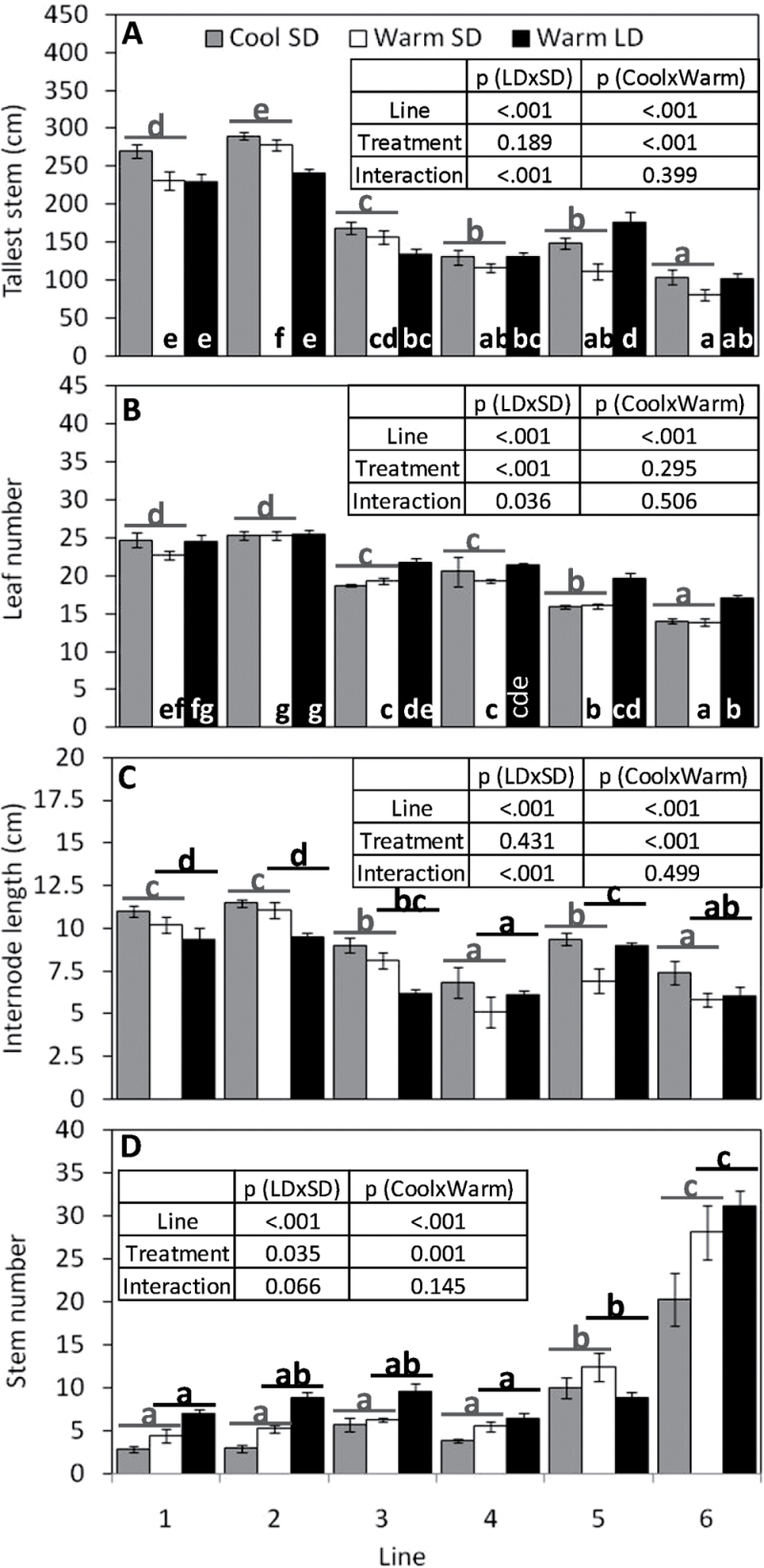
There were significant intereactions between line and daylength for both stem height and leaf number (see Fig. 4A, B for groupings) and, based on least significant differences, only line 5 had stems that were significantly taller under LDs, compared with SDs (Fig. 4A; Supplementary Table S2 at JXB online), whereas leaf numbers were significantly greater under LDs for lines 1, 3, 5, and 6 (Fig. 4B; Supplementary Table S2), compared with SDs (Supplementary Table S3).
Fig. 4.
Impacts of short day (SD), long day (LD), and temperature (warm/cool) (see Fig. 1) on stem height (A), leaf number (B), average internode length(stem height/leaf number) (C), and stem number (D), in six Miscanthus sacchariflorus lines grown in a controlled glasshouse experiment ±SE (n=7). Where ANOVA indicated that treatment, line, or the interaction thereof were significant (inserted table), groupings from multiple range tests are indicated as lower case letters either inside bars (where interactions were present), or spanning cool/warm (grey) or SD/LD (black) treatments (where interactions were not present).
The rank order of flowering and biomass, along with the associated photoperiod and degree days at the estimated time of floral initiation (using data from the first experiment), is reported in Table 2. A general trend was observed between latitude and rank order of flowering and biomass, where flowering order progressed from a northerly to southerly cline, and higher biomass corresponded with later flowering times (and thus lower latitudes). Line 4 (from South Korea) deviated from this trend, however, and, despite originating from a mid range latitude, it flowered later than all other lines.
Table 2.
Summary flowering and yield data for Miscanthus sacchariflorus lines grown in controlled glasshouse conditions at Aberystwyth, Wales, UK.
| Line | Latitude (°N) | Altitude (m) | Country of origin | Flowering rank order | Biomass rank order | Photoperiod at initiationa | Degree days at initiationa |
|---|---|---|---|---|---|---|---|
| 6 | 39.3 | 30 | China | 1 | 6 | 13.8±0.111 | 568±17.5 |
| 5 | 38.4 | 1000 | Japan | 2 | 4 | 13.0±0.078 | 689±21.48 |
| 3 | 34.1 | 22 | Japan | 3 | 3 | 12.8±0.027 | 752±22.85 |
| 1 | 28.8 | 33 | China | 4 | 2 | 12.7±0.028 | 782±26.49 |
| 2 | 29.4 | 27 | China | 5 | 1 | 12.7±0.034 | 787±16.34 |
| 4 | 34.5 | 50 | S. Korea | 6 | 5 | 12.5±0.057 | 873±36.46 |
a Mean of warm and cool temperature treatments.
Floral initiation was calculated from Experiment 1.
Stem height and leaf number were used to calculate the average internode length for each line. The two thick-stemmed M. sacchariflorus types had the highest biomass and tallest stems, as well as the greatest number of leaves and average internode length (Fig. 4A–C). However, stem height alone was not a good overall predictor of biomass, which was greatest under warm LDs whereas stem height was not (Figs 3B, 4A).
Simple linear regression using line as the grouping factor showed a negative correlation between stem height and stem number (R 2=0.79, P < 0.001). Stem numbers were higher in warm versus cool treatments (Fig. 4D; Supplementary Table S3 at JXB online) and, in all but line 5, were higher under warm LDs compared with warm SDs. The only line in which stem height was not greater under LDs compared with SDs was line 5 (Fig. 4D).
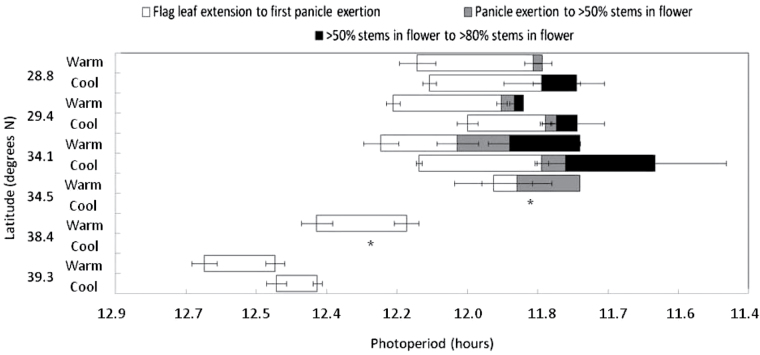
Flowering synchronicity within whole plants varied considerably, as shown in Fig. 5. Lines 5 and 6 were the earliest to flower, but, despite this, did not progress to panicle exertion in >50% of the plant. Lines 1, 2, and 3 flowered more uniformly, and in the case of the two thick-stemmed M. sacchariflorus types plants exposed to the cooler, but not the warmer, thermal regime reached panicle exertion in >80% stems (Fig. 5). In the case of line 2, this process took only 8 d from flag leaf emergence. The duration from flag leaf emergence to panicle exertion did not differ significantly between the two temperature regimes, with the exception of lines 4 and 5, where panicle exertion was not reached in either case in the cool treatment (Fig. 5).
Fig. 5.
Onset and development of flowering (first stem exerting flag leaf, first stem exerting panicle, >50% stems exerting panicles, and >80% stems exerting panicles) in individual plants of Miscanthus sacchariflorus from six latitudes grown in a controlled environment in response to warm and cool temperature regimes from Experiment 2 ±SE (n=7). Temperature accumulated was 180 °Cd more in the warm, compared with cool treatment. An asterisk indicates that flag leaf exertion was the furthest developmental stage reached.
Trait phenology was generally consistent across all treatments. For example, line 2 was the tallest line, with the highest leaf count and biomass under all three treatments. Line 6 was consistently the plant with the lowest biomass, with the shortest but most numerous stems. Stem number was lowest across every treatment in line 1.
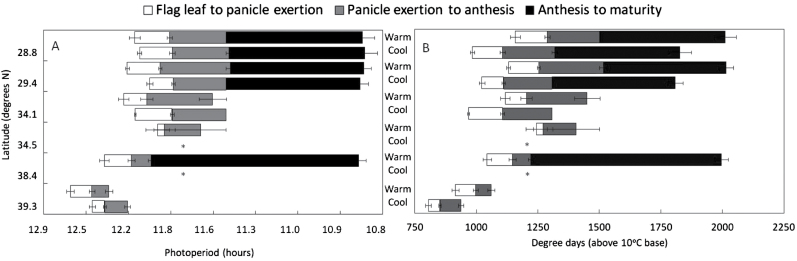
In labelled stems (first stem to reach flag leaf emergence), it was possible to compare the development of panicles to maturity (seed set) across both warm and cool SD treatments. Lines 1 and 2 both reached maturity under warm and cool SDs, while line 5 reached maturity under warm SDs only, and lines 3, 4, and 6 did not reach anthesis (Fig. 6). Although the number of days to flag leaf emergence was generally earlier under warm, compared with cool, SDs, the actual °Cd required were less (Fig. 6A, B). Overall °Cd values from flag leaf emergence to panicle emergence were 128±8.3 and 112±7.5 under warm and cool SDs, respectively, and from panicle emergence to anthesis were 197±18.0 and 184±10.5, respectively. Assuming an average of 18 d from initiation to flag leaf emergence, as indicated by the first experiment, floral initiation to anthesis was 610±6.3 °Cd under warm SDs and 585±7.5 °Cd under cool SDs.
Fig. 6.
Onset and completion of four stages of flowering [flag leaf exertion, panicle exertion, anthesis, and maturity (seed set)] in an individual stem of each of six Miscanthus sacchariflorus lines from different latitudes ±SE (n=7). Data are shown in response to warm and cool temperature regimes from Experiment 2 that differed in accumulated temperature by 180 °Cd. An asterisk indicates that flag leaf exertion was the furthest developmental stage reached. (A) Photoperiod at each flowering stage. (B) Degree days at each flowering stage.
Discussion
Miscanthus sacchariflorus is a quantitative short-day plant
The first hypothesis tested was that the selected lines were obligate SD plants that would not flower when daylengths exceeded a certain critical daylength (estimated at ~12.5h). A number of developmental stages of floral primordia that occurred between photoperiods of 11.7h and 13.2h were observed (Fig. 2). These stages are comparable with those described in Sorghum (Lane, 1963) and, to the authors’ knowledge, represent the first such report of floral transition with associated photoperiods in Miscanthus. Flowering was also observed in constant LD photoperiods of 15.3h, but with a highly significant delay (P < 0.001) when compared with declining SD treatments (Fig. 3A).
Data from Experiment 1 indicated that the duration from floral initiation to flag leaf emergence was ~18 d under the conditions tested. This information was used to predict flag leaf emergence in Experiment 2 for the lines where developing primordia were only observed upon excision of the stem apex at harvest. The highly significant delay in flowering in the LD treatment strongly indicates the existence in M. sacchariflorus of a quantitative SD requirement for flowering, which was delayed under LDs by a minimum of 51 d (in line 3) and by a maximum of 83 d (in line 1). The presence of a few exerted panicles as well as a number of floral primordia in various stages of development in the LD-treated plants at harvest indicated that floral transition, rather than panicle exertion, was delayed under LDs. In many species of the Gramineae, including Sorghum and Saccharum, both panicle initiation and the subsequent differentiation of floral primordia are sensitive to photoperiod, while elongation of the differentiated panicle is usually only temperature sensitive (Julien, 1973; Ritchie and Alagarswamy, 1989). Flowering time did not appear to be affected by static photoperiods, in contrast to that reported for Saccharum (Chilton and Moreland, 1954), since flag leaf emergence occurred at similar times in the sloped, stepped, and static photoperiods of Experiment 1.
Two Chinese M. sacchariflorus lines from 28.8°N and 29.4°N were used in both experiments (lines 1 and 2). Flag leaf emergence for these lines occurred at photoperiods of 12.7h and 12.3h in Experiments 1 and 2, respectively. If floral transition occurred 18 d previously, these lines appear to initiate flowering at photoperiods between 13h and 13.2h. The associated accumulated temperatures at the time of transition were upwards of 800 °Cd in Experiment 1, and upwards of 1087 °Cd in Experiment 2 (Fig. 1A, B). Also included in Experiment 2 was a thin-stemmed M. sacchariflorus line (line 6) from China. Flag leaf emergence was first observed in this line, from 39.3°N, at photoperiods of 12.8h and 845 °Cd, corresponding to floral transition at 14.2h and 570 °Cd (Fig. 1B). Photoperiod is first perceived in the leaf, rather than the developing primordia, and the signal to flower is subsequently transmitted to the shoot apex (Corbesier et al., 2007). Grafting experiments in Arabidopsis have demonstrated that this signal becomes detectable in the apex within 24–48h (Notaguchi et al., 2008). Assuming a similarly rapid mechanism in Miscanthus, data from both experiments indicate that the ‘critical’ daylength under which flowering is accelerated compared with LDs for the thick-stemmed types is ~13.2h, and ~14.2h for thin-stemmed M. sacchariflorus types. The critical daylength suggested for the latter is supported by field observations in Aberystwyth, where flowering occurred during natural daylengths of 14h (Jensen et al., 2011a ). The putative critical daylength of the thick-stemmed types is consistent with a highly photoperiod-sensitive Sorghum variety under field conditions, where photoperiods ranging from 13.03h to 13.37h induced flowering, and the duration from floral initiation to flag leaf emergence was reported to vary between 24 d and 33 d (Folliard et al., 2004).
Impact of photoperiod and temperature on biomass
Previous trials in Miscanthus have reported a trend towards reduced biomass in early flowering lines (Clifton-Brown and Lewandowski, 2002). In Arabidopsis, mutations in the flowering gene fve/ms14 result in significantly delayed flowering and increased biomass in both LDs and SDs (Morel et al., 2009). The hypothesis tested here was therefore that delayed flowering would result in increased biomass.
In the present study biomass was significantly increased (P < 0.001) in all lines under static LDs. The average increase was 51.8%, with a minimum increase of 40.2% in line 5 and a maximum of 60.1% in line 2 (Fig. 3B; Supplementary Table S2 at JXB online). The plants with the highest biomass were the two thick-stemmed M. sacchariflorus from the most southerly latitudes. Over all treatments these types had the tallest stems and longest internode length (Fig. 3A, C). This type of M. sacchariflorus have been observed to reach heights of 7 m in China, and to exceed the biomass accumulation of all other Miscanthus species endemic to China (Chen and Renvoize, 2006; Yan et al., 2011). Leaf number significantly increased in all except line 2 under LDs, and was accompanied by a significant increase in stem number (Fig. 4D), which may have contributed to the increase in biomass. The absence of a significant difference in leaf number under different photoperiod regimes in line 2 suggests that floral transition in this line may be less strongly regulated by an environmental signal, rather than reaching a specific developmental stage, but that photoperiod may still play a role in panicle exertion. It is worth noting that stem height may have significantly increased under LDs compared with SDs if the experiment had continued to run. This is because rapid elongation of the upper internode occurs with flag leaf emergence in many Miscanthus lines. Since plants under LDs were reaching this point by the end of the experiment, this late stage elongation would not yet have occurred. In line 4, the biomass increase under LDs was not associated with an increase in leaf or stem number, indicating an increase in stem diameter and/or density (not measured).
A trend in tillering (stem number), where numbers were lowest under cool SDs and highest under warm LDs, was identified in all except line 5. Line 5 contrasted starkly with other lines by producing the fewest and tallest (by 58%) stems under LDs (Fig. 4A, D). Flowering is known to release apical dominance in many plants, promoting the production of further tillers. Apical dominance may operate strongly in line 5, so that under LDs tillering remains suppressed for an extended period. As the only line to originate from altitude (at 1000 m), where conditions are more changeable and weather is more extreme, stronger apical dominance would perhaps direct photosynthates toward viable seed, rather than rhizome, to survive harsh winters.
Despite only 189 °Cd difference between the two temperature treatments, stem number was significantly lower in the cool, compared with the warm, treatment (Fig. 4D). Tiller formation is known to be impacted strongly by temperature and, in Sorghum, the relationship between stem number and temperature has been shown to be linear (Lafarge et al., 2002). In rice, ambient temperatures have been shown to make the highest direct contribution to stem number out of a range of factors tested (Yoshida, 1973).
Impact of ambient temperature on floral initiation and panicle development
In Arabidopsis, a plant that flowers preferentially under LDs, a temperature increase to 25 °C or 27 °C under SDs can trigger flowering at the same time as at 16 °C under LDs (Balasubramanian et al., 2006). Temperature has also been shown to influence flowering time in Sorghum where flowering is promoted under 22–26 °C, compared with 17–20 °C warmer temperatures (Craufurd and Qi, 2001; Kumar et al., 2009; Araldi et al., 2010). It was hypothesized that flowering in Miscanthus would be accelerated under 28 °C/24 °C, compared with 20 °C/16 °C regimes.
Flowering time was promoted under warm, compared with cool, SDs in only three out of six lines (Fig. 3A; Supplementary Table S3 at JXB online). However, temperature regimes differed only during phase 1 of the experiment, and the rapid growth of plants during this phase prompted the photoperiod phase of the experiment to be entered sooner than planned and after the accrual of only 189 °Cd difference between the two temperature treatments. It is anticipated that flowering time (and biomass) would have been significantly impacted by temperature in all lines, were the degree day differences between treatments greater.
Although anthesis was reached by every line in at least one of the SD treatments, only three lines reached seed set. It was found that under cool SDs, lines 4 and 5 from 34.5°N and 38.4°N, respectively, did not reach panicle exertion, and also that lines 5 and 6 did flower in >50% of stems. This was surprising as lines 5 and 6 were the first to exhibit floral initiation, although line 4 was the last. The origin of these lines was the three most northerly latitudes where daylengths decline more rapidly, which might suggest a more uniform flowering response. However, due to concerns over the rate of canopy growth on starting the second phase (photoperiod ‘inductive’ phase) of the experiment, the photoperiod was reduced more quickly (Fig. 1B). Furthermore, the transition from phase 1 (temperature phase) to phase 2 required the transfer of the cooler treated plants to an increase in temperature gradient at 28 °C/24 °C, from 20 °C/16 °C. In Sorghum and other grasses, floral transition can be highly sensitive to stresses (Ong and Monteith, 1985; Craufurd and Peacock, 1993), and one explanation for the incomplete flowering of the above lines is the movement of these plants during a sensitive phase of development. These lines also exhibited greater numbers of stems than those which flowered more synchronously. Some of the stems may have been physically immature and unable to perceive the flowering signal at the point at which the optimal photoperiod was reached. In Sorghum and Saccharum, a juvenile phase exists, which is controlled by both line and temperature (Coleman, 1969; Ellis et al., 1997). During this phase, flowering cannot occur and photoperiod has no effect. The present study has not addressed the issue of juvenility in Miscanthus, although mitigation of such a mechanism was made by permitting the plants to grow to the five leaf stage before transferring to photoperiod treatment. In sugar cane, floral induction is thought to be possible only after the maturation of 2–4 internodes (Coleman, 1969).
With respect to phenology, panicle development in Miscanthus appeared similar to that in Sorghum, where studies show a thermal requirement of 450 °Cd from floral initiation to flag leaf emergence, a further 270 °Cd from flag leaf emergence to panicle emergence, and an additional 150 °Cd to anthesis (Ritchie and Alagarswamy, 1989), totalling 870 °Cd. Assuming an average duration of initiation to flag leaf emergence in Miscanthus of ~18 d, the total requirement from initiation to anthesis was calculated to be ~25 d, and 600 °Cd under SDs. Commercial sugar cane clones induced to flower in Lucknow, India, took 28–35 d from transition to flag leaf emergence (Swapna and Singh, 2008). However, in a separate study using 12 sugar cane varieties, floral initiation to panicle emergence took 45–82 d (Maccoll, 1977), so the speed of development can vary considerably.
Flowering order follows a geographical trend that is not strictly based on latitude
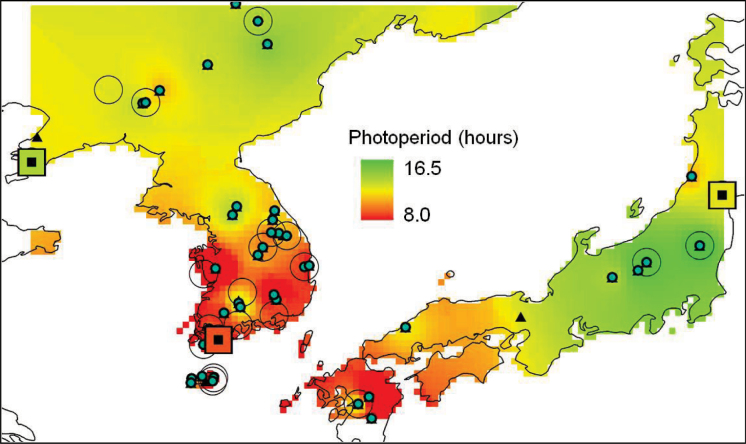
Although the sample size is small, there was some indication of a trend in flowering rank order according to latitude in all but the South Korean line (which flowered later than latitude alone might indicate) (Table 2). Previous field observations of Miscanthus flowering times in Aberystwyth, Wales, when superimposed onto a map of the provenance of those lines, reflected trends in temperature, degree days, and precipitation of those locations, rather than latitude alone (Jensen et al., 2011a ). Three lines used in the present study originated from locations within or near those on the aforementioned map. These lines were therefore superimposed onto the same map and it was found that the photoperiods corresponding to their flowering times in glasshouses in Aberystwyth fitted the reported trend (Fig. 7). In this case, the line from South Korea responded consistently with lines from that area (Fig. 7). The influence of climatic factors such as altitude and precipitation on flowering time patterns reported in Jensen et al. (2011a) has also been observed in ecotypes of Arabidopsis in Norway, where distance from the coast, and altitude, were found to explain much of the variation in flowering times following vernalization (Lewandowska-Sabat et al., 2012). A similar effect in Miscanthus can be seen, where flowering variation is better explained as a function of altitude, precipitation, and temperature, rather than simply by latitude and longitude. This potential predictability in flowering based on location of origin appears to contrast with many domesticated crops, including sugar cane, where the relationship between flowering time and latitude of origin does not generally hold (Maccoll, 1977). However, many species, such as sugar cane, have been highly bred and hybridized, whereas Miscanthus is still undomesticated. There is also the possibility of a residual epigenetic effect on flowering time where the environment of origin may have a lasting impact on phenotypes as a consequence of localized climate at the site of collection, as in Norway Spruce (Yakovlev et al., 2010). The possible impacts of such a phenomenon are outside the remit of the present study.
Fig. 7.
Modified kriged map of flowering times in the present study against previously reported geographical trends in Asia, reflecting temperature, degree days, and precipitation of those locations (Jensen et al., 2011a ). Lines from the present study (squares) are included with those from a previous study where flowering times of M. sinensis (small blue circle) and M. sacchariflorus (large open circle) observed in field trials in Aberystwyth, Wales, were superimposed onto locations of origin in Asia.
Conclusion
Experimental manipulation of the daylength and climate conditions performed in a modified glasshouse has provided the first report of the independent effects of photoperiod and temperature in a diverse selection of M. sacchariflorus lines. This information is important for the domestication of this species, and for highlighting consistencies with Sorghum, which is the closest relative to Miscanthus for which a reference genome exists. Maturity locus Ma1, the major repressor of flowering under LDs in Sorghum, has recently been positionally cloned, and considerable progress has been made in understanding the regulation of flowering in this species (Murphy et al., 2011). The conservation and synteny recently identified between Sorghum and Miscanthus (Ma et al., 2012) indicate that mapping of genes involved in the control of flowering time will be greatly assisted.
Environmental conditions that successfully initiate flowering have been defined and strong evidence is provided that M. sacchariflorus lines operate a quantitative SD flowering response. For the first time the impact that flowering can have on biomass in Miscanthus is demonstrated, showing that delaying flowering by an average of 61 d can increase biomass by an average of 52%. Germplasm from a wide latitudinal range has shown extensive phenotypic plasticity. However, flowering times were consistent with trends previously reported using geographical and climate data from the collection sites of the germplasm (Jensen et al., 2011a ). This study supports the domestication of highly productive Miscanthus cultivars and contributes towards an understanding of the likely impacts of climate change on yield through the provision of data regarding flowering times under differing photoperiods and temperatures. Where flowering time is photoperiod sensitive, planting in latitudes where flowering is induced before the accumulation of maximum biomass is likely to impact yield significantly. Optimal planting zones must therefore be determined according to photoperiod sensitivity. However, the impact of temperature on flowering times was also highly significant (P < 0.001), despite differences between treatments of only 180 °Cd. Modelling flowering times under varied climate scenarios will therefore be an important factor in Miscanthus commercialization. The identification of similarities between the flowering phenologies of M. sacchariflorus and Sorghum indicates the existence of comparable flowering pathways. Encouragingly, this suggests that the mapping of genes controlling flowering time in Miscanthus will be expedited through genotyping by sequencing and the use of Sorghum as a reference genome.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Leaf formation, stem elongation, floral transition, and flag leaf exertion in lines 1 and 2 from Experiment 1, under sloped, stepped, and constant photoperiods ±SE (n=5).
Table S1. Summary statistics for Experiment 1, impact of sloped, stepped, and static photoperiods on leaf number and stem length in two lines of Miscanthus sacchariflorus.
Table S2. Summary of ANOVA results for Experiment 2 for the impact of long-day versus short-day photoperiods on six traits in Miscanthus sacchariflorus in a controlled glasshouse.
Table S3. Summary of ANOVA results for Experiment 2 for the impact of warm versus cool temperatures on six traits in Miscanthus sacchariflorus in a controlled glasshouse.
Acknowledgements
The authors wish to thank Marc Loosley for support in phenotyping, Brian Jones and Sian Thomas-Jones for the production and maintenance of clonal material, and Brian Jones and Laurence Jones for technical assistance in modifying glasshouse compartments. We thank Michal Mos for assistance with plant maintenance and harvest. The authors also thank Dr Astley Hastings for the generation of Fig. 7. This work was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) Grant BB/E014933/1, a BBSRC Institute Strategic Programme Grant on Bioenergy and Biorenewables (BBSEG00003134), and a BBSRC Institute career path fellowship BB/E024319/2; and the Department for Environment Food and Rural Affairs (DEFRA) (grant NF0426).
References
- Araldi R, Silva FML, Ono EO, Rodrigues JD. 2010. Flowering in sugarcane. Ciencia Rural 40, 694–702 [Google Scholar]
- Balasubramanian S, Sureshkumar S, Lempe J, Weigel D. 2006. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genetics 2, 980–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez MA, Ahn JH, Weigel D. 2003. A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nature Genetics 33, 168–171 [DOI] [PubMed] [Google Scholar]
- Caddel JL, Weibel DE. 1971. Effect of photoperiod and temperature on the development of sorghum. Agronomy Journal 63, 799–803 [Google Scholar]
- Chen SL, Renvoize SA. 2006. Miscanthus. In: Wu ZY, Raven PH, Hong DY, eds. Flora of China. Beijing/St Louis: Science Press/Missouri Botanical Garden Press, 581–583 [Google Scholar]
- Chilton S, Moreland C. 1954. Experiments on the flowering of sugarcane. Sugar Bulletin 32, 165–169 [Google Scholar]
- Clifton-Brown JC, Lewandowski I. 2002. Screening Miscanthus genotypes in field trials to optimise biomass yield and quality in Southern Germany. European Journal of Agronomy 16, 97–110 [Google Scholar]
- Clifton-Brown JC, Lewandowski I, Andersson B, et al. 2001. Performance of 15 Miscanthus genotypes at five sites in Europe. Agronomy Journal 93, 1013–1019 [Google Scholar]
- Coleman RE. 1969. Physiology of flowering in sugarcane. Proceedings of the International Society of Sugarcane Technologists; 13th Congress, Taiwan, 992ld–1000
- Corbesier L, Vincent C, Jang SH, et al. 2007. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316, 1030–1033 [DOI] [PubMed] [Google Scholar]
- Craufurd PQ, Flower DJ, Peacock JM. 1993. Effect of heat and drought stress on sorghum (Sorghum bicolor). 1. Panicle development and leaf appearance. Experimental Agriculture 29, 61–76 [Google Scholar]
- Craufurd PQ, Peacock JM. 1993. Effect of heat and drought stress on sorghum (Sorghum bicolor). 2. Grain-yield. Experimental Agriculture 29, 77–86 [Google Scholar]
- Craufurd PQ, Qi AM. 2001. Photothermal adaptation of sorghum (Sorghum bicolor) in Nigeria. Agricultural and Forest Meteorology 108, 199–211 [Google Scholar]
- Deuter M. 2000. Breeding approaches to improvement of yield and quality in Miscanthusgrown in Europe. In: Lewandowski I, Clifton-Brown J. European Miscanthus improvement (FAIR3 CT-96–1392) Final Report. Stuttgart, 28–52 [Google Scholar]
- Ellis RH, Qi A, Craufurd PQ, Summerfield RJ, Roberts EH. 1997. Effects of photoperiod, temperature and asynchrony between thermoperiod and photoperiod on development to panicle initiation in Sorghum. Annals of Botany 79, 169–178 [Google Scholar]
- Folliard A, Traoré PCS, Vaksmann M, Kouressy M. 2004. Modeling of sorghum response to photoperiod: a threshold-hyperbolic approach. Field Crops Research 89,:59–70 [Google Scholar]
- Hammer GL, Vanderlip RL, Gibson G, Wade LJ, Henzell RG, Younger DR, Warren J, Dale AB. 1989. Genotype-by-environment interaction in grain—Sorghum. 2. Effects of temperature and photoperiod on ontogeny. Crop Science 29, 376–384 [Google Scholar]
- Hodkinson TR, Renvoize SS, Chase MW. 1997. Systematics of Miscanthus. Aspects of Applied Biology 49, 189–198 [Google Scholar]
- Jensen E, Farrar K, Thomas-Jones S, Hastings A, Donnison I, Clifton-Brown J. 2011. a Characterization of flowering time diversity in Miscanthus species. GCB Bioenergy 3, 387–400 [Google Scholar]
- Jensen E, Squance M, Hastings A, Jones S, Farrar K, Huang L, King R, Clifton-Brown J, Donnison I. 2011. b Understanding the value of hydrothermal time on flowering in Miscanthus species. Aspects of Applied Biology 112, 181–189 [Google Scholar]
- Julien MHR. 1973. Physiology of flowering in Saccharum. 1. Daylength control of floral initiation and development in S. spontaneum L. Journal of Experimental Botany 24, 549–557 [Google Scholar]
- Julien R. 1968. Reports of the Mauritius Sugar Industry Research Institute 39–51 [Google Scholar]
- Koornneef M, Alonso-Blanco C, Peeters AJM, Soppe W. 1998. Genetic control of flowering time in arabidopsis. Annual Review of Plant Physiology and Plant Molecular Biology 49, 345–370 [DOI] [PubMed] [Google Scholar]
- Kumar SR, Hammer GL, Broad I, Harland P, McLean G. 2009. Modelling environmental effects on phenology and canopy development of diverse sorghum genotypes. Field Crops Research 111, 157–165 [Google Scholar]
- Lafarge TA, Broad IJ, Hammer GL. 2002. Tillering in grain sorghum over a wide range of population densities: identification of a common hierarchy for tiller emergence, leaf area development and fertility. Annals of Botany 90, 87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane HC. 1963. Effect of light quality on maturity in the milo group of sorghum. Crop Science 3, 496–499 [Google Scholar]
- Lewandowska-Sabat AM, Fjellheim S, Rognli OA. 2012. The continental–oceanic climatic gradient impose clinal variation in vernalization response in Arabidopsis thaliana. Environmental and Experimental Botany 78, 109–116 [Google Scholar]
- Ma X-F, Jensen E, Alexandrov N, et al. 2012. High resolution genetic mapping by genome sequencing reveals genome duplication and tetraploid genetic structure of the diploid Miscanthus sinensis . PLoS One 7, e33821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccoll D. 1977. Some aspects of the flowering of sugar cane in Barbados and its control in a breeding programme. Annals of Botany 41, 191–201 [Google Scholar]
- Morel P, Trehin C, Breuil-Broyer S, Negrutiu I. 2009. Altering FVE/MSI4 results in a substantial increase of biomass in Arabidopsis—the functional analysis of an ontogenesis accelerator. Molecular Breeding 23, 239–257 [Google Scholar]
- Murphy RL, Klein RR, Morishige DT, Brady JA, Rooney WL, Miller FR, Dugas DV, Klein PE, Mullet JE. 2011. Coincident light and clock regulation of pseudoresponse regulator protein 37 (PRR37) controls photoperiodic flowering in sorghum. Proceedings of the National Academy of Sciences, USA 108, 16469–16474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaguchi M, Abe M, Kimura T, Daimon Y, Kobayashi T, Yamaguchi A, Tomita Y, Dohi K, Mori M, Araki T. 2008. Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant and Cell Physiology 49, 1922–1922 [DOI] [PubMed] [Google Scholar]
- Ong CK, Monteith JL. 1985. Response of pearl-millet to light and temperature. Field Crops Research 11, 141–160 [Google Scholar]
- Ritchie JT, Alagarswamy G. 1989. Simulation of sorghum and pearl millet phenology. In: Virmani SM, Tandon HLS, Alagarswamy G, eds. Modeling the growth and development of sorghum and pearl millet. Andhra Pradesh: International Crops Research Institute for the Semi-Arid Tropics, 24–26 [Google Scholar]
- Rooney WL, Blumenthal J, Bean B, Mullet JE. 2007. Designing sorghum as a dedicated bioenergy feedstock. Biofuels Bioproducts & Biorefining 1, 147–157 [Google Scholar]
- Swapna M, Singh PK. 2008. Shoot apex development at various stages of flowering in sugarcane (Saccharum spp. hybrid). Cytologia 73, 173–177 [Google Scholar]
- Wingler A, Purdy SJ, Edwards SA, Chardon F, Masclaux-Daubresse C. 2009. QTL analysis for sugar-regulated leaf senescence supports flowering-dependent and -independent senescence pathways. New Phytologist 185, 420–433 [DOI] [PubMed] [Google Scholar]
- Yakovlev IA, Fossdal CG, Johnsen O. 2010. MicroRNAs, the epigenetic memory and climatic adaptation in Norway spruce. New Phytologist 187, 1154–1169 [DOI] [PubMed] [Google Scholar]
- Yan J, Chen W, Luo FAN, et al. 2011. Variability and adaptability of Miscanthus species evaluated for energy crop domestication. GCB Bioenergy 4, 49–60 [Google Scholar]
- Yoshida S. 1973. Effects of temperature on the growth of the rice plant (Oryza sativa L.) in a controlled environment. Soil Science and Plant Nutrition 19, 299–310 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.