Abstract
Three phytohormone molecules – ethylene (ET), jasmonic acid (JA) and salicylic acid (SA) – play key roles in mediating disease response to necrotrophic fungal pathogens. This study investigated the roles of the ET, JA, and SA pathways as well as their crosstalk during the interaction between tomato (Solanum lycopersicum) plants and a necrotrophic fungal pathogen Alternaria alternata f. sp. lycopersici (AAL). Both the ET and JASMONIC ACID INSENSITIVE1 (JAI1) receptor-dependent JA signalling pathways are necessary for susceptibility, while SA response promotes resistance to AAL infection. In addition, the role of JA in susceptibility to AAL is partly dependent on ET biosynthesis and perception, while the SA pathway enhances resistance to AAL and antagonizes the ET response. Based on these results, it is proposed that ET, JA, and SA each on their own can influence the susceptibility of tomato to AAL. Furthermore, the functions of JA and SA in susceptibility to the pathogen are correlated with the enhanced or decreased action of ET, respectively. This study has revealed the functional relationship among the three key hormone pathways in tomato defence against AAL.
Key words: AAL, ethylene, hormonal interactions, jasmonic acid, salicylic acid, tomato
Introduction
Plants have evolved complex defence mechanisms to defend themselves against different pathogens, which are encountered throughout their lifetimes (Nürnberger and Lipka, 2005). Pathogen attack triggers complex signalling cascades regulated by signalling molecules such as jasmonic acid (JA), salicylic acid (SA), and ethylene (ET), resulting in plant defence responses (Sheard et al., 2010). These defence responses are considered to be dependent on specific plant–attacker interactions. In general, SA-mediated signalling pathways are implicated in resistance to biotrophs or hemibiotrophic pathogens, whereas JA- and ET-dependent responses are effectively against necrotrophs and herbivorous insects (McDowell and Dangl, 2000). For example, Arabidopsis mutants that are impaired in SA production, as well as NahG transgenic plants lacking SA, exhibit enhanced disease susceptibility to a variety of biotrophic pathogens, including the fungal pathogen Hyaloperonospora arabidopsidis and the bacterial pathogen Pseudomonas syringae (Kunkel and Brooks, 2002). In contrast, the JA-perception mutant coronatine insensitive 1 (coi1) shows severely compromised resistance to the necrotrophic fungal pathogens Alternaria brassicicola and Botrytis cinerea and the bacterial leaf pathogen Erwinia carotovora (Thomma et al., 1998).
Plant hormones usually do not regulate defence responses via a linear mode of action but through complex network of regulatory interactions. One of the best-studied examples is the interaction between the SA and JA signalling pathways (Kunkel and Brooks, 2002; Koornneef and Pieterse, 2008). For example, exogenous SA application inhibits JA-induced proteinase inhibitor gene expression and protein accumulation in tomato (Solanum lycopersicum) (Doares et al., 1995), whereas methyl jasmonate (MeJA) treatment inhibits SA-induced acidic pathogenesis-related (PR) gene expression in tobacco (Niki et al., 1998). Reduced susceptibility to the coronatine-producing stains of P. syringae in Arabidopsis mutants coi1 and jin1, which are defective in JA signalling, is correlated with elevated PR1 expression and is dependent on SA accumulation (Kloek et al., 2001; Laurie-Berry et al., 2006). Coronatine promotes susceptibility by stimulating JA signalling and inhibiting SA-mediated defence that normally limits the growth of P. syringae (Kunkel and Brooks, 2002; Laurie-Berry et al., 2006). Infection with P. syringae, which induces SA-mediated defence, renders plants more susceptible to the necrotrophic pathogen Alternaria brassicicola by suppression of the JA signalling pathway (Spoel et al., 2007). The above pharmacological and genetic evidence shows that the crosstalk between SA and JA is antagonistic. However, cooperation and sequential positive interactions have also been reported between the SA and JA pathways (Mur et al., 2006). JA and ET are considered to act synergistically in response to pathogens and activate defence-related gene expression in Arabidopsis (Thomma et al., 1999). Microarray data has revealed that nearly half of the genes induced by ET are also induced by JA (Schenk et al., 2000). Lorenzo et al. (2003) reported that JA and ET pathways converge in the transcriptional activation of ETHYLENE RESPONSE FACTOR1 (ERF1), which encodes a transcription factor that regulates the expression of pathogen response genes.
Most knowledge about crosstalk in plant hormone signalling is from Arabidopsis, mainly due to the availability of genetic and genomic resources. It will be interesting to investigate the response of plants to diverse pathogens in other plant species. Tomato is not only a major vegetable crop, but also a useful model plant for studying the complex network of hormone pathways involved in host defence response, for many mutants or transgenic plants that are altered in hormonal metabolism or signalling have been characterized. In tomato, damage to a single leaflet by mechanical wounding or an insect results in production of systemin, an 18-amino-acid peptide (Pearce et al., 1991). Interaction of systemin with its proposed receptor triggers the activation of the octadecanoid pathway for JA biosynthesis, which induces defence gene expression (Sun et al., 2011). JA-deficient mutants def1 (defenseless-1, with a defective octadecanoid synthesis pathway) and spr2 (a suppressor of (pro)systemin-mediated responses2 mutation with reduced levels of trienoic fatty acids) have been characterized (Li et al., 2003). In addition, the 35S::prosystemin transgenic line (35S::prosys), which overexpresses prosystemin, constitutively accumulates high levels of proteinase inhibitor proteins throughout the entire plant (Howe and Ryan, 1999). The F-box protein CORONATINE INSENSITIVE 1 (COI1) is a key player in the JA pathway (Sheard et al., 2010). A JA-insensitive mutant jai1 (jasmonic acid insensitive1) contains a mutation in the tomato homologue of Arabidopsis COI1 and fails to express JA-regulated genes in response to wounding and MeJA (Li et al., 2004). Moreover, several ET mutants in tomato, such as Nr (Never ripe) and epi (epinastic), have been characterized. Nr is an ET-insensitive mutant, caused by a single base substitution in the N-terminal coding region of the ET receptor gene ETR3 (i.e. NR) that is homologous to ETR1 in Arabidopsis (Wilkinson et al., 1995). The ET-overproducing mutant epi is constitutively activated in a subset of ET responses (Fujino et al., 1988). To study SA responses, transgenic NahG plants expressing the enzyme salicylate hydroxylase were used. The enzyme converts endogenous SA immediately to inactive catechol, therefore the plants are deficient in SA accumulation (Brading et al., 2000).
The necrotrophic pathogen Alternaria alternata f. sp. lycopersici (AAL) causes Alternaria stem canker on some susceptible tomato cultivars. The disease is characterized by dark-brown canker formation on stems and leaf tissue necrosis between the veins (Brandwagt et al., 2002). The fungus produces a group of mycotoxins, AAL toxins, which have been shown as a host-specific pathogenicity factor (Brandwagt et al., 2002). AAL toxins, together with another group of structurally related mycotoxins, fumonisins, are classified as sphinganine-analogue mycotoxins due to their structural similarity to sphinganine (Brandwagt et al., 2002). Resistance to AAL or insensitivity to AAL toxins in tomato is determined by the single co-dominant Alternaria Stem Canker (ASC) locus. The Asc-1 gene is homologous to the yeast Longevity Assurance Gene 1 (LAG1) (Brandwagt et al., 2002). Using a protoplast model system, Asai et al. (2000) showed that fumonisin B1-induced cell death in Arabidopsis requires the JA-, SA-, and ET-mediated signalling pathways, but not the signal transmitter NPR1. Previous reports indicated that an ET-dependent pathway is involved in AAL-toxin-induced cell death (Moore et al., 1999; Asai et al., 2000). Recently, Zhang et al. (2011) studied the role of JA and ET in AAL-toxin-induced tomato cell death and showed that both JA and ET promote AAL-toxin-induced cell death alone and that JASMONIC ACID INSENSITIVE1 (JAI1) receptor-dependent JA signalling promotes programmed cell death through ET action. Egusa et al. (2009) also reported that JA promotes the susceptibility of detached tomato leaves to AAL infection. However, the role of plant hormones and their crosstalk in the interaction between tomato plants and AAL remain to be investigated.
The present study, using ET, JA, and SA mutants combined with exogenous application, demonstrates that both the ET response and the JA-dependent signalling pathway are necessary for susceptibility of tomato to AAL fungus, while the SA pathway is responsible for the resistance to this pathogen. Furthermore, it is observed that the role of JA and SA in susceptibility to the pathogen is associated with the action of ET the pathway. The significance of these findings is also discussed.
Materials and methods
Plant materials and growth conditions
Tomato cultivar Castlemart (CM) is the parental line of JA mutants spr2 and jai1 as well as the transgenic line 35S::prosys. Homozygous jai1 seedlings were selected from F2 populations as described previously (Li et al., 2004). 35S::prosys seeds were collected from a 35S::prosys homozygote (Howe and Ryan, 1999) that had been backcrossed five times to its wild-type (WT) line cv. CM as the recurrent parent (Li et al., 2002). The ET-overproducing mutant epinastic (epi), which is constitutively activated in a subset of ET responses (Fujino et al., 1988), and its WT line, VFN8, were obtained from the Tomato Genetics Resource Center (University of California, Davis, CA, USA). Tomato seeds of NahG transgenic line and its WT cv. Moneymaker (MM) were kindly provided by Dr. Jonathan D. G. Jones (John Innes Centre, Norwich, UK).
Seeds were sown in seedling trays containing a rich soil mixture after germination on filter paper. Seedlings were grown in a greenhouse, with temperature ranging from 22 to 28 °C (night and day air temperature, respectively) and a 16/6 light/dark cycle. Three weeks after germination, seedlings were transplanted to plastic pots (12cm diameter, 15cm depth) filled with perlite and turfy soil (3:1, v/v), which were watered daily and fertilized weekly with a half-strength Enshi nutrient solution (Zhang et al., 2011). Tomato plants of 7 weeks of age were used in this study.
Fungal infection assay
Fungal culture and infection were performed as described by Brandwagt et al. (2002). In brief, the AAL isolates were grown for 14 days on corn meal agar (CMA) at 25 °C in the dark. Spores were washed by water, filtered, and washed twice in tap water to remove partial in-vitro-produced AAL toxins. The spore quantity was counted using a haemocytometer. Tomato plants of 7 weeks of age were sprayed with spore suspensions containing approximately 1.0×106 spores ml–1 and 0.1% (v/v) Tween 20 and then covered with plastic domes to keep high humidity at 24–26 °C for 48h. Then, the domes were removed and disease symptoms emerged 3–4 days after infection.
Chemical pretreatments
Tomato plants were subjected to different chemical pretreatments before AAL inoculation. Solutions of the ET-action inhibitor silver thiosulphate were prepared by mixing AgNO3 and sodium thiosulphate at a concentration ratio of 1:4 (Bellés et al., 1993). Solutions of the ET-biosynthesis inhibitor CoCl2 were prepared by dissolving in ultrapure water. 1-Aminocyclopropane-1-carboxylic acid (ACC, Sigma, St Louis, MO, USA) was prepared by dissolving in ultrapure water. Solutions containing 0.1mM ACC, 1mM silver thiosulphate, and 0.1mM CoCl2 (all containing 0.1% Tween 20) were applied to plants 24h before AAL infection, while controls were treated with equal amount of 0.1% Tween 20.
1-Methylcyclopropene (1-MCP) treatment was performed as described previously (Diaz et al., 2002). AAL infection was conducted after 48h of 1-MCP treatment.
SA was dissolved in absolute ethanol then added drop wise to 20mM phosphate buffer solution (pH 7.0) (ethanol/phosphate buffer, 1:1000, v/v). For SA pretreatment, tomato seedlings were soil-drenched with 50ml of 0.2, 1, or 2mM SA, respectively, and 20mM phosphate buffer solution (pH 7.0) was used as a control.
For methyl jasmonate (MeJA) treatment, plants were transferred into a lucite container (84L) containing 18 µl MeJA (Sigma). Six cotton wicks were distributed evenly throughout the box and each containing 300 µl MeJA solution (3 µl MeJA, diluted into 297 µl ethanol). Twenty-four hours after MeJA treatment, the cotton wicks were removed and the plants were acclimated to ambient humidity for an additional 24h before AAL infection. Control plants were incubated in a separate container in which ethanol was applied to cotton wicks.
Preparation of endogenous ET-deficient plants and ET-insensitive plants
Tomato WT cv. CM seedlings were divided equally into three groups and sprayed with different solutions to the whole plants weekly from the cotyledon stage to the five-leaf stage. The first group was sprayed with distilled water and used as a control; the second and the third groups were sprayed with 0.1mM CoCl2 and 1mM silver thiosulphate, respectively. The plants in the second and the third groups were used as endogenous ET-deficient and ET-insensitive plants, respectively.
Real-time quantitative PCR (qPCR) analysis
Tomato leaves (at least five leaves from independent plants) were harvested following AAL infection at different time points (0, 1, 3, and 5 d) and immediately immersed in liquid nitrogen. Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, and then treated with RNA free DNase I (Takara, Japan) to remove genomic DNA contamination. For reverse transcription, oligo(dT)18 primers, 10mM dNTP mix, and water were added to 5 µg RNA, following the manufacturer’s protocol (Fermentas, Canada). Quantitative PCR was performed as described previously (Zhang et al., 2011). All experiments were repeated twice, with similar results. For each experiment, data were analysed separately. Results of one representative experiment are shown. The primer sequences used are listed in Table 1.
Table 1.
Primer sequences used for real-time quantitative PCR.
| Gene | GenBank accession | Forward primer (5’–3’) | Reverse primer (5’–3’) | Product (bp) |
|---|---|---|---|---|
| Actin | AB199316 | TGGTCGGAATGGGACAGAAG | CTCAGTCAGGAGAACAGGGT | 190 |
| LOXD | U37840 | GGCTTGCTTTACTCCTGGTC | AAATCAAAGCGCCAGTTCTT | 72 |
| AOS2 | AF230371 | CGATTACCTCCGATTCTGGT | AAATCTTCATCCCACCGAAG | 172 |
| PI-II | K03291 | TGATGAACCCAAGGCAAATA | ACACAACTTGATGCCCACAT | 154 |
| PAL | M83314 | ACAGAATTGTTGACGGGTGA | CCATTCCAGCTCTTCAGACA | 122 |
| ICS | DQ984132 | TCCAGGCTGAAGATGATGAG | TTATTCCAACCGCAAATTCA | 183 |
| NPR1 | AY640378 | GGAAACTTCACTGGCAGACGTC | GTGTCTCTGAAACTTGTCGACC | 415 |
| PR1a | X71592 | TGGTATGGCGTAAGTCGGTA | CTTGGAATCAAAGTCCGGTT | 152 |
| PR2a | M80604 | AGATCTTGAAGCCCTAGCCA | TGGATCAACTTCGTTTCCAA | 118 |
| ACO1 | X58273 | TTGCTCATTTCCTTTGTGGA | GGAAGCTAGCAAAGCAAACC | 122 |
| ACS2 | X59139 | ATCCACCTTGTTTGTGACGA | TGTTCATCGAGGATTTCAGC | 86 |
| ACS4 | X59146 | AATTGCTCGGAGGTAGGATG | TTCCTCTTCCATTGTGCTTG | 154 |
| ERF1 | AY044236 | ATTGGAGTTAGAAAGAGGCCAT | CTCATTGATAATGCGGCTTG | 143 |
| NR | U38666 | GCGGTTATGGTTCTGGTTCT | TGTCGAGCTACATCCAAAGC | 194 |
| ETR4 | AF118843 | CTGCAGATTGGAATGAATGG | ATAAGGCACCGTCAACATCA | 123 |
Fungal biomass measurement
Fungal biomass was measured according to Egusa et al. (2009) with minor modifications. Total genomic DNA was extracted from 1.0g tomato leaves (from independent plants) at 6 d post inoculation using 2 % CTAB method. Extracted DNA was diluted 1/100 and used for qPCR template. The primer pairs (DeH-F, 5’-CTCCGCCTGCCAATGTGATTAC-3’ and E8T7, 5’-GCGTACCAAGGCACGTGCTCAA-3’) designed for the amplification of the gene for AAL toxin biosynthesis (ALT1) were used for detecting the tomato pathotype of AAL (Egusa et al., 2009).
Ethylene quantification
Ethylene was quantified with a gas chromatograph (HP5890, Agilent Technologies, USA) as described previously (Lund et al., 1998).
Statistical analysis
Data were analysed using Statistica (SAS Institute, http://www.statsoft.com). Differences in expanding lesions per plant at each time point in each figure (except for Fig. 3A) and the differences in relative fungal DNA in each figure were analysed by one-way analysis of variance (ANOVA). If the ANOVA analysis was significant (P < 0.05), Duncan’s multiple range test was used to detect significant differences between groups. In Fig. 3A and Supplementary Fig. S2, the differences in expanding lesions per plant at each time point were analysed by Student’s t-test.
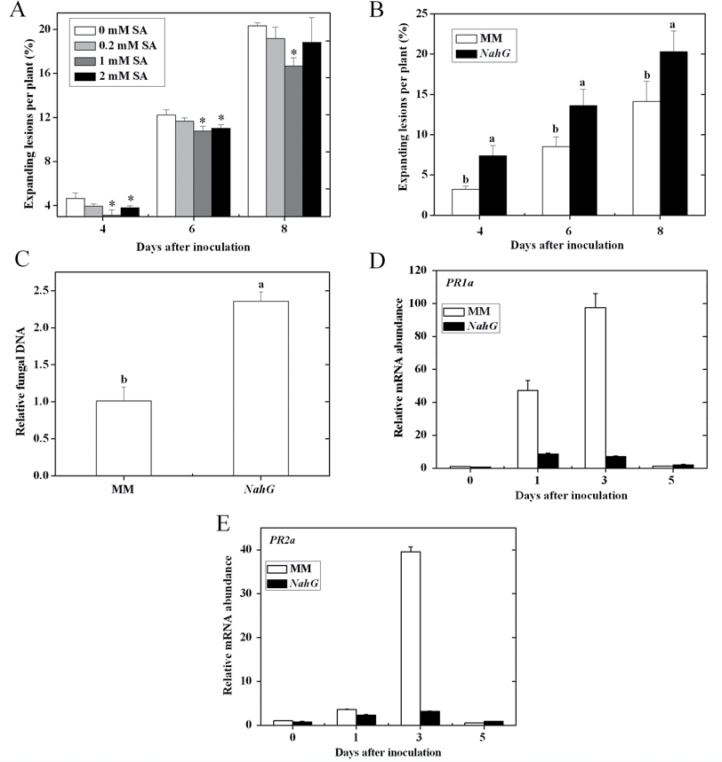
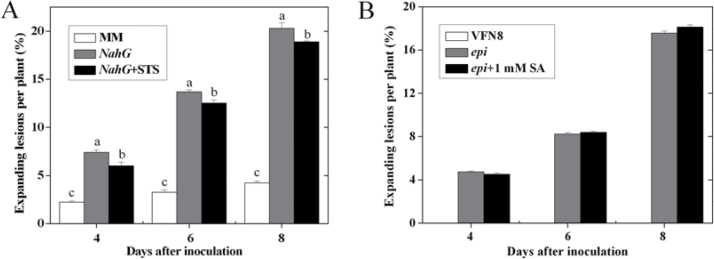
Fig. 3.
The role of the SA pathway in tomato resistance to A. alternata f. sp. lycopersici. (A) Effects of various concentrations of SA application on the disease development in CM plants. (B) Disease development in the NahG transgenic line and MM plants. (C) Fungal biomass in MM and NahG transgenic plants at 6 d post inoculation. Error bars indicate standard deviation from the mean of three replicates. In A, asterisks indicate significant differences as compared with the water-treated control at the same time point (P < 0.05; Student’s t-test), the experiment was repeated twice and similar results were obtained; for each time point in B, letters indicate significant differences among treatments (P < 0.05, Duncan’s multiple range test); in C, letters indicate significant differences among treatments (P < 0.05, Duncan’s multiple range test). (D, E) Transcription patterns of SA-regulated PR genes PR1a and PR2a, respectively, in MM and NahG plants at 0, 1, 3, and 5 d post inoculation.
Results
ET modulates tomato susceptibility to AAL
Previous reports revealed that ET is involved in AAL-toxin-induced tomato cell death (Moore et al., 1999; Zhang et al., 2011). This led the present study to test the role of ET in modulating defence of tomato plants to the toxigenic AAL fungus using the ET action inhibitors silver thiosulphate and 1-MCP and the ET precursor ACC to establish the effect of reduced ET perception or increased ET production, respectively.
Typical necrotic lesions appeared on stems at approximately 3 or 4 d after AAL infection, while the foliar symptom appearance was later than that of the stem. Expanding lesions on stem and leaves per plant were recorded at 4, 6, and 8 d after AAL inoculation. As shown in Fig. 1A and B, fewer lesions were observed in silver thiosulphate- or 1-MCP-pretreated plants at each time point examined, while more spreading lesions were found in ACC-pretreated plants at 4 and 6 d following the fungus infection compared with control plants. Fungal biomass in the infected plants was estimated by quantifying fungal DNA with qPCR using specific primers for ALT1, which is involved in AAL toxin biosynthesis and pathogenicity (Egusa et al., 2009). Consistently with disease symptoms, the amount of fungal DNA was increased by ACC pretreatment and reduced by 1-MCP treatment as compared with the control (Fig. 1C), suggesting that ET plays an important role in susceptibility of tomato to AAL.
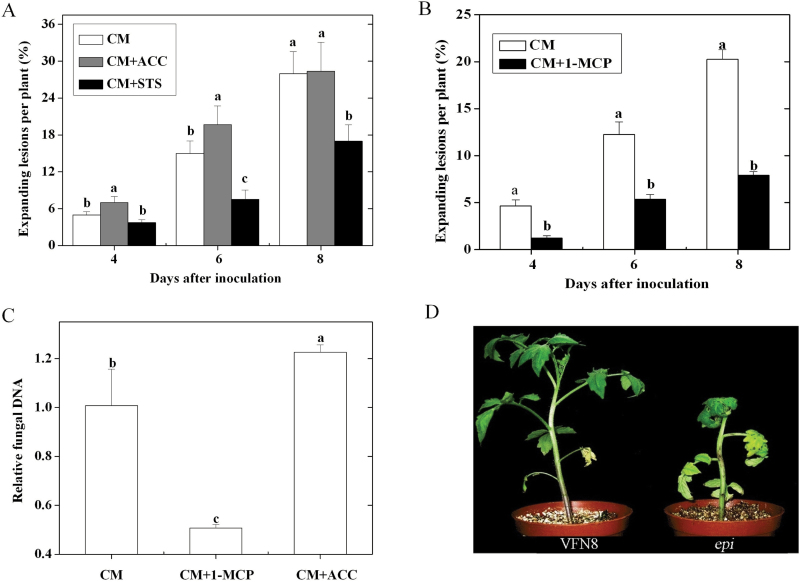
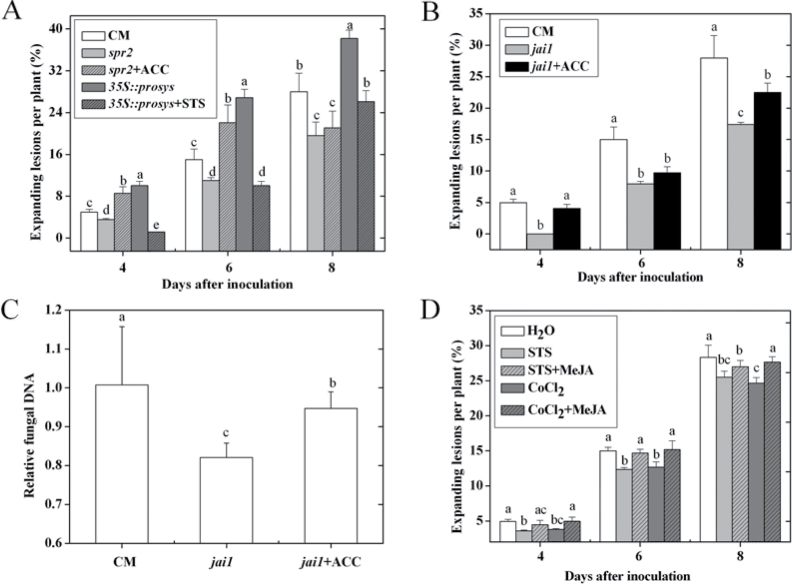
Fig. 1.
Role of ET in tomato susceptibility to A. alternata f. sp. lycopersici. (A) Effect of exogenous ET precursor ACC (0.1mM) and ET action inhibitor silver thiosulphate (1mM) on disease development in CM plants. (B) Effect of ET receptor inhibitor MCP (10 nl l–1) on the disease development in CM plants. (C) Effect of 10 nl l–1 1-MCP and 0.1mM ACC on the relative fungal DNA in CM leaves at 6 d post inoculation; the amount of fungal DNA was quantified by qPCR using ALT1 primers. (D) Disease symptoms in epi and VFN8 plants at 7 d post inoculation. Error bars indicate standard deviation from the mean of three replicates. For each time point in A and B, letters indicate significant differences among treatments; in C, letters indicate significant differences among treatments (P < 0.05, Duncan’s multiple range test). The experiment was repeated three times and similar results were obtained. For each experiment, data were analysed separately. Results of one representative experiment are shown.
To investigate the role of ET response on the susceptibility of tomato plants to AAL in detail, an inoculation assay was conducted on a tomato ET-overproducing mutant epinastic (epi) and its WT line cv. VFN8. The results showed that small lesions appeared on the stem and leaves of epi at 4 d, and the lesions increased in size over time. The disease symptoms of epi and VFN8 plants at 7 d post inoculation are shown in Fig. 1D. The epi plants wilted and died at 3 weeks after infection, while no lesion was observed in VFN8 plants even after 1 month post inoculation (data not shown).
Involvement of JA signalling pathway in susceptibility of tomato to AAL
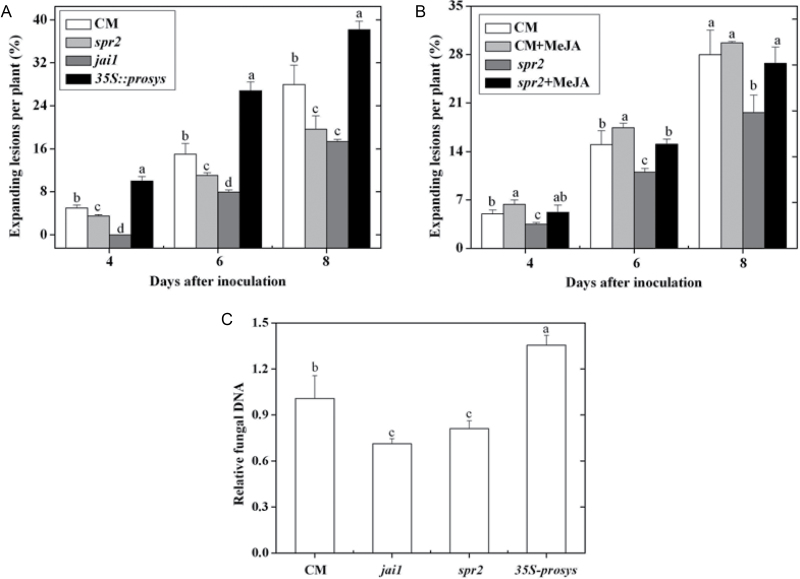
To investigate the role of JA signalling in the interaction between tomato and AAL, JA-deficient mutant spr2, JA-insensitive mutant jai1, the transgenic line 35S::prosys, and their WT cv. CM were used for AAL inoculation experiments. Compared with CM, jai1 and spr2 plants showed enhanced resistance to the fungus (Fig. 2A, B) and there were decreased expression levels of LOXD and AOS2 and abolished PI-II expression in infected jai1 leaves (Supplementary Fig. S1, available at JXB online). However, there were more lesions on the stems and leaves of 35S::prosys (Fig. 2A). Pretreatment with MeJA, on the other hand, increased the number of necrotic lesions on spr2 and CM plants, and exogenous MeJA application restored the disease symptom of spr2 to CM level (Fig. 2B). A positive correlation was observed between fungal biomass and the percentage of expanding lesions, the fungal biomass of spr2 and jai1 was significantly lower, whereas the fungal biomass of 35S::prosys was higher than that of CM plants (Fig. 2C). These results suggest that the JA signalling pathway is involved in tomato susceptibility to AAL, which is dependent on the JAI1 receptor.
Fig. 2.
The role of the JA pathway in tomato susceptibility to A. alternata f. sp. lycopersici. (A, B) Disease development in CM, spr2, jai1, and 35S::prosys plants (A) and effect of application of MeJA to CM and spr2 plants (B) at 4, 6, and 8 d post inoculation. (C) Fungal biomass in CM, spr2, jai1, and 35S::prosys plants at 6 d post inoculation. The amount of fungal DNA was quantified by qPCR using ALT1 gene primers. Error bars indicate standard deviation from the mean of three replicates. For each time point in A and B, letters indicate significant differences among treatments; in C, letters indicate significant differences among treatments (P < 0.05, Duncan’s multiple range test).
SA enhances the resistance of tomato plants to AAL
SA has a significant role in the induction of host response to pathogens. The establishment of systemic acquired resistance is associated with enhanced biosynthesis of SA and activated expression of PR genes, which requires the function of Arabidopsis NPR1 (Yu et al., 2001). Here, to assess the potential role of SA in tomato resistance to AAL, CM plants were soil-drenched with different concentrations of SA solutions. The results show that 1mM SA pretreatment greatly decreased lesion occurrence at each time point examined, while drenching with 2mM SA reduced disease symptom at 4 and 6 d post inoculation (Fig. 3A). Transgenic NahG plants exhibited more expanding lesions than the WT line MM (Fig. 3B). The amount of fungal DNA in NahG plant was markedly higher than that in MM (Fig. 3C).
Arabidopsis NahG plants enhanced susceptibility to P. syringae pv. phaseolicola strain 3121, and exogenous application of catechol, the immediate SA-degradation product of NAHG activity, caused wild-type Arabidopsis to lose resistance to this strain, suggesting that this susceptibility in NahG plants might be due to catechol production (van Wees and Glazebrook, 2003). To investigate this possibility, MM tomato plants were treated with different concentrations of catechol (0, 0.1, 0.5, 1, 2.5, 5mM) before AAL inoculation. No significant difference in amount of expanding lesions was observed among different treatments (data not shown), suggesting that catechol accumulation is not the direct cause of the enhanced susceptibility to AAL in NahG plants.
Several SA-dependent PR genes are commonly used as reporters of SA-dependent defence (Kunkel and Brooks, 2002). SA-mediated acidic PR gene expression in MM and NahG plants was also detected, the results showing that transcript levels of PR1a and PR2a in the NahG plant were greatly depressed compared with MM plants at 1 and 3 d post inoculation (Fig. 3D, E). The above results suggest that the SA-mediated response enhances the resistance of tomato plants to AAL infection.
Enhanced jai1 plant resistance is correlated with decreased ET production and signalling
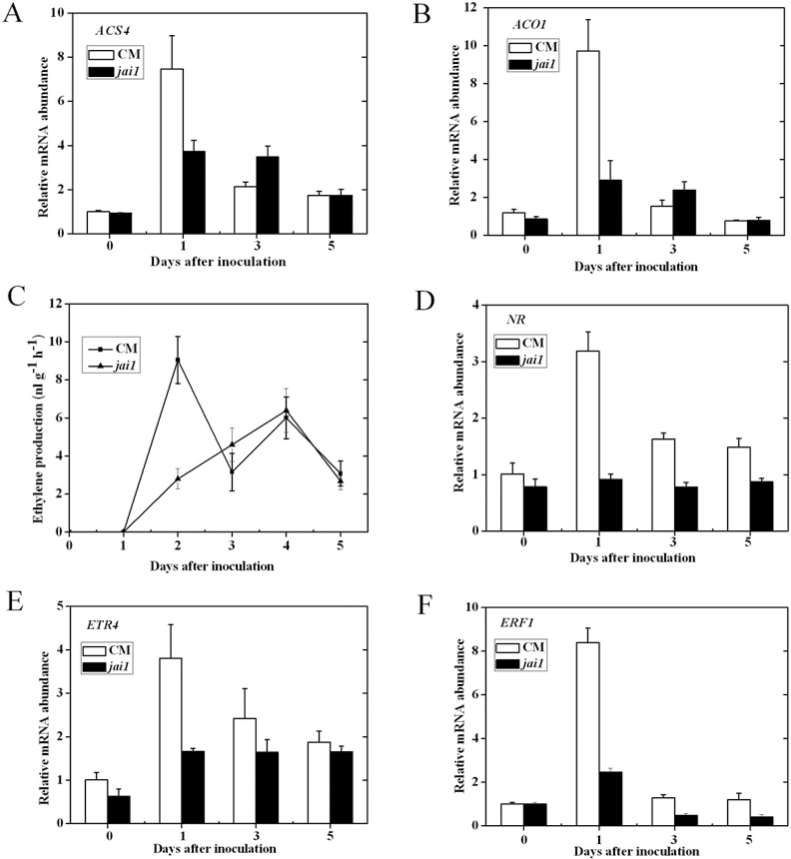
To determine whether the reduced disease symptom in jai1 plants was correlated with altered ET signalling, the expression of genes encoding ET biosynthesis or signalling in jai1 and CM leaves was profiled after AAL infection. ACC synthase (ACS) and ACC oxidase (ACO) are two key enzymes involved in ET biosynthesis (Yang and Hoffman, 1984) and the present study detected the expression levels of ACS4 and ACO1. As shown in Fig. 4A and B, the transcript levels of ACS4 and ACO1 were highly induced in both CM and jai1 plants at 1 d after AAL infection. Moreover, the expression levels of both genes were markedly lower in jai1 at 1 d in comparison with CM (Fig. 4A, B). Then, ET production was assessed in both CM and jai1 leaves after AAL infection. Fig. 4C shows that ET emission in jai1 was suppressed at an early stage of infection and that the ET burst in jai1 was delayed compared with CM.
Fig. 4.
Transcription patterns of ET-related genes and ET production in jai1 and CM plants in response to A. alternata f. sp. lycopersici. Transcription patterns of ACS4 (A) ACO1 (B), NR (D), ETR4 (E), and ERF1 (F) in CM and jai1 plants at 0, 1, 3, and 5 d post infection. (C) ET production in jai1 and CM leaves during infection in a time-course experiment.
Tomato ETR3 (NR) and ETR4, but not the other ET receptor genes, are significantly induced by pathogen infection (Wilkinson et al., 1995; Klee, 2002). Transcription factor ERF1-dependent gene induction is controlled by the combined action of JA and ET in response to pathogen attack (Koornneef and Pieterse, 2008). As shown in Fig. 4D and E, the expression of NR and ETR4 was strongly induced and reached a maximum at 1 d, declining but still remaining at significantly higher levels than controls at 3 and 5 d post inoculation in CM plants. In contrast, induced ETR4 mRNA accumulation in jai1 was lower than in CM, whereas the expression of NR in jai1 was not obviously altered in comparison with control plants at 0h. The expression of ERF1 in CM was strongly induced and peaked at 1 d and the ERF1 expression in jai1 was greatly reduced at all the time points examined compared with CM (Fig. 4F). Together, these results indicate that both ET biosynthesis and the signalling pathway are strongly decreased in jai1 leaves inoculated with AAL as compared to wide-type CM.
Enhanced susceptibility to AAL by JA is partly dependent on ET signal
To further test whether ET and JA act independently or cooperatively during AAL infection, spr2 and 35S::prosys plants were sprayed with ACC or silver thiosulphate before AAL inoculation, respectively. As shown in Fig. 5A, as with the similar trends for CM with ACC or silver thiosulphate pretreatment (Fig. 1A), spr2 pretreated with ACC exhibited enhanced susceptibility at 4 and 6 d after inoculation, whereas application of silver thiosulphate enhanced the resistance of 35S::prosys to AAL, indicating that the enhanced susceptibility to this fungus by ET is not dependent on JA accumulation or response. Furthermore, there were increased expanding lesions and fungal biomass in ACC-pretreated jai1 plants compared with untreated jai1 (Fig. 5B, C), indicating that the role of ET in susceptibility to AAL is independent of the JAI1 receptor.
Fig. 5.
Effects of ET response to JA mutants or 35S::prosys and MeJA application to ET-biosynthetic or -insensitive tomato plants on susceptibility to A. alternata f. sp. lycopersici. (A) The effect of exogenous ET precursor ACC on spr2 and ET action inhibitor silver thiosulphate on 35S::prosys susceptibility to infection. (B) The effect of ACC on jai1 susceptibility to infection. (C) Fungal biomass in CM, jai1, and ACC-pretreated jai1 plants at 6 d post inoculation. (D) The effect of exogenous MeJA application to ET-biosynthetic or -insensitive tomato plants on disease development. Error bars indicate standard deviation from the mean of three replicates. For each time point in A, B, and D, letters indicate significant differences among treatments; in C, letters indicate significant differences among treatments (P < 0.05, Duncan’s multiple range test). The experiment was repeated once and similar results were obtained.
To obtain endogenous ET-deficient or ET-insensitive tomato plants, this study repetitively pretreated of CM plants from the cotyledon stage to the five-leaf stage, with ET biosynthesis inhibitor CoCl2 or ET action inhibitor silver thiosulphate. The test showed that moderate concentrations of CoCl2 at 0.1mM and silver thiosulphate at 1mM are most effective (Supplementary Fig. S2). Subsequently, the ET-deficient and ET-insensitive plants were pretreated with MeJA or H2O alone to assess the interaction between the two signalling pathways. As shown in Fig. 5D, blocking ET perception or biosynthesis markedly reduced the expanding lesions, and pretreatment of ET-deficient or -insensitive plants with exogenous MeJA resulted in an increase of necrotic lesion formation and restored disease symptoms to nearly the water-treated control level, suggesting that the role of JA response in tomato susceptibility is partly dependent on the ET signal and that the JA pathway also can promote tomato susceptibility to AAL alone.
SA suppresses ET signalling pathway during AAL infection
In Arabidopsis, SA and ET may function together to coordinate the induction of several defence-related genes (reviewed in Kunkel and Brooks, 2002). Here, since the role of ET signalling in tomato susceptibility to AAL were opposite to SA, it was of interest to test whether the SA-enhanced resistance to this fungus was correlated with reduced ET signalling.
To determine the role of SA in ET-induced susceptibility to AAL, NahG transgenic plants were sprayed with silver thiosulphate before AAL inoculation. Silver thiosulphate application markedly decreased the expanding lesions in NahG (Fig. 6A). In addition, application of 1mM SA did not significantly influence the disease symptoms in the ET-overproducing mutant epi (Fig. 6B), suggesting that the ET signal might be an effective factor in SA-induced defence response.
Fig. 6.
Effects of ET action inhibitor silver thiosulphate application to NahG transgenic line (A) and SA treatment of epi mutant (B) on disease development in tomato plants. Error bars indicate standard deviation from the mean of three replicates. In A, letters indicate significant differences among treatments (P < 0.05, Duncan’s multiple-range test). The experiment was repeated once and similar results were obtained. For each experiment, data were analysed separately. Results of one representative experiment are shown.
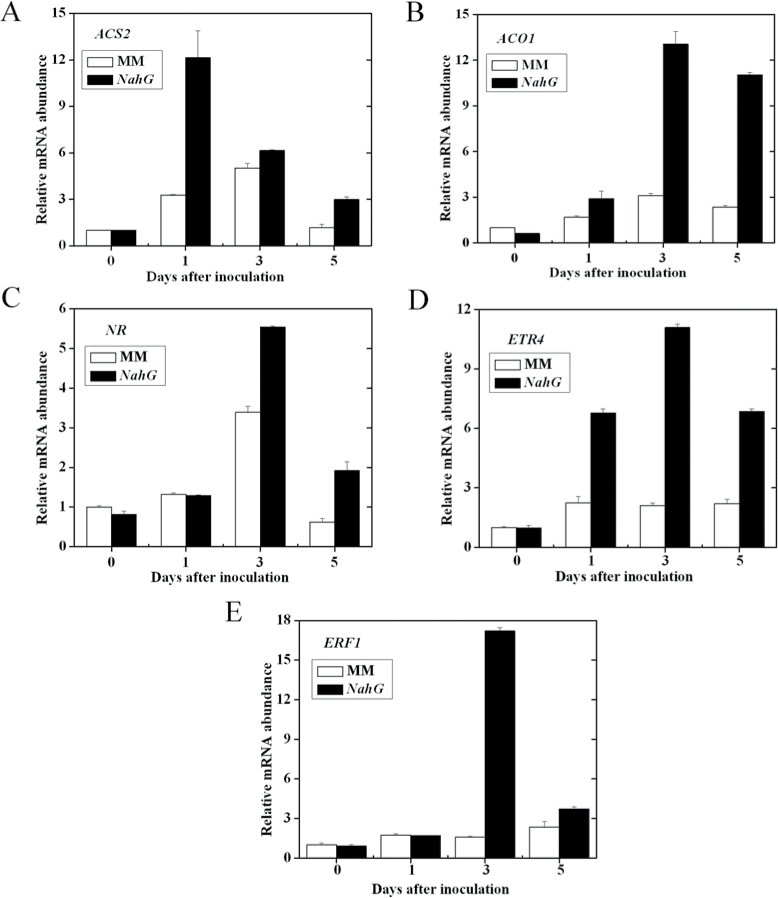
Furthermore, this study detected the expression of genes involved in ET biosynthesis or signalling in NahG and its WT line cv. MM after AAL infection. As shown in Fig. 7A and B, the transcript levels of ET biosynthetic genes ACS2 and ACO1 were upregulated in NahG as compared with MM, clearly indicating that SA suppresses ET biosynthesis during AAL infection. Similarly to ACS2 and ACO1, the expression abundance of the ET-responsive genes NR, ETR4, and ERF1 was increased in NahG relative to MM (Fig. 7C–E). The above results suggest that enhanced resistance to AAL by SA might be associated with decreased ET response.
Fig. 7.
Transcription patterns of ET-biosynthetic genes ACS2 and ACO1 and ET-regulated genes NR, ETR4, and ERF1 in NahG and MM plants in response to A. alternata f. sp. lycopersici. Transcript accumulation of ACS2 (A), ACO1 (B), NR (C), ETR4 (D), and ERF1 (E) in A. alternata f. sp. lycopersici-infected MM and NahG plants at 0, 1, 3, and 5 d post infection.
Discussion
Upon pathogen attack, infected plant cells generate signalling molecules to initiate defence mechanisms in surrounding cells to limit pathogen spread (Kunkel and Brooks, 2002; Koornneef and Pieterse, 2008). Three signalling molecules, SA, JA, and ET, play key roles in mediating disease resistance (e.g. against necrotrophic fungal pathogens) (Asai et al., 2000). The disease of Alternaria stem canker is a problem primarily for coastal-grown tomatoes in California and some areas in China. There is limited information on the role of plant hormones in plant defence against this toxin-producing fungus. The present study investigated the roles of ET, JA, and SA as well as their interactions in modulating susceptibility of tomato plants to AAL.
The plant hormone ET is an important signal molecule in plant–pathogen interaction but the role of ET is largely controversial (Diaz et al. 2002). The Arabidopsis ethylene insensitive2 (ein2) mutant exhibits increased susceptibility to B. cinerea and E. carotovora, but decreased symptoms when infected with P. syringae or Xanthomonas campestris pv. campestris (Kunkel and Brooks, 2002). Lund et al. (1998) reported that ET-insensitive tomato Nr shows reduced disease symptoms upon infection by the bacterial pathogens P. syringae pv. tomato and Xanthomonas campestris pv. vesicatoria. The studies using Arabidopsis and tomato ET mutants demonstrated that both ET perception and signalling are required for resistance against or susceptibility to pathogens, depending on the plant–pathogen interaction (Lund et al., 1998; Thomma et al., 1999; Diaz et al., 2002). In the present study, the results suggest a novel role for ET in modulating susceptibility of tomato plants to AAL (Fig. 1). ET action can be regulated by changes of its biosynthesis and perception (Klee, 2002). The present study also observed that the production of ET is one of the earliest plant responses (Fig. 4C). Combined with the previous reports (Moore et al., 1999; Zhang et al., 2011), it seemed that production of ET could be induced by the pathogen or the AAL toxin at the earliest stage, which promoted AAL-toxin-induced cell death. Then, the necrotrophic pathogen AAL extracted nutrients from the dead host cells and produced more AAL toxin, which in turn enhanced cell death.
Necrotrophic fungal pathogens infect and kill host tissue and extract nutrients from the dead host cells, while biotrophic fungal pathogens colonize living plant tissue and obtain nutrients from living host cells. Classically, SA signalling triggers resistance against biotrophic and hemibiotrophic pathogens, whereas a combination of JA and ET signalling activates resistance against necrotrophic pathogens (Glazebrook, 2005). However, from the present data, it can be concluded that the JA signalling pathway is involved in tomato susceptibility to AAL (Fig. 2). In a recent report by Egusa et al. (2009), the detached tomato leaflets of JA-deficient mutant def1 show increased resistance to AAL, which is consistent with the present results. Additionally, it is shown here that SA mediates resistance of tomato plants to AAL (Fig. 3A–C). Egusa et al. (2009) reported that the exogenous addition of SA to spore suspensions does not affect susceptibility of detached tomato leaves to AAL, which is contradictory to the present results, and it should be noted that their methods of fungal inoculation and SA application were different from this study’s. Furthermore, exogenous 0.2mM SA application significantly inhibited AAL-toxin-induced cell death in detached leaflets (Supplementary Fig. S3), suggesting that the enhanced resistance to AAL by SA might be associated with the inhibition of cell death. In several previously reported studies, elevated SA often enhances susceptibility to necrotrophic pathogens but promotes resistance to hemibiotrophs (Veronese et al., 2004, 2006; El Oirdi et al., 2011). In the present study, the roles of JA and SA in susceptibility of tomato plants to AAL were different from other plant and necrotrophic pathogen interactions (Thomma et al., 1998; Diaz et al., 2002; El Oirdi et al., 2011). There could be two possible reasons for the discrepancies. First, most of the studies on the roles of JA and SA in plant resistance to necrotrophic pathogens have been carried out in the model plant Arabidopsis and there could be significance differences between Arabidopsis and tomato in their signalling pathways or defence mechanisms against necrotrophic pathogens. Second, most of the reported studies on the roles of JA and SA in plant responses to necrotrophic pathogens have used broad host-range necrotrophs such as B. cinerea. AAL, on the other hand, is a host-specific necrotroph, which produces a group of mycotoxins as a host-specific pathogenicity factor (Brandwagt et al., 2002). The host-specific nature of the toxins and the pathogens may imply specific plant–pathogen interactions that require manoeuvring host physiology and viability at some stages of the infection cycle, a feature also found with hemibiotrophic pathogens.
The JA, SA, and ET pathways are involved in a complex signalling network in which the different pathways influence each other through positive or negative regulatory interactions. It allows plants to minimize energy costs and create a flexible signalling network for the defence responses to different attackers (Kunkel and Brooks, 2002; Koornneef and Pieterse, 2008). Several studies provide evidence for positive interactions between JA and ET signalling (Kunkel and Brooks, 2002). For example, ET is required together with JA for defence against necrotrophic pathogens and for systemic resistance induced by root-colonizing bacteria, and they stimulate each other’s biosynthesis in wound responses (reviewed in Tuominen et al., 2004). Transcription factor ERF1 acts as an important signalling node integrating signals from the JA and ET pathways (Koornneef and Pieterse, 2008). Berrocal-Lobo and Molina (2004) demonstrated that ERF1 is induced upon infection with Fusarium oxysporum and depends on both functionally intact ET and JA pathways. Similar results were observed in this study, in which the resistance of jai1 was enhanced and ET response was reduced in jai1 as compared to CM, suggesting that the ET and JA pathways probably cooperatively regulate tomato susceptibility to AAL. ACC application potentiated disease symptom development in the spr2 mutants and silver thiosulphate alleviated the development of disease symptoms in the 35S::prosys plants (Fig. 5A). Furthermore, exogenous MeJA application to ET-insensitive plants (obtained by repetitive pretreatment of CM plants with silver thiosulphate) and to ET-deficient plants (obtained by repetitive pretreatment of CM plants with CoCl2) before AAL inoculation both resulted in similar percentages of expanding lesions to the untreated control (Fig. 5D), but less than MeJA treatment alone (Fig. 2B), indicating that the effect of JA on susceptibility to AAL is not dependent on ET signalling completely.
Although the induction of SA-dependent PR gene expression does not require an intact ET signalling in Arabidopsis, ACC application enhanced the level of SA-induced PR1 expression in a dose-dependent manner (De Vos et al., 2006). Moreover, ET was shown to be essential for the onset of SA-dependent systemic acquired resistance against tobacco mosaic virus in tobacco (Verberne et al., 2003). The accumulation of SA in X. campestris pv. vesicatoria-infected tomato plants is dependent on ET synthesis (O’Donnell et al., 2003). These results suggest that ET positively regulates the SA-induced defence response. However, the ET signalling also negatively affects SA-dependent responses: the basal level of PR-1 mRNA appears to be significantly elevated in Arabidopsis ein2 plants (reviewed in Kunkel and Brooks, 2002). Here in the interaction between tomato and AAL, they seem to be antagonistic. First, compared with MM, NahG transgenic plants exhibited higher expression of ET-biosynthetic and -responsive genes (Fig. 7), indicating that SA-enhanced resistance to AAL might be correlated with a decrease in the ET signalling pathway. Secondly, pretreatment of NahG plants with silver thiosulphate significantly alleviated the development of disease symptoms (Fig. 6A), while exogenous application of SA to the epi mutant had no significant effect on disease development (Fig. 6B), indicating that ET might play an important role in SA-induced defence response during AAL infection.
There is evidence for both positive and negative interactions between the SA and JA pathways. JA antagonizes SA-dependent pathogen defences in the course of P. syringae infection in tomato (Kunkel and Brooks, 2002). In Arabidopsis, the reduced susceptibility of jin1, a JA-insensitive mutant to P. syringae, is caused by hyperactivation of PR and elevated SA accumulation (Laurie-Berry et al., 2006). In this study, to test the interactions between the SA and JA pathways, the expression of SA-biosynthetic or -regulated genes were detected in jai1 and CM following AAL infection. The results showed that there were similar expression patterns of PAL (encoding phenylalanine ammonialyase, Asai et al., 2000) or ICS (isochorismate synthase, Uppalapati et al., 2007) in jai1 and CM, and no obvious differences were observed between them following AAL infection (Supplementary Fig. S4A, B). Moreover, the expression of the SA-regulated genes NPR1 and PR1a was downregulated in jai1 compared with CM (Supplementary Fig. S4C, D), suggesting that SA-mediated defence signalling is reduced in jai1 relative to CM during AAL infection. Collectively, these results suggest that enhanced resistance against AAL in jai1 plants is independent of activated SA-meditated defence response. A similar result was observed in Arabidopsis, in which COI1 receptor-mediated F. oxysporum resistance was not attributed to SA-dependent defence (Thatcher et al., 2009).
In conclusion, a hypothetical model to summarize the roles of the ET, JA, and SA pathways as well as the signalling crosstalk during the tomato–AAL interaction is proposed (Fig. 8). Challenge of tomato plants by fungal AAL starts a cascade of signalling events that involve the synthesis and subsequent activation of the ET, JA, and SA pathways. The ET response and JAI1-dependent JA signalling enhance the susceptibility of tomato plants to AAL, while SA pathway is involved in resistance against AAL. Furthermore, the JA and ET pathways act synergistically to promote tomato susceptibility, while SA promotes tomato resistance to AAL and antagonizes ET signalling during AAL infection. JA and SA affect the susceptibility of tomato to the pathogen partly through the action of ET. ET-mediated signalling might be more effective in determining tomato susceptibility to AAL.
Fig. 8.
A proposed model for the JA, SA, and ET defence signalling pathways and their interactions during A. alternata f. sp. lycopersici infection. Both ET and JAI1 receptor-dependent JA pathways are necessary for susceptibility, while SA signalling promotes resistance of tomato plants to infection. Furthermore, the JA and ET pathways act synergistically to promote the susceptibility, while SA promotes tomato resistance to AAL and antagonizes ET signalling during AAL infection. JA and SA affect tomato susceptibility to the pathogen partly through the action of ET. ET-mediated signalling plays a central role in determining the susceptibility of tomato to A. alternata f. sp. lycopersici.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. Transcription patterns of the JA-biosynthetic genes AOS2 and LOXD and the JA-regulated gene PI-II in the leaves of jai1 and CM post AAL infection.
Supplementary Fig. S2. Effects of various concentrations of CoCl2 and silver thiosulphate application on disease development in CM plants.
Supplementary Fig. S3. Symptom of CM leaflets after 0.2 µM AAL toxin application or co-treatment with AAL toxin and 0.2mM SA.
Supplementary Fig. S4. Transcription patterns of SA- biosynthetic genes PAL and ICS and JA-regulated genes NPR1 and PR1a in the leaves of jai1 and CM post AAL infection.
Acknowledgements
The authors are grateful to Tomato Genetics Resource Center (University of California, Davis, CA, USA) for providing epi and VFN8 tomato seeds, Dr Liangcheng Du (University of Nebraska, Lincoln) for providing Alternaria alternata f. sp. lycopersici, and Dr. Jonathan D.G. Jones (John Innes Centre, Norwich, UK) for providing tomato seeds of NahG transgenic line and its WT cv. Moneymaker (MM). The authors also thank Dr. Sixue Chen (University of Florida, Gainesville) for critical reading of the manuscript. This work was supported by the National Basic Research Program of China (2009CB119000) and the National Science Foundation of China (30970244, 30571269).
References
- Asai T, Stone JM, Heard JE, Kovtun Y, Yorgey P, Sheen J, Ausubel FM. 2000. Fumonisin B1-induced cell death in Arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signaling pathways. The Plant Cell 12, 1823–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellés JM, Pérez-Amador MA, Carbonell J, Conejero V. 1993. Correlation between ornithine decarboxylase and putrescine in tomato plants infected by citrus exocortis viroid or treated with ethephon. Plant Physiology 102, 933–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocal-Lobo M, Molina A. 2004. Ethylene response factor 1 mediates Arabidopsis resistance to the soilborne fungus Fusarium oxysporum . Molecular Plant–Microbe Interactions 17, 763–770 [DOI] [PubMed] [Google Scholar]
- Brading PA, Hammond-Kosack KE, Parr A, Jones JD. 2000. Salicylic acid is not required for Cf-2- and Cf-9-dependent resistance of tomato to Cladosporium fulvum . The Plant Journal 23, 305–318 [DOI] [PubMed] [Google Scholar]
- Brandwagt BF, Kneppers TJA, Nijkamp HJJ, Hille J. 2002. Overexpression of the tomato Asc-1 gene mediates high insensitivity to AAL toxins and fumonisin B1 in tomato hairy roots and confers resistance to Alternaria alternata f. sp. lycopersici in Nicotiana umbratica plants. Molecular Plant–Microbe Interactions 15, 35–42 [DOI] [PubMed] [Google Scholar]
- De Vos M, Van Zaanen W, Koornneef A, Korzelius JP, Dicke M, Van Loon LC, Pieterse CMJ. 2006. Herbivore-induced resistance against microbial pathogens in Arabidopsis . Plant Physiology 142, 352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, ten Have A, van Kan JAL. 2002. The role of ethylene and wound signaling in resistance of tomato to Botrytis cinerea . Plant Physiology 129, 1341–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doares SH, Navaraez-Vasquez J, Conconi A, Ryan CA. 1995. Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiology 108, 1741–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egusa M, Ozawa R, Takabayashi J, Otani H, Kodama M. 2009. The jasmonate signaling pathway in tomato regulates susceptibility to a toxin-dependent necrotrophic pathogen. Planta 229, 965–976 [DOI] [PubMed] [Google Scholar]
- El Oirdi M, El Rahman TA, Rigano L, El Hadrami A, Rodriguez MC, Daayf F, Vojnov A, Bouarab K. 2011. Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato. The Plant Cell 23, 2405–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino DW, Burger DW, Yang SF, Bradford KJ. 1988. Characterization of an ethylene overproducing mutant of tomato (Lycopersicon esculentum Mill. cultivar VFN8). Plant Physiology 88, 774–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology 43, 205–227 [DOI] [PubMed] [Google Scholar]
- Howe GA, Ryan CA. 1999. Suppressors of systemin signaling identify genes in the tomato wound response pathway. Genetics 153, 1411–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ. 2002. Control of ethylene-mediated processes in tomato at the level of receptors. Journal of Experimental Botany 53, 2057–2063 [DOI] [PubMed] [Google Scholar]
- Kloek AP, Verbsky ML, Sharma SB, Schoelz JE, Vogel J, Klessig DF, Kunkel BN. 2001. Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. The Plant Journal 26, 509–522 [DOI] [PubMed] [Google Scholar]
- Koornneef A, Pieterse CMJ. 2008. Cross talk in defense signaling. Plant Physiology 146, 839–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM. 2002. Cross talk between signaling pathways in pathogen defense. Current Opinion in Plant Biology 5, 325–331 [DOI] [PubMed] [Google Scholar]
- Laurie-Berry N, Joardar V, Street IH, Kunkel BN. 2006. The Arabidopsis thaliana jasmonate insensitive 1 gene is required for suppression of salicylic acid-dependent defenses during infection by Pseudomonas syringae . Molecular Plant–Microbe Interactions 19, 789–800 [DOI] [PubMed] [Google Scholar]
- Li CY, Liu G, Xu C, Lee GI, Bauer P, Ling HQ, Ganal MW, Howe GA. 2003. The tomato suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. The Plant Cell 15, 1646–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Williams MM, Loh YT, Lee GI, Howe GA. 2002. Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiology 130, 494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, Pichersky E, Howe GA. 2004. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. The Plant Cell 16, 126–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. 2003. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. The Plant Cell 15, 165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund ST, Stall RE, Klee HJ. 1998. Ethylene regulates the susceptible response to pathogen infection in tomato. The Plant Cell 10, 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JM, Dangl JL. 2000. Signal transduction in the plant immune response. Trends in Biochemical Sciences 25, 79–82 [DOI] [PubMed] [Google Scholar]
- Moore T, Martineau B, Bostock RM, Lincoln JE, Gilchrist DG. 1999. Molecular and genetic characterization of ethylene involvement in mycotoxin-induced plant cell death. Physiological and Molecular Plant Pathology 54, 73–85 [Google Scholar]
- Mur LAJ, Kenton P, Atzorn R, Miersch O, Wasternack C. 2006. The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiology 140, 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki T, Mitsuhara I, Seo S, Ohtsuba N, Ohashi Y. 1998. Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco leaves. Plant Cell Physiology 39, 500–507 [Google Scholar]
- Nürnberger T, Lipka V. 2005. Non-host resistance in plants: new insights into an old phenomenon. Molecular Plant Pathology 6, 335–345 [DOI] [PubMed] [Google Scholar]
- O’Donnell PJ, Schmelz E, Block A, Miersch O, Wasternack C, Jones JB, Klee HJ. 2003. Multiple hormones act sequentially to mediate a susceptible tomato pathogen defense response. Plant Physiology 133, 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G, Strydom D, Johnson S, Ryan CA. 1991. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 253, 895–897 [DOI] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM. 2000. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proceedings of the National Academy of Sciences, USA 97, 11655–11660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard LB, Tan X, Mao H, et al. 2010. Jasmonate perception by inositol phosphate-potentiated COI1-JAZ co-receptor. Nature 468, 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Johnson JS, Dong X. 2007. Regulation of tradeoffs between plant defense against pathogens with different lifestyles. Proceedings of the National Academy of Sciences, USA 104, 18842–18847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JQ, Jiang HL, Li CY. 2011. Systemin/jasmonate-mediated systemic defense signaling in tomato. Molecular Plant 4, 607–615 [DOI] [PubMed] [Google Scholar]
- Thatcher LF, Manners JM, Kazan K. 2009. Fusarium oxysporum hijacks COI1-mediated jasmonate signaling to promote disease development in Arabidopsis . The Plant Journal 58, 927–939 [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF. 1998. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proceedings of the National Academy of Sciences, USA 95, 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Tierens KFMJ, Broekaert WF. 1999. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea . Plant Physiology 121, 1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H, Overmyer K, Keinänen M, Kollist H, Kangasjärvi J. 2004. Mutual antagonism of ethylene and jasmonic acid regulates ozone-induced spreading cell death in Arabidopsis . The Plant Journal 39, 59–69 [DOI] [PubMed] [Google Scholar]
- Uppalapati SR, Ishiga Y, Wangdi T, Kunkel BN, Anand A, Mysore KS, Bender CL. 2007. The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Molecular Plant–Microbe Interactions 20, 955–965 [DOI] [PubMed] [Google Scholar]
- Verberne MC, Hoekstra J, Bol JF, Linthorst HJM. 2003. Signaling of systemic acquired resistance in tobacco depends on ethylene perception. The Plant Journal 35, 27–32 [DOI] [PubMed] [Google Scholar]
- Veronese P, Chen X, Bluhm B, Salmeron J, Dietrich R, Mengiste T. 2004. The BOS loci of Arabidopsis are required for resistance to Botrytis cinerea infection. The Plant Journal 40, 558–574 [DOI] [PubMed] [Google Scholar]
- Veronese P, Nakagami H, Bluhm B, AbuQamar S, Chen X, Salmeron J, Dietrich RA, Hirt H, Mengiste T. 2006. The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. The Plant Cell 18, 257–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wees SC, Glazebrook J. 2003. Loss of non-host resistance of Arabidopsis NahG to Pseudomonas syringae pv. phaseolicola is due to degradation products of salicylic acid. The Plant Journal 33, 733–742 [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ. 1995. An ethylene-inducible component of signal transduction encoded by Never-ripe . Science 270, 1807–1809 [DOI] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. 1984. Ethylene biosynthesis and its regulation in higher plants. Annual Review of Plant Physiology 35, 155–189 [Google Scholar]
- Yu D, Chen C, Chen Z. 2001. Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. The Plant Cell 13, 1527–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LP, Jia CG, Liu LH, Zhang ZM, Li CY, Wang QM. 2011. The involvement of jasmonates and ethylene in Alternaria alternata f. sp. lycopersici toxin-induced tomato cell death. Journal of Experimental Botany 62, 5405–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.