Abstract
Insect egg deposition activates plant defence, but very little is known about signalling events that control this response. In Arabidopsis thaliana, oviposition by Pieris brassicae triggers salicylic acid (SA) accumulation and induces the expression of defence genes. This is similar to the recognition of pathogen-associated molecular patterns (PAMPs), which are involved in PAMP-triggered immunity (PTI). Here, the involvement of known signalling components of PTI in response to oviposition was studied. Treatment with P. brassicae egg extract caused a rapid induction of early PAMP-responsive genes. In addition, expression of the defence gene PR-1 required EDS1, SID2, and, partially, NPR1, thus implicating the SA pathway downstream of egg recognition. PR-1 expression was triggered by a non-polar fraction of egg extract and by an oxidative burst modulated through the antagonistic action of EDS1 and NUDT7, but which did not depend on the NADPH oxidases RBOHD and RBOHF. Searching for receptors of egg-derived elicitors, a receptor-like kinase mutant, lecRK-I.8, was identified which shows a much reduced induction of PR-1 in response to egg extract treatment. These results demonstrate the importance of the SA pathway in response to egg-derived elicitor(s) and unravel intriguing similarities between the detection of insect eggs and PTI in Arabidopsis.
Key words: Arabidopsis thaliana, oviposition, PAMP-triggered immunity, Pieris brassicae, PR-1 expression, SA pathway.
Introduction
Eggs from herbivorous insects pose a serious threat for plants as they develop into feeding larvae. Consequently, plants have evolved exquisite strategies to respond to oviposition by producing direct and indirect defences (Hilker and Meiners, 2006). Several plant species develop a necrotic zone at the oviposition site, and this is often associated with increased egg mortality or a reduced larval survival rate (Shapiro and DeVay, 1987; Balbyshev and Lorenzen, 1997; Hilker and Meiners, 2006; Bruessow and Reymond, 2007). Indirect defences consist of the emission of volatiles in response to insect eggs, resulting in the attraction of egg parasitoids (Hilker et al., 2002). Modifications of plant surface chemistry by egg deposition can also be used by egg parasitoids as a cue to locate their host (Fatouros et al., 2008).
Plants are thus able to perceive egg deposition, but the chemical nature of egg-derived elicitors that trigger plant responses is still largely unknown (Hilker and Meiners, 2010). An elicitor responsible for the release of volatiles by the pine sawfly was isolated from oviduct secretions (Hilker et al., 2005). Formation of tumour-like structures on pea pods is caused by bruchins, long-chain fatty acid-derived molecules found in eggs of bruchid beetles (Doss et al., 2000). Anti-aphrodisiac male pheromones in the accessory gland secretions from mated female pierid butterflies cause leaf surface changes that attract egg parasitoids (Fatouros et al., 2008). In addition, very little is known about signalling pathways that control plant responses to oviposition. Eggs of the phytophagous mites Tetranychus urticae develop faster in the tomato mutant def-1, which is deficient in jasmonic acid (JA) accumulation (Ament et al., 2004). JA treatment triggered the emission of volatiles that attract egg parasitoids, mimicking the response of plants to oviposition (Hilker et al., 2002). However, it is still unclear how other responses to oviposition, including necrosis and defence gene expression, are regulated.
Pieris brassicae, the Large White butterfly, is distributed worldwide, but is mainly found throughout Europe and Asia. It is a serious pest of cultivated Brassica vegetables and can cause substantial yield losses (Feltwell, 1978; Kular and Kumar, 2011). In recent years, Arabidopsis thaliana has become a useful model to gain molecular insights into the plant response to Pieris species (Reymond et al., 2004; de Vos et al., 2005). For the response to oviposition, it was shown that P. brassicae eggs trigger localized cell death, accumulation of callose, and production of reactive oxygen species (ROS) on leaves (Little et al., 2007). Importantly, the Arabidopsis transcriptome signature after oviposition was strikingly different from that observed after feeding by chewing larvae. Pieris brassicae eggs triggered expression changes similar to those observed during infection with biotroph pathogens, including the induction of defence genes (Little et al., 2007). Furthermore, it was found that eggs from distantly related insect species induce the expression of similar genes and that this activity is enriched in egg lipids (Little et al., 2007; Bruessow et al., 2010). This indicated that some generic egg-derived molecules are recognized by the plant and induce a conserved response, in analogy with the detection of pathogen-associated molecular patterns (PAMPs) by the plant innate immune system (Boller and Felix, 2009). The recognition of PAMPs by specific cell surface receptors initiates convergent signalling cascades that ultimately result in the expression of defence genes, a process called PAMP-triggered immunity (PTI) (Jones and Dangl, 2006). Early PAMP-responsive genes are regulated by a combined activation of mitogen-activated protein kinases (MAPKs) and calcium-dependent protein kinases (CDPKs) (Boudsocq et al., 2010). In addition, PAMP recognition leads to a rapid oxidative burst and the production of ROS that are required for defence gene induction (Suzuki et al., 2011). In plants, ROS are often generated by the action of NADPH oxidases, also known as respiratory burst oxidase homologues (RBOHs), which produce O2 – in the apoplast. Two Arabidopsis NADPH oxidases, RBOHD and RBOHF, play a key role in innate immunity and cell death regulation (Torres et al., 2002; Suzuki et al., 2011; Marino et al., 2012).
PAMP recognition is generally followed by the activation of the salicylic acid (SA) pathway (Vlot et al., 2009). Treatment with the bacterial PAMP flagellin induces SA accumulation, and the expression of a major proportion of PAMP-induced genes requires a functional SA pathway (Tsuda et al., 2008). One key upstream component of the SA pathway is Enhanced Disease Susceptibility1 (EDS1), which is an important regulator of PTI (Wiermer et al., 2005). EDS1 controls SA biosynthesis by isochorismate synthase SID2 (ICS1) (Wildermuth et al., 2001) and subsequent induction of defence genes against biotroph pathogens (Wiermer et al., 2005). In addition, EDS1 regulates the production of ROS that are often associated with biotic and abiotic stresses (Straus et al., 2010). Signalling downstream from SA is controlled by the key regulator Nonexpressor of PR genes1 (NPR1). Upon SA accumulation, a change in the cellular redox state triggers monomerization of NPR1 that is subsequently translocated to the nucleus where it activates transcription of defence genes (Vlot et al., 2009). NPR1 interacts with TGA bZIP transcription factors that bind to the PR-1 promoter and have either positive or negative transcriptional activity (Kesarwani et al., 2007).
Having observed that oviposition in Arabidopsis is associated with the release of egg-derived elicitors, a strong SA accumulation, and the up-regulation of similar sets of genes to those up-regulated during infection by biotroph pathogens (Little et al., 2007; Bruessow et al. 2010), it was decided to investigate whether perception of insect eggs shares common signalling components with PTI. Here, it is revealed that plants use a similar, but not identical, signalling machinery to respond to pathogens and insect eggs.
Materials and methods
Plant and insect growth conditions
Arabidopsis thaliana (L.) Heynh. ecotype Columbia (Col-0) plants were grown in a growth chamber as described previously (Reymond et al., 2004). The sid2-1 mutant was obtained from Christiane Nawrath (University of Lausanne), rbohD/F from Miguel Angel Torres (Polytechnic University of Madrid), eds1-2 and nudt7-1 from Jane Parker (MPI for Plant Breeding Research, Koln), tga2356 from Corné Pieterse (Utrecht University), and npr1-1, rbohD (SALK_109396), rbohF (SALK_034674), and lecrk-I.8 (SALK_0066416) from the Nottingham Arabidopsis Stock Centre, All mutants are in the Col-0 background.
Pieris brassicae was reared on Brassica oleracea in a greenhouse (Reymond et al., 2000).
Treatments with egg extracts
Pieris brassicae eggs were collected and crushed with a pestle in Eppendorf tubes. After centrifugation (15 000 g, 3min), the supernatant (‘egg extract’) was stored at –20 °C. Plants were 4 weeks old at the time of treatment. For each plant, two leaves were treated with 2 µl of egg extract. A total of four plants were used for each experiment. After the appropriate time, egg extract was gently removed with a paintbrush and treated leaves were stored in liquid nitrogen. Untreated plants were used as controls.
For extraction of total lipids, 1ml of P. brassicae egg extract was transferred into a 50ml Falcon tube and was mixed dropwise with 6.25ml of CHCl3/EtOH (1:1, v/v). The solution was placed on a shaker for 30min and mixed with an additional 15ml of CHCl3/EtOH. The supernatant was evaporated in a speedvac and the dried material was dissolved in 25ml of CHCl3 and filtered through a funnel packed with cotton. After evaporation, the dried lipid extract (~18mg) was then resuspended in 100 µl of dimethylsulphoxide (DMSO) and diluted with water to a final volume of 1ml.
Solid-phase extraction (SPE) fractionation of egg lipids was done on a Sep-Pak C18-reverse phase cartridge (Waters AG, Baden, Switzerland). A 4mg aliquot of lipids dissolved in 10% DMSO was loaded on the cartridge and eluted with 2ml of 50% MeOH, followed by 2ml of 80% MeOH, 2ml of 100% MeOH, and 2ml of 100% tetrahydrofuran. Fractions of 2ml were collected, dried under a nitrogen flux, and resuspended with DMSO to a final concentration of 100mg ml–1. Each fraction was diluted 10× with water before treatment.
For treatment with pyrrolidine dithiocarbamate (PDTC), leaves were dipped for 10 s in a 100 µM solution 1h before treatment with egg extract. PDTC treatment was then repeated every 24h for 72h. Untreated plants and plants only treated with PDTC were used as controls.
Insect bioassays
For oviposition tests, eight 5-week-old Arabidopsis Col-0 plants, eight eds1-2 plants, and eight nudt7-1 plants were placed in one cage containing P. brassicae butterflies for 4h in the greenhouse. Plants were then transferred to a growth chamber for 5 d (20 °C, 65% relative humidity, 100 µmol m–2 s–1, 10/14h photoperiod) and the number of hatched eggs was measured. For each experiment, three cages were used and each experiment was replicated three times independently.
To test for the effect of the oviposition host genotype on subsequent larval performance, 30 freshly hatched larvae from eggs oviposited on Col-0, eds1-2, and nudt7-1 were placed on 22 Col-0 plants in transparent plastic boxes and were allowed to feed for 7 d in a growth chamber. Larvae were then collected and weighed with a precision balance. This experiment was repeated three times independently.
Quantitative real-time PCR analysis
RNA extraction and the quantitative PCR procedure were published previously (Bruessow et al., 2010). The list of gene-specific primers can be found in Supplementary Table S1 available at JXB online.
Histochemical stainings
Superoxide radical (O2 −) was visualized with the sensitive dye nitroblue tetrazolium (NBT; Sigma, http://www.sigmaaldrich.com). After removal of egg extract, leaves were submerged in a solution containing 0.02% NBT and 10mM NaN3 for 4h at room temperature in the dark. Hydrogen peroxide (H2O2) accumulation was measured with 3,3-diaminobenzidine (DAB; Sigma). Leaves were submerged in a 1.0mg ml–1 DAB solution and incubated in the dark at room temperature for 6–8h. For visualization of cell death, leaves were submerged in lactophenol trypan blue solution [5ml of lactic acid, 10ml of 50% glycerol, 1mg of trypan blue (Sigma), and 5ml of phenol] at 30 °C for 2–3h. After each staining, leaves were destained for 10min in boiling 95% ethanol.
Microscope images were saved as TIFF files and processed for densitometric quantification with ImageJ version 1.64 (NIH).
Results
Eggs up-regulate early PAMP-responsive genes
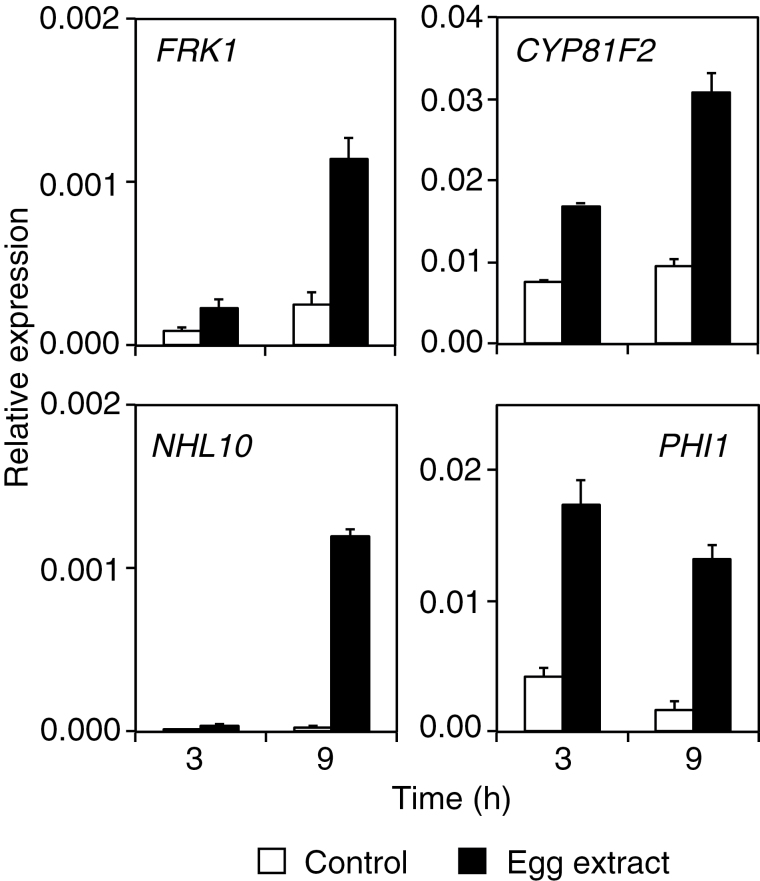
Since recognition of insect eggs by Arabidopsis displayed some elements of a PAMP response (Little et al., 2007), the expression of early responsive genes specific for either the MAPK or the CDPK branch of the PTI response was measured. It was shown previously that treatment with egg extract closely mimics the effect of oviposition by P. brassicae (Bruessow et al., 2010). Arabidopsis plants were thus treated with P. brassicae egg extract and RNA was extracted a few hours later. Up-regulation of FRK1, a MAPK-specific gene, of CYP81F2, a MAPK-dominant gene, of NHL10, a MAPK- and CDPK-regulated gene, and of PHI1, a CDPK-specific gene (Boudsocq et al., 2010) was observed already 3h after egg extract application and was stronger after 9h of treatment (Fig. 1). Thus, these data indicate that P. brassicae egg-derived elicitors activate early genes that are common to the PTI response.
Fig. 1.
Expression of early PAMP-responsive genes. Relative expression levels of FRK1 (At2g19190), CYP81F2 (At5g57220), NHL10 (At2g35980), and PHI1 (At1g35140) were analysed in Arabidopsis by quantitative PCR. Leaves were treated with 2 µl of Pieris brassicae egg extract for 3h and 9h before RNA extraction. Expression levels were normalized with respect to the housekeeping gene EIF4A (At3g13920). Data bars represent the mean (±SE) of three technical repeats. This experiment was repeated once with similar results.
EDS1 is crucial for egg-induced defence gene expression
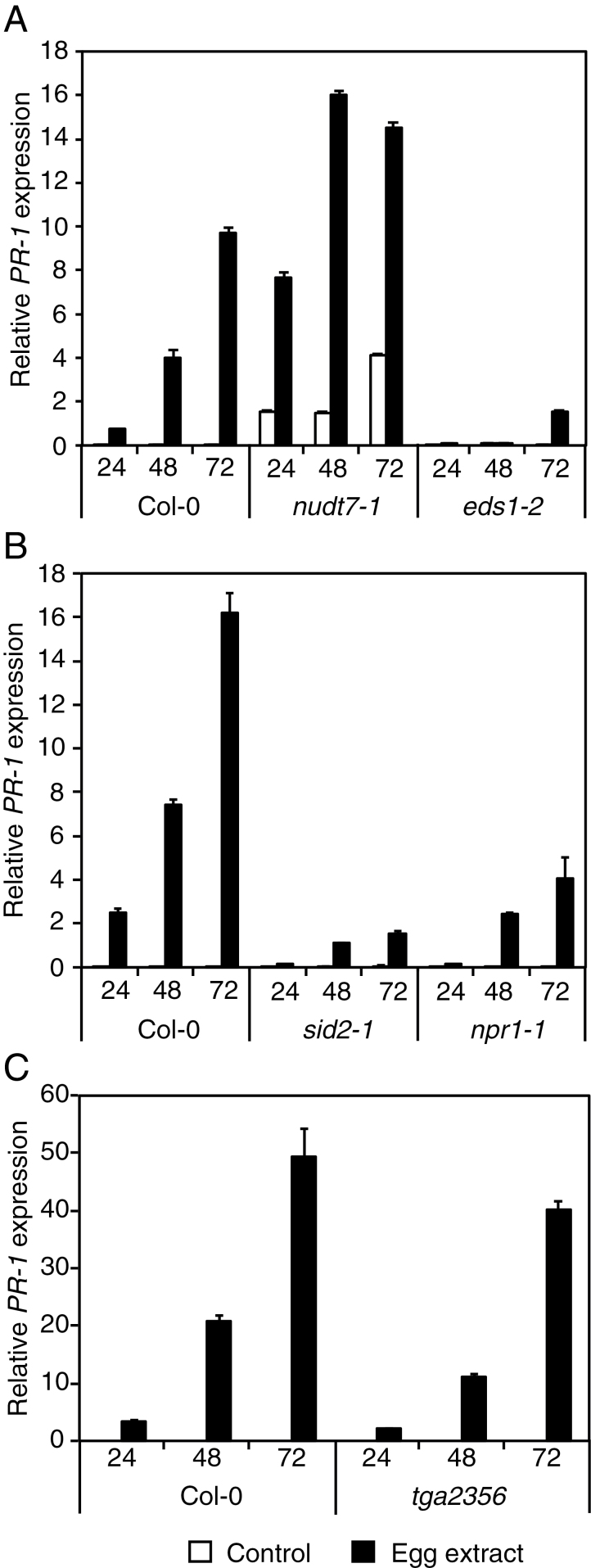
EDS1 activity is crucial for PTI-related ROS production and SA-dependent defence gene activation. This activity is antagonized by Nudix hydrolase7 (NUDT7), a pyrophosphohydrolase that is important for limiting oxidative stress (Straus et al., 2010). The expression of PR-1, a well known marker of the SA pathway that is strongly up-regulated by oviposition (Little et al., 2007), was thus followed in wild-type and eds1-2 plants after treatment with P. brassicae egg extract. In Col-0, PR-1 expression increased gradually from 24h to 72h after egg extract treatment, whereas this induction was almost completely abolished in eds1-2 (Fig. 2A). In contrast, nudt7-1 exhibited a much greater up-regulation of PR-1 than Col-0, in line with the known antagonistic effect of NUDT7 on EDS1 activity. Similarly, the expression of several oviposition-induced genes was strongly reduced in eds1-2, including ICS1, a gene that is required for SA synthesis, thioredoxin-H5 (TRX5), a gene that regulates an important step of the SA-mediated defence response, a trypsin inhibitor (TI), and a chitinase (CHIT) (Supplementary Fig. S1 at JXB online). These data indicate that EDS1 plays a key role in Arabidopsis response to oviposition and that EDS1 acts in concert with NUDT7 to control the expression of SA-dependent genes.
Fig. 2.
PR-1 expression in SA signalling mutants. The relative expression level of PR-1 (At2g14610) was analysed by quantitative PCR. Leaves from Col-0, nudt7-1, and eds1-2 (A), Col-0, sid2-1, and npr1-1 (B), and Col-0 and the tga2356 quadruple mutant (C) were treated with 2 µl of P. brassicae egg extract for 24, 48, and 72h before RNA extraction. Expression levels were normalized with respect to the housekeeping gene EIF4A. Data bars represent the mean (±SE) of three technical repeats. Each experiment was repeated once with similar results.
NPR1 controls egg-induced PR-1 expression independently of TGAs
NPR1 interacts with TGA transcription factors to control the expression of pathogenesis-related genes. Six members of this family have been shown to play a role in response to bacterial pathogens (Zhang et al., 2003; Kesarwani et al., 2007). Specifically, TGA2, TGA5, and TGA6 have a significant and redundant function in PR-1 expression but require TGA3 for a more stringent activation (Zhang et al., 2003; Kesarwani et al., 2007; Blanco et al., 2009). PR-1 expression was analysed in npr1-1 and in the quadruple mutant tga2356 in response to egg extract treatment. First, npr1-1 showed a much reduced induction of PR-1 compared with Col-0, although this reduction was not as pronounced as in sid2-1 (Fig. 2B). Surprisingly, PR-1 expression in tga2356 followed wild-type accumulation from 1 d to 3 d after egg treatment (Fig. 2C). These results show that up-regulation of PR-1 in response to egg treatment requires NPR1 but not TGA2, TGA3, TGA5, and TGA6.
EDS1 and SA modulate ROS accumulation
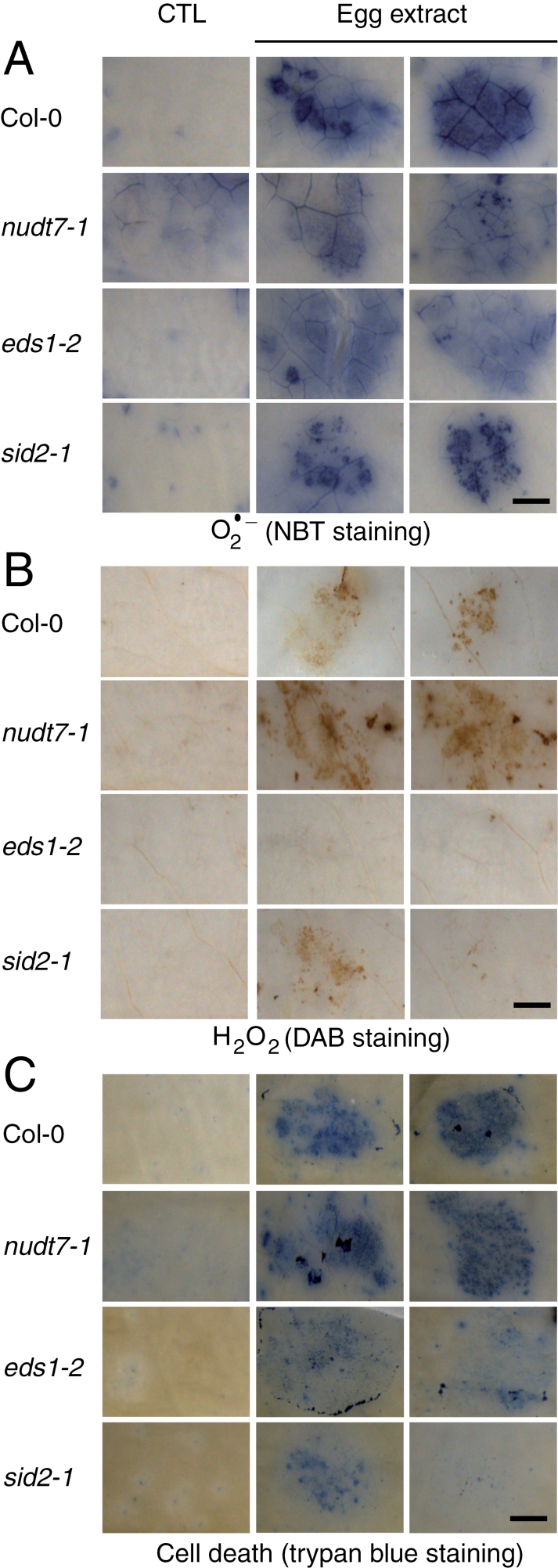
The production of two major ROS, O2 – and H2O2, as well as cell death, in response to egg extract treatment was further explored. Leaves were stained with NBT, which preferentially detects O2 –, with DAB, which reveals the presence of H2O2, or with trypan blue, which accumulates in dead cells. Treatment with egg extract triggered a strong accumulation of O2 –, H2O2, and cell death in Col-0 (Fig. 3). Whereas O2 – accumulation was similar to that in the wild type in eds1-2, nudt7-1, and in the SA-deficient mutant sid2-1, H2O2 and cell death were significantly diminished in eds1-2 and sid2-1 and, in contrast, significantly increased in nudt7-1 (Fig. 3; Supplementary Fig. S3 at JXB online). These results indicate that both EDS1 and SA are required to generate H2O2 downstream of O2 –, and that this process is under the negative regulation of NUDT7.
Fig. 3.
Accumulation of reactive oxygen species (ROS) and cell death in SA signalling mutants. Leaves from Col-0, nudt7-1, eds1-2, and sid2-1 plants were treated with 2 µl of P. brassicae egg extract for 72h. Histochemical staining of leaves with nitroblue tetrazolium (NBT) to detect O2 – (A), 3,3-diaminobenzidine (DAB) to detect H2O2 (B), and trypan blue to detect cell death (D) was performed. Control plants (CTL) were not treated and stained after 72h. For all stainings, leaves from 3–5 different plants per genotype were used. Panels are close-up photographs of the spotted area. This experiment was repeated twice with similar results. All images were taken with the same magnification. Bar=1mm.
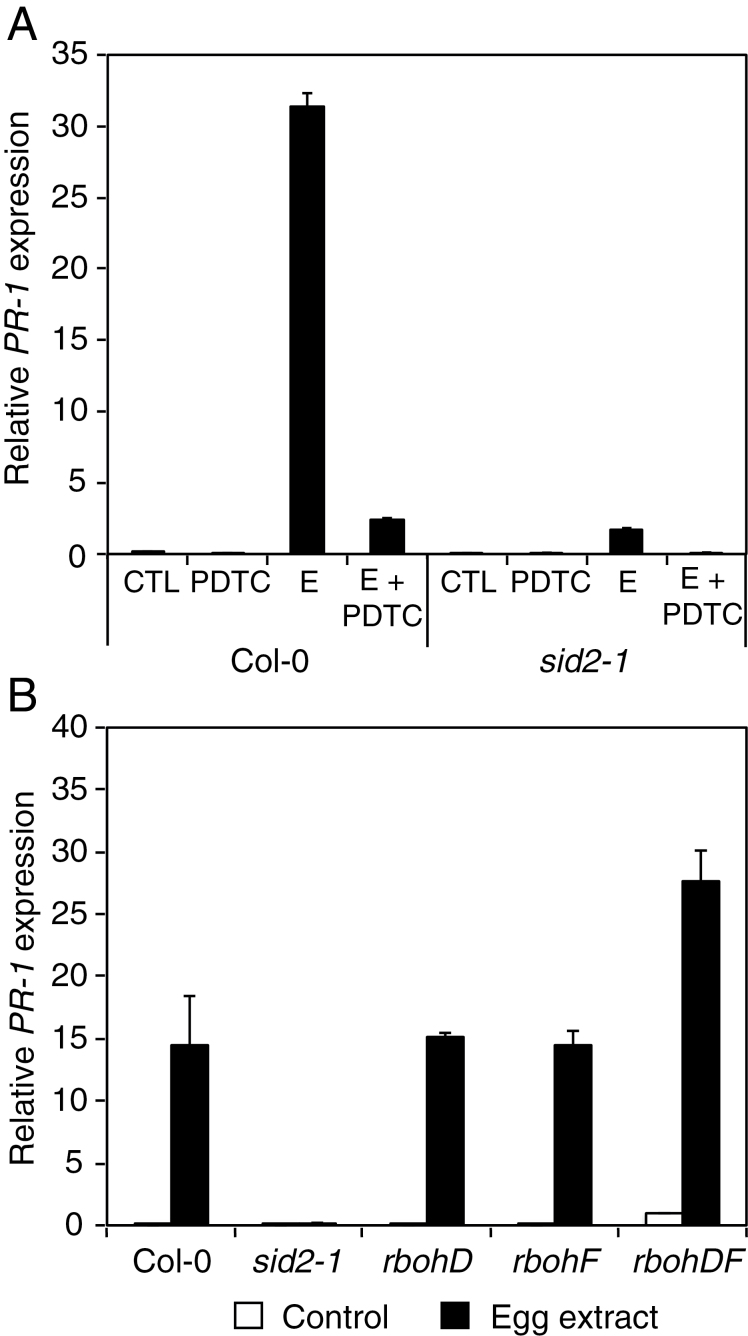
To investigate the relationship between ROS production and PR-1 expression after egg treatment, the ROS scavenger PDTC was used. PDTC strongly diminished egg-induced up-regulation of PR-1, suggesting that ROS production is required for defence gene expression in response to oviposition (Fig. 4A). To confirm that PR-1 induction was dependent on SA-derived ROS accumulation, sid2-1 was treated with egg extract in the presence or absence of PDTC. There was only a weak induction of PR-1 by egg extract treatment in sid2-1 compared with Col-0, and this weak expression was further attenuated by PDTC treatment, indicating that this residual induction was also dependent on ROS accumulation (Fig. 4A). The implication of RBOHD and RBOHF was then tested. Single mutants rbohD and rbohF as well as the double mutant rbohD/F exhibited wild-type production of ROS and dead cells in response to egg extract (Supplementary Figs S2, S3 at JXB online). In addition, up-regulation of PR-1 was not affected in these mutants, suggesting that RBOHD and RBOHF do not play a role in signalling events triggered by oviposition (Fig. 4B). Thus, the data show that defence gene expression in response to P. brassicae eggs requires an oxidative burst that depends on EDS1 and SA.
Fig. 4.
Role of ROS in egg-induced PR-1 expression. The relative expression level of PR-1 was analysed by quantitative PCR. Expression levels were normalized with respect to the housekeeping gene EIF4A. Data bars represent the mean (±SE) of three technical repeats. Each experiment was repeated once with similar results. (A) Leaves from Col-0 and sid2-1 plants were treated with 2 µl of P. brassicae egg extract for 72h in the presence or absence of the ROS scavenger pyrrolidine dithiocarbamate (PDTC). CTL, untreated plants; PDTC, plants treated with PDTC; E, plants treated with egg extract; E + PDTC, plants treated with both egg extract and PDTC. (B) Leaves from Col-0, rbohD, rbohF, double mutant rbohD/F, and sid2-1 plants were treated with 2 µl of P. brassicae egg extract for 72h.
Partial purification of P. brassicae egg extract
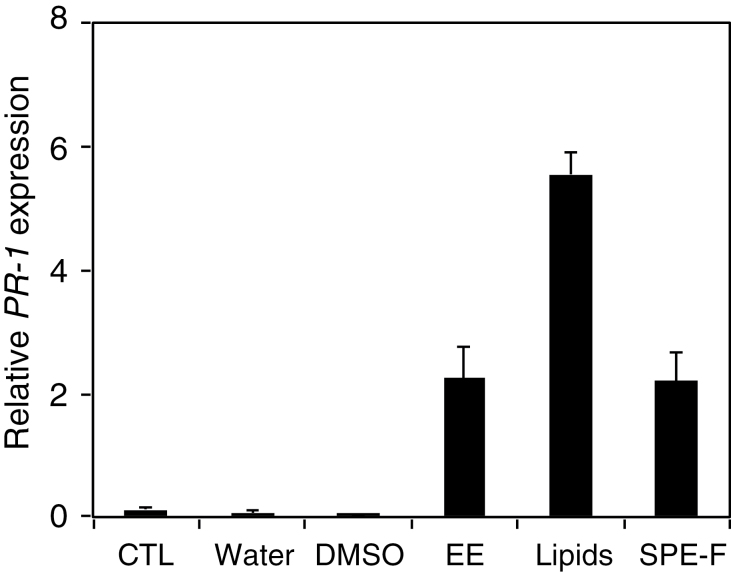
In a preliminary attempt to characterize the chemical nature of egg-derived elicitors, lipids were extracted from P. brassicae eggs and fractionated by SPE. Similarly to crude egg extract, total lipids and a fraction eluted with 100% MeOH strongly activated Arabidopsis β-glucuronidase (GUS) reporter lines containing the promoter of PR-1, TI, and SAG13, which are genes induced by egg extract treatment (Bruessow et al., 2010). GUS staining was precisely confined to the site of application, suggesting that egg-derived elicitors are recognized at the site of oviposition (Supplementary Fig. S4 at JXB online). In addition, PR-1 expression was quantitated and it was shown that P. brassicae egg extract, total egg lipids, and the active SPE fraction induced the expression of PR-1 after 24h, whereas a treatment with either water or 10% DMSO was inactive (Fig. 5). The active SPE fraction was also shown to induce early PAMP-responsive genes (Supplementary Fig. S5).
Fig. 5.
Purified egg lipids activate PR-1 gene expression. Plants were treated with 2 µl of P. brassicae egg extract, total egg lipids, and a fraction eluting at 100% MeOH from a solid-phase extraction column (SPE-F) for 24h. Untreated plants (CTL) and plants treated with 2 µl of water or 10% DMSO were used as controls. The relative expression level of PR-1 was analysed by quantitative PCR. Expression levels were normalized with respect to the housekeeping gene EIF4A. Data bars represent the mean (±SE) of three technical repeats. The experiment was repeated once with similar results.
Involvement of a L-type lectin receptor kinase in egg perception
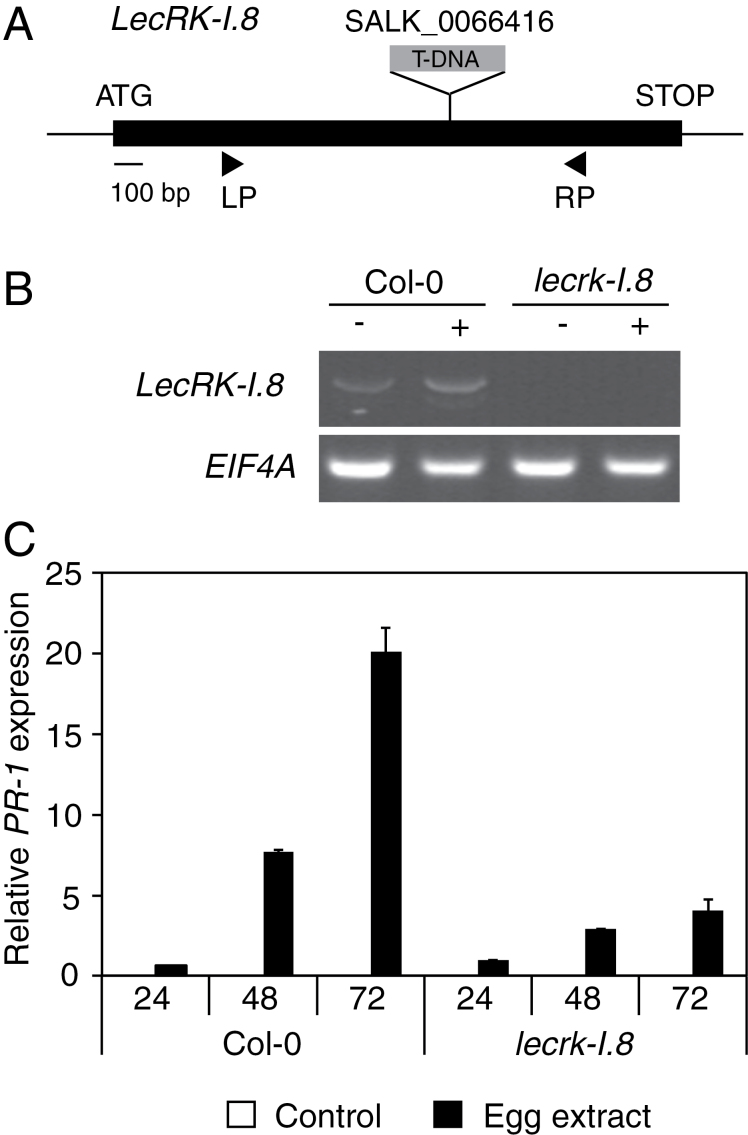
PAMPs are generally perceived by receptor-like kinases (RLKs) at the plasma membrane. FLS2, the receptor for flagellin, was identified through a genetic screen (Gomez-Gomez and Boller, 2000), whereas CERK1, the receptor for chitin, was identified through a homology search with a rice oligosaccharide-binding protein (Miya et al., 2007). For the bacterial PAMP EF-Tu, its receptor EFR was discovered based on the observation that the EFR gene was induced after treatment with EF-Tu (Zipfel et al., 2006). Given that >600 RLKs are present in Arabidopsis (Shiu et al., 2004) and without having a chemically characterized egg elicitor, it was reasoned that one strategy to search for a potential receptor was to concentrate on RLKs that are induced by egg extract. It was previously reported that P. brassicae egg extract triggers a significant induction of 41 RLK genes (Little et al., 2007). T-DNA insertion lines were thus obtained from all candidate genes and PR-1 expression was monitored in response to egg extract treatment (not shown). From all tested mutant lines, only one showed a clear and consistent reduced response to egg extract. The mutated gene encodes LecRK-I.8, which is an L-type lectin receptor kinase. The lecrk-I.8 mutant has a T-DNA inserted in the middle of the LecRK-I.8 coding sequence and has no detectable transcript (Fig. 6A, B). When treated with egg extract for 24, 48, and 72 h, lecrk-I.8 plants showed a strong, although not complete, reduction of PR-1 expression compared with Col-0 (Fig. 6C), suggesting that this RLK plays a role in the perception of egg-derived elicitors.
Fig. 6.
Involvement of an L-type lectin receptor kinase in egg perception. (A) Gene structure of LecRK-I.8 (At5g60280) showing the T-DNA insertion site of the mutant studied here. The positions of primers used for RT-PCR are shown. (B) Analysis of LecRK-I.8 expression in Col-0 and in the T-DNA insertion line lecrk-I.8 by RT-PCR. EIF4A was used as a control. Plants were treated with 2 µl of P. brassicae egg extract for 72h (+). Untreated plants were used as controls (–). (C) The relative expression level of PR-1 was analysed by quantitative PCR. Leaves from Col-0 and lecrk-I.8 were treated with 2 µl of P. brassicae egg extract for 24, 48, and 72h before RNA extraction. Expression levels were normalized with respect to the housekeeping gene EIF4A. Data bars represent the mean (±SE) of three technical repeats. This experiment was repeated once with similar results.
Discussion
Pieris brassicae butterflies deposit eggs underneath Arabidopsis leaves by gently gluing them to the surface without apparent damage. Here it was shown that treatment with egg extract induces the expression of early PAMP-responsive genes within 3h, indicating that there is a fast recognition of egg-derived elicitors by the plant. In addition, it was found that a fraction from purified egg lipids is able to induce PR-1 and early PAMP-responsive genes, strongly suggesting that the activation of the SA pathway is triggered by egg-derived elicitor(s) of a non-polar nature. Since P. brassicae egg deposition does not cause wounding, these egg elicitors must freely cross the hydrophobic layer of plant cuticle, move through the cell wall, and reach the plasma membrane where potential receptors are located. Although previous observations that P. brassicae eggshells do not activate PR-1::GUS and that egg extracts from widely divergent insect species are similarly active (Bruessow et al., 2010) strongly suggest that egg-derived elicitors must be contained in the inner part of the egg and do not come from surface contaminants, it cannot be formally excluded that conserved endosymbionts or endogenous viruses are the source of elicitors. Further purification and identification of a pure elicitor will answer this question.
In an attempt to identify a receptor for egg-derived elicitors, it was found that the RLK LecRK-I.8 is required for egg-induced PR-1 induction. This receptor belongs to a class of L-type lectin receptor kinases consisting of 45 members. Several LecRKs are differentially expressed during growth and development and are induced upon treatment with elicitors and pathogens (Bouwmeester and Govers, 2009). Considering the lectin nature of the extracellular domain, LecRKs are postulated to interact with carbohydrate-containing ligands, but the presence of a conserved hydrophobic pocket does not exclude other ligands (Barre et al., 2002). The observation that lecrk-I.8 does not display a complete lack of PR-1 induction suggests that other RLKs are required for egg perception. Genetic redundancy might explain this finding since LecRK-I.8 is part of a cluster of five closely related LecRLK genes on chromosome 5. Alternatively, heterodimers between LecRK-I.8 and other co-receptors might form a ligand–receptor complex, and the disruption of one component might not totally abolish the response. Further characterization of egg-derived elicitors and the demonstration of their interaction with LecRK-I.8 or other RLKs will be crucial to understand early phases of egg detection by the plant.
Although the JA pathway is central for response to chewing larvae in Arabidopsis (Reymond et al., 2004; Howe and Jander, 2008), SA accumulation under P. brassicae eggs (Bruessow et al., 2010) suggested, however, that this pathway was involved in response to oviposition. Indeed, it is shown here that EDS1, SID2, and NPR1, essential components of the SA pathway, are necessary for the induction of defence genes in response to egg treatment. This is remarkable in the sense that two developmental stages of the same species, P. brassicae eggs and larvae, trigger two antagonistic signal transduction pathways. There are, however, examples where egg deposition is accompanied by wounding of the leaf. In pine trees, oviposition by the pine sawfly induces the release of plant volatiles that are attractive to egg parasitoids (Hilker et al., 2002). In this case, needle slitting by gravid females occurs prior to egg deposition. The observation that JA treatment mimics the release of volatiles suggested that this hormone may be involved in indirect defence against eggs (Hilker et al., 2002). However, SA accumulation was not measured in these plants and, since wounding activates the JA pathway, the specific contribution of JA to the signalling of oviposition remains to be determined.
In contrast to the well established involvement of ROS in plant–pathogen interactions, evidence for a role for ROS in plant–insect interactions is still preliminary (Giovanini et al., 2006; Maffei et al., 2006; Kerchev et al., 2012). Although RBOHD and RBOHF have been implicated in PTI (Torres et al., 2002; Marino et al., 2012), it was found that treatment with egg extract induced wild-type ROS production and PR-1 gene expression in a rbohD/F double mutant. There are 10 RBOHs in Arabidopsis but, unlike RBOHD and RBOHF, other RBOH homologues are mainly expressed in roots and rarely respond to stress in aerial parts (Suzuki et al., 2011). Reduction of molecular oxygen to O2 – can, however, occur through other mechanisms in plant cells. Photosystem I in the chloroplasts and Complex I and III of the electron transport chain in mitochondria generate O2 – in response to excessive electron flow (Mittler et al., 2004), whereas cell wall class III peroxidases can catalyse O2 – production in the apoplast (Liszkay et al., 2003). It was found that egg-induced accumulation of H2O2 and cell death, but not O2 –, requires EDS1 and is under the negative control of NUDT7. This result is strikingly similar to the role of EDS1 and NUDT7 in photo-oxidative stress responses where they modulate the balance between chloroplast-derived O2 – and H2O2 through SA (Straus et al., 2010). Interestingly, insect egg deposition was shown to reduce photosynthetic activity (Schröder et al., 2005) and the expression of photosynthesis-related genes (Little et al., 2007). A decreased photosynthetic efficiency at the site of oviposition might lead to incomplete conversion of absorbed light energy by the photosystems, with the consequence that excess excitation energy would be dissipated in the form of ROS, including O2 –. Clearly, the data link ROS accumulation to egg-induced gene expression, but more work will be needed to identify the source and mechanisms of ROS production in response to oviposition.
It was found that NPR1 is a major regulator of egg-induced gene expression. Induction of PR-1 by egg extract was severely compromised in npr1-1 but, however, not to the extent observed in sid2-1. A residual PR-1 expression in npr1-1 could be due to partial genetic redundancy. There are five NPR1 paralogues in Arabidopsis and they were shown to contribute quantitatively to SA responses (Canet et al., 2010). For instance, NPR3 and NPR4 negatively regulate PR gene expression (Zhang et al., 2006), illustrating a complex interplay of positive and negative activities of NPR factors. Indeed, NPR3 and NPR4 were recently found to bind SA directly with different affinities, and NPR3 and NPR4 are postulated to mediate NPR1 stability differently depending on SA levels (Fu et al., 2012).
Although NPR1 was reported to interact with TGA factors to activate SA-dependent gene expression, egg extract treatment induced PR-1 to wild-type levels in the tga2356 quadruple mutant, suggesting that factors other than TGAs are required for this induction. WRKY proteins represent another class of transcription factors that are involved in defence responses, and some WRKYs are direct targets of NPR1 (Wang et al., 2006). It was previously found that several WRKYs, including some NPR1 targets, are induced by P. brassicae oviposition (Little et al., 2007). These factors might thus play a specific role in egg-induced defence gene expression.
It was recently reported that egg-induced SA accumulation leads to a suppression of defence against chewing larvae by negatively interfering with the JA pathway (Bruessow et al., 2010). This finding raised the intriguing hypothesis that during evolution eggs have hijacked a pre-existing SA pathway for the benefit of their progeny. However, this left open the question on the initial role of SA in response to oviposition. Egg viability was tested in SA signalling mutants but, surprisingly, it was observed that the egg hatching rate was not significantly different between Col-0, eds1-2, and nudt7-1 plants (Supplementary Fig. S6A at JXB online). In addition, P. brassicae larvae that emerged from eggs oviposited on these plants did not show any significant difference in weight gain when transferred to Col-0 plants for 7 d (Supplementary Fig. S6B). Thus, it looks like the activation of SA-dependent defences in Col-0 is not crucial for embryo development and further performance of hatching larvae. Although Arabidopsis does not display a strong necrotic zone at the oviposition site, other plant species develop a more intense response that can lead to egg mortality, egg dropping from the leaf, or a reduced larval survival rate (Shapiro and DeVay, 1987; Balbyshev and Lorenzen, 1997; Hilker and Meiners, 2006). One important role for the SA pathway might be to control the development of a hypersensitive-like response under the eggs. Activation of the SA pathway might be attenuated in Arabidopsis and not efficient enough to have measurable effects on egg viability. Whether the egg-induced SA pathway is only beneficial for the attacker or makes a significant contribution to defence against eggs will need to be further addressed.
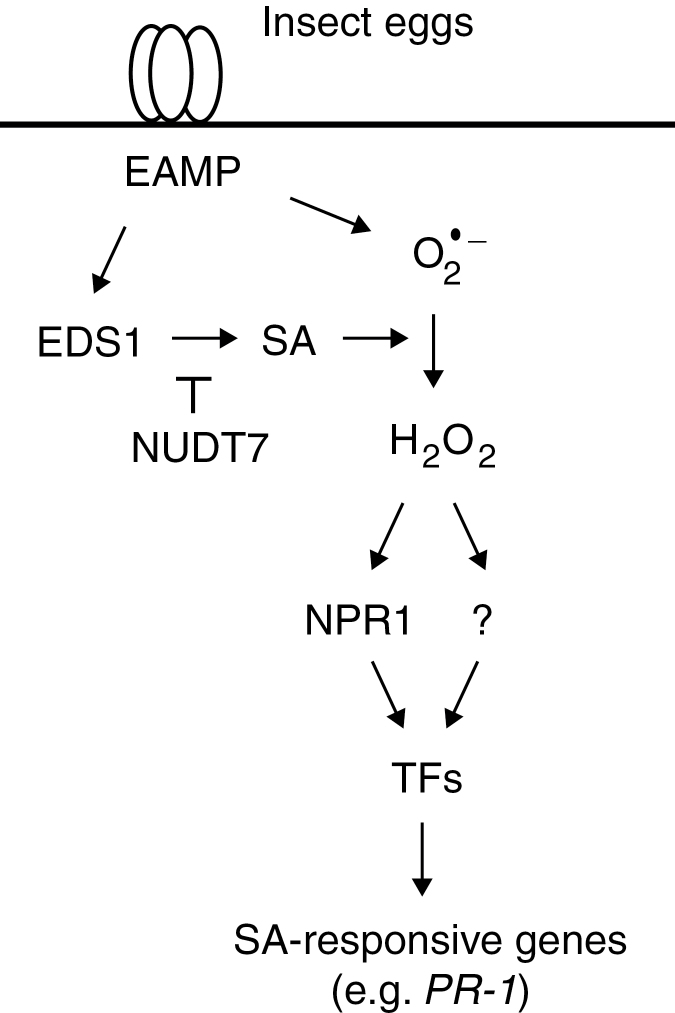
In conclusion, a model depicting the current understanding of oviposition signalling in Arabidopsis is proposed that is based on existing knowledge of PTI and the SA pathway (Fig. 7). Eggs deposited on leaves release as yet unknown egg-associated molecular patterns (EAMPs) that are recognized by cell surface receptor(s), including potentially LecRK-I.8. This triggers a PTI-like response that involves early MAPK- and CPK-dependent signalling and a burst of O2 –. EDS1 stimulates the accumulation of SA, thus favouring the conversion of O2 – to H2O2. This leads to a change in the cellular redox state that alters NPR1 conformation, which is then translocated to the nucleus where its association with transcription factors controls the expression of defence genes. Although this pathway shares several known components of PTI, there are some differences that distinguish egg perception from bacterial infection. Indeed, it is shown here that RBOHD and RBOHF are not responsible for O2 – accumulation and that TGA factors are dispensable for PR-1 induction. Future work will be necessary to identify the chemical nature of EAMPs and their plant receptors, how and where ROS are generated, and which downstream transcription factors regulate the expression of SA-dependent genes. It is however remarkable that plants have evolved a similar perception machinery to detect insect eggs, fungi, and bacteria.
Fig. 7.
Model for signalling of egg-induced gene expression in Arabidopsis. Upon egg oviposition, as yet unknown egg-associated molecular patterns (EAMP) are recognized by plant surface receptors and activate EDS1-dependent SA accumulation. SA promotes the conversion of EAMP-induced superoxide (O2 –) to hydrogen peroxide (H2O2), which in turn leads to the translocation of NPR1 to the nucleus. Association of NPR1 with as yet unknown transcription factors (TFs) controls the up-regulation of PR-1 and other SA-dependent genes. Eggs also activate an NPR1-independent induction of SA-responsive genes. In the model, NUDT7 acts as a negative regulator of EDS1 activity.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. EDS1 is required for egg-induced expression of SA signalling genes.
Figure S2. Egg-induced ROS and cell death accumulation in rbohD and rbohF mutants.
Figure S3. Quantification of ROS and cell death accumulation.
Figure S4. Purified egg extracts activate the expression of reporter genes.
Figure S5. Purified egg extracts activate the expression of early PAMP-responsive genes.
Figure S6. Egg viability in SA signalling mutants.
Table S1. List of primers used in this study.
Acknowledgements
This project was supported by Swiss SNF grant 31003A_132915. We thank Blaise Tissot for maintenance of the plants, Dr Jane Parker for the eds1-2 and nudt7-1 mutants, Dr Miguel Angel Torres for the rbohD/F mutant, and Dr Corné Pieterse for the tga2356 mutant. We thank O. Hilfiker for the preparation of egg lipids.
References
- Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC. 2004. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiology 135, 2025–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbyshev N, Lorenzen J. 1997. Hypersensitivity and egg drop: a novel mechanism of host plant resistance to Colorado potato beetle (Coleoptera: Chrysomelidae). Journal of Economic Entomology 90, 652–657 [Google Scholar]
- Barre A, Hervé C, Lescure B, Rougé P. 2002. Lectin receptor kinases in plants. Critical Reviews in Plant Sciences 21, 379–399 [Google Scholar]
- Blanco F, Salinas P, Cecchini NM, Jordana X, van Hummelen P, Alvarez ME, Holuigue L. 2009. Early genomic responses to salicylic acid in Arabidopsis . Plant Molecular Biology 70, 79–102 [DOI] [PubMed] [Google Scholar]
- Boller T, Felix G. 2009. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annual Review of Plant Biology 60, 379–406 [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng S-H, Sheen J. 2010. Differential innate immune signalling via Ca(2+) sensor protein kinases. Nature 464, 418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester K, Govers F. 2009. Arabidopsis L-type lectin receptor kinases: phylogeny, classification, and expression profiles. Journal of Experimental Botany 60, 4383–4396 [DOI] [PubMed] [Google Scholar]
- Bruessow F, Gouhier-Darimont C, Buchala A, Metraux J-P, Reymond P. 2010. Insect eggs suppress plant defence against chewing herbivores. The Plant Journal 62, 876–885 [DOI] [PubMed] [Google Scholar]
- Bruessow F, Reymond P. 2007. Oviposition-induced changes in Arabidopsis genome expression: anticipating your enemy?. Plant Signaling and Behavior 2, 165–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canet JV, Dobón A, Roig A, Tornero P. 2010. Structure–function analysis of npr1 alleles in Arabidopsis reveals a role for its paralogs in the perception of salicylic acid. Plant, Cell and Environment 33, 1911–1922 [DOI] [PubMed] [Google Scholar]
- De Vos M, Van Oosten VR, Van Poecke RMP, et al. 2005. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Molecular Plant-Microbe Interactions 18, 923–937 [DOI] [PubMed] [Google Scholar]
- Doss RP, Oliver JE, Proebsting WM, Potter SW, Kuy S, Clement SL, Williamson RT, Carney JR, DeVilbiss ED. 2000. Bruchins: insect-derived plant regulators that stimulate neoplasm formation. Proceedings of the National Academy of Sciences, USA 97, 6218–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros NE, Broekgaarden C, Bukovinszkine’kiss G, van Loon JJA, Mumm R, Huigens ME, Dicke M, Hilker M. 2008. Male-derived butterfly anti-aphrodisiac mediates induced indirect plant defense. Proceedings of the National Academy of Sciences, USA 105, 10033–10038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltwell J. 1978. The depredations of the Large White Butterfly (Pieris brassicae) (Pieridae). Journal of Research on the Lepidoptera 17, 218–225 [Google Scholar]
- Fu ZQ, Yan S, Saleh A, et al. 2012. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486, 228–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanini MP, Puthoff DP, Nemacheck JA, Mittapalli O, Saltzmann KD, Ohm HW, Shukle RH, Williams CE. 2006. Gene-for-gene defense of wheat against the Hessian fly lacks a classical oxidative burst. Molecular Plant-Microbe Interactions 19, 1023–1033 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. 2000. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis . Molecular Cell 5, 1003–1011 [DOI] [PubMed] [Google Scholar]
- Hilker M, Kobs C, Varama M, Schrank K. 2002. Insect egg deposition induces Pinus sylvestris to attract egg parasitoids. Journal of Experimental Biology 205, 455–461 [DOI] [PubMed] [Google Scholar]
- Hilker M, Meiners T. 2006. Early herbivore alert: insect eggs induce plant defense. Journal of Chemical Ecology 32, 1379–1397 [DOI] [PubMed] [Google Scholar]
- Hilker M, Meiners T. 2010. How do plants ‘notice’ attack by herbivorous arthropods?. Biological Reviews 85, 267–280 [DOI] [PubMed] [Google Scholar]
- Hilker M, Stein C, Schröder R, Varama M, Mumm R. 2005. Insect egg deposition induces defence responses in Pinus sylvestris: characterisation of the elicitor. Journal of Experimental Biology 208, 1849–1854 [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G. 2008. Plant immunity to insect herbivores. Annual Review of Plant Biology 59, 41–66 [DOI] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. 2006. The plant immune system. Nature 444, 323–329 [DOI] [PubMed] [Google Scholar]
- Kerchev PI, Fenton B, Foyer CH, Hancock RD. 2012. Plant responses to insect herbivory: interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant, Cell and Environment 35, 441–453 [DOI] [PubMed] [Google Scholar]
- Kesarwani M, Yoo J, Dong X. 2007. Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis . Plant Physiology 144, 336–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kular JS, Kumar S. 2011. Quantification of avoidable yield losses in oilseed Brassica caused by insect pests. Journal of Plant Protection Research 51, 38–43 [Google Scholar]
- Liszkay A, Kenk B, Schopfer P. 2003. Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta 217, 658–667 [DOI] [PubMed] [Google Scholar]
- Little D, Gouhier-Darimont C, Bruessow F, Reymond P. 2007. Oviposition by pierid butterflies triggers defense responses in Arabidopsis . Plant Physiology 143, 784–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei ME, Mithoefer A, Arimura G-I, et al. 2006. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiology 140, 1022–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino D, Dunand C, Puppo A, Pauly N. 2012. A burst of plant NADPH oxidases. Trends in Plant Sciences 17, 9–15 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. 2004. Reactive oxygen gene network of plants. Trends in Plant Sciences 9, 490–498 [DOI] [PubMed] [Google Scholar]
- Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N. 2007. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis . Proceedings of the National Academy of Sciences, USA 104, 19613–19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE. 2004. A conserved transcript pattern in response to a specialist and a generalist herbivore. The Plant Cell 16, 3132–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE. 2000. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis . The Plant Cell 12, 707–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder R, Forstreuter M, Hilker M. 2005. A plant notices insect egg deposition and changes its rate of photosynthesis. Plant Physiology 138, 470–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AM, DeVay JE. 1987. Hypersensitivity reaction of Brassica nigra L. (Cruciferae) kills eggs of Pieris butterflies (Lepidoptera: Pieridae). Oecologia 71, 631–632 [DOI] [PubMed] [Google Scholar]
- Shiu S-H, Karlowski WM, Pan R, Tzeng Y-H, Mayer KFX, Li W-H. 2004. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. The Plant Cell 16, 1220–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus MR, Rietz S, Ver Loren E, van Themaat, Bartsch M, Parker JE. 2010. Salicylic acid antagonism of EDS1-driven cell death is important for immune and oxidative stress responses in Arabidopsis . The Plant Journal 62, 628–640 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. 2011. Respiratory burst oxidases: the engines of ROS signaling. Current Opinion in Plant Biology 14, 691–699 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG. 2002. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proceedings of the National Academy of Sciences, USA 99, 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Sato M, Glazebrook J, Cohen JD, Katagiri F. 2008. Interplay between MAMP-triggered and SA-mediated defense responses. The Plant Journal 53, 763–775 [DOI] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF. 2009. Salicylic acid, a multifaceted hormone to combat disease. Annual Review of Phytopathology 47, 177–206 [DOI] [PubMed] [Google Scholar]
- Wang D, Amornsiripanitch N, Dong X. 2006. A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathogens 2, e123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiermer M, Feys BJ, Parker JE. 2005. Plant immunity: the EDS1 regulatory node. Current Opinion in Plant Biology 8, 383–389 [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. 2001. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414, 562–565 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cheng YT, Qu N, Zhao Q, Bi D, Li X. 2006. Negative regulation of defense responses in Arabidopsis by two NPR1 paralogs. The Plant Journal 48, 647–656 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tessaro MJ, Lassner M, Li X. 2003. Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. The Plant Cell 15, 2647–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G. 2006. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125, 749–760 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.