Abstract
Herbivory initiates a shift in plant metabolism from growth to defence that may reduce fitness in the absence of further herbivory. However, the defence-induced changes in carbon assimilation that precede this reallocation in resources remain largely undetermined. This study characterized the response of photosynthesis to herbivore induction of jasmonic acid (JA)-related defences in Nicotiana attenuata to increase understanding of these mechanisms. It was hypothesized that JA-induced defences would immediately reduce the component processes of photosynthesis upon attack and was predicted that wild-type plants would suffer greater reductions in photosynthesis than plants lacking JA-induced defences. Gas exchange, chlorophyll fluorescence, and thermal spatial patterns were measured together with the production of defence-related metabolites after attack and through recovery. Herbivore damage immediately reduced electron transport and gas exchange in wild-type plants, and gas exchange remained suppressed for several days after attack. The sustained reductions in gas exchange occurred concurrently with increased defence metabolites in wild-type plants, whereas plants lacking JA-induced defences suffered minimal suppression in photosynthesis and no increase in defence metabolite production. This suppression in photosynthesis occurred only after sustained defence signalling and defence chemical mobilization, whereas a short bout of feeding damage only transiently altered components of photosynthesis. It was identified that lipoxygenase signalling interacted with photosynthetic electron transport and that the resulting JA-related metabolites reduced photosynthesis. These data represent a metabolic cost to mounting a chemical defence against herbivory and link defence-signalling networks to the differential effects of herbivory on photosynthesis in remaining leaf tissues in a time-dependent manner.
Key words: chlorophyll fluorescence, defence, lipoxygenase, nicotine, plant–insect interaction
Introduction
Plants optimize resource use when attacked by herbivores to minimize reductions in fitness (e.g. McKey, 1974), but the mechanisms that regulate these physiological trade-offs are not well understood. Feeding by arthropod herbivores reduces growth in part through the loss of photosynthetic tissue (e.g. Welter, 1989). Tissues adjacent to feeding damage also may exhibit reduced photosynthesis, further contributing to reductions in productivity (Aldea et al., 2006; Nabity et al., 2009, 2012). Feeding elicits the production of a complex network of hormone signalling that induces defences and initiates the trade-off between using resources for growth or defence. The manifestation of these defences can be specific to the herbivore–plant interaction (Walling, 2000), but the generic response for chewing herbivores is the elicitation of the jasmonic acid (JA) signalling pathway. This pathway regulates indirect and direct defences that vary in their resource requirements and fitness costs (Kessler et al., 2004). Gene expression and proteomic surveys of JA elicitation implicate candidate mechanisms regulating the switch from growth to defence (Heidel and Baldwin, 2004; Giri et al., 2006; Maserti et al., 2010; Chen et al., 2011), but the link between defence production and reduced photosynthesis is only beginning to be understood (Zangerl et al., 1997, 2002; Tang et al., 2009; Halitschke et al., 2011).
In the absence of substantial herbivore damage, the elicitation of JA-related defences reduces fitness in many species (Cipollini, 2007; Kim et al., 2009; Kessler et al., 2011); however, the magnitude of this reduction varies with the type of defensive compound produced. Suppression of trypsin protease inhibitors (TPIs) in Nicotiana attenuata Torr. ex Watson increases growth and fitness (Zavala et al., 2004), but plants silenced in nicotine production function similarly to wild-type plants (Steppuhn et al., 2004). As such, TPIs may incur greater resource costs than nicotine. The reallocation of resources to produce TPIs, nicotine, or other defence compounds does not fully account for the ultimate fitness cost, as plants lacking these defences still grow more slowly when attacked by chewing herbivores (van Dam et al., 2001; Zavala and Baldwin, 2004; Zavala et al., 2004). This persistent reduction in fitness cannot be explained by the synthesis or lack of individual defence products and suggests that other aspects of physical damage or JA signalling reduce photosynthesis and growth.
The reduction in photosynthesis in remaining tissues following herbivory may yield a significant and often unaccounted for reduction in carbon assimilation. When caterpillar feeding removed 5% of the leaf area of wild parsnip (Pastinaca sativa L.), photosynthesis declined by 20% in the remaining foliage for at least 3 d as a result of ruptured oil tubes containing autotoxic terpenes (Zangerl et al., 2002; Gog et al., 2005). Defoliation in other species that lack autotoxic defences also reduces photosynthesis (Aldea et al., 2005, 2006; Nabity et al., 2012). In some cases, the loss of photosynthesis in remaining tissue can equal the amount of tissue consumed, effectively doubling fitness costs for each bite consumed (Aldea et al., 2006). Defoliation by the tobacco hornworm (Manduca sexta L.) in N. attenuata reduces the operating efficiency of photosystem II (PSII) (F q’/F m’) in remaining tissue, indicating that electron transport may limit carbon assimilation after short-term feeding (Halitschke et al., 2011). However, feeding damage by piercing sucking insects does not reduce photosynthesis, nor do these insects increase JA or nicotine levels (Heidel and Baldwin, 2004; Halitschke et al., 2011; Donovan et al., 2012). As such, it is hypothesized that herbivore-specific elicitors may determine the photosynthetic suppression in remaining leaf tissue (Halitschke et al., 2011).
The objective of this study was to determine whether induction of JA defences by herbivore attack suppressed component processes of photosynthesis. We measured photosynthesis, hormone levels, and the production of defence compounds immediately after attack and for several days thereafter in wild-type plants and plants with impaired JA signalling. We hypothesized that an inducible JA-related defence signalling network would immediately reduce leaf photosynthesis upon attack and predicted that wild-type plants would suffer greater reductions in photosynthesis than plants lacking JA-related defence production.
Materials and methods
Plants and insects
To determine whether defence signalling alters the component processes of photosynthesis, we examined the response of plants with and without genetically suppressed JA defence signalling to herbivory. Wild-type N. attenuata plants were compared with a genetically altered strain, antisense LOX3 (asLOX), in which the primary lipoxygenase initiating the JA signalling cascade is suppressed. This genetic modification results in a 45–65% decrease in JA burst upon herbivore attack, >25% decrease in nicotine, and up to 50% decrease in TPIs (Halitschke and Baldwin, 2003).
Seeds obtained from the Max Planck Institute for Chemical Ecology (Jena, Germany) were prepared and germinated on agar in an environmental growth chamber (25 °C 16:8h light:dark) following the method of Krugel et al. (2002) and then transplanted to 2 l pots containing soilless medium (Sunshine LC1, SunGro Horticulture, Bellevue, WA, USA). Plants were grown in a glasshouse with supplemental lighting (28:22±2 °C, 16:8h light:dark), watered when necessary, and infested >2 weeks after transplanting. Eggs of M. sexta were obtained from an in-house colony, and after hatching, neonate (<1 d old) larvae were caged on +1 source leaves as determined by Giri et al. (2006) and allowed to feed for 1 or 3 d. These source leaves are the first fully expanded leaf of the developing rosette and were used in place of stalk leaves to test how early herbivory events contribute to lasting reductions in growth and fitness. Plants lacking herbivores were fitted with the same fine mesh cages but without insects. Larvae grew as they fed and after 3 d were first instars.
Gas exchange, chlorophyll fluorescence, and thermal imaging
To capture rapid responses reflecting the direct effect of leaf damage on photosynthesis, gas exchange and chlorophyll fluorescence were measured after 1 d of herbivory. We assumed that this rapid response did not include indirect suppression of photosynthesis associated with an induced defence response. In a subsequent experiment, plants were exposed to 3 d of herbivory to capture potential effects related to synthesis and translocation of the major direct defences (~3 d for TPIs, Van Dam et al., 2001; ~5 d for nicotine, Shi et al., 2006). Two cohorts of plants were used in the 3 d feeding experiment to provide sufficient tissue for analysis of plant metabolites.
Photosynthesis was measured on the first cohort of plants immediately after feeding and after a 2-d recovery time (no insect feeding). Photosynthesis was measured on the second cohort of plants immediately after feeding damage and after a 3 and 7 d recovery following a random design blocked by time of measurement within each day. For all experiments, each genotype (n=5) was assessed at each herbivore treatment (+ or − herbivore) under the conditions described. Immediately after measurements, leaves were clipped, frozen in liquid N2, and stored at −80 °C. Leaves examined over the time course were collected at the last time point. All frozen leaf samples were ground using a mortar and pestle under liquid N2, divided into aliquots of known mass (~150mg), and stored at −80 °C until hormone and metabolite analysis.
Gas exchange was measured with an infrared gas analysis system (LI-6400; Licor, Lincoln, NE, USA) with a modified cuvette allowing simultaneous imaging of chlorophyll fluorescence with a CFImager (Technologica, UK). Leaves were dark-adapted (>30min), imaged after an 800ms saturating pulse (>6000 µmol m–2 s–1, photosynthetically active radiation), light adapted (350 µmol m–2 s–1, photosynthetically active radiation) for 20min until gas exchange parameters stabilized, and then imaged after another saturating pulse. This allowed images of maximal (F m), minimal (F o), and variable (F v=F m−F o) fluorescence, and calculation of the maximal efficiency of PSII (F v/F m), as defined by Baker (2008). Once a light-adapted steady state was achieved, gas exchange values were recorded, and images for the operating efficiency of PSII (F q’/F m’) were taken.
Spatial patterns in leaf temperature were measured immediately after fluorescence using an infrared thermal camera (ThermaCAM T400, FLIR Systems, Portland, OR, USA). Leaves were illuminated under the above light conditions and then imaged at ambient conditions (350 µmol m−2 s−1, 25±2°C).
To characterize herbivore-induced spatial heterogeneity in leaf fluorescence, we counted the number of pixels for each leaf image that were reduced by >5% for F v/F m and by >10% for F q’/F m’ relative to the mean value of undamaged tissue in the same leaf. This value of undamaged tissue did not differ from mean values of separate, undamaged leaves assessed concurrently with damaged leaves. We expressed the damaged area as a percentage of the remaining tissue for each image type. Images were made under low irradiance to maximize electron transfer efficiency (Baker et al., 2007). This procedure optimized the assessment of pulse amplitude-modulated fluorescence and strengthened the correlation between fluorescence and photosynthetic rate (Longstaff et al., 2002).
Spatial patterns in thermal images were assessed by subsampling pixels of undamaged leaf tissue and damaged (chewed) edge tissue using the ‘spot’ tool within the thermal imaging software (FLIR Quick Report, 1.2 SP2; FLIR Systems, Portland, OR, USA).
Plant hormones
To quantify how defence-related plant hormones interact with alterations in photosynthesis, we measured JA and salicylic acid (SA) concentrations for individual leaves. Hormones were analysed by high-performance liquid chromatography mass spectrometry (HPLC-MS) (2010 EV HPLC/MS; Shimadzu, Columbia, MD, USA) using a protocol modified from Wang et al. (2007). Aliquots of frozen leaf tissue were extracted with 1ml ethyl acetate spiked with deuterated internal standards. For JA, five carbons were enriched with 13C (5JA) and added at 200ng ml–1, whereas for SA only four carbons were enriched (4SA) and added at 50ng ml–1 to each sample. Samples were vortexed for 5min and centrifuged at 13 000rpm for 20min at 4 °C, and the supernatants were transferred to new tubes. Each sample was re-extracted with 0.5ml of unlabeled ethyl acetate, shaken, and centrifuged under the same conditions. The supernatants were combined and evaporated to dryness at 30 °C under a vacuum concentrator. The dried residue was resuspended in 0.5ml 70% (v/v) methanol, vortexed for >5min, and centrifuged at 13 000rpm for 10min, and 0.4ml was transferred to vials for analysis by HPLC-MS
HPLC-MS was carried out in negative electrospray ionization mode with a mobile phase of 0.05% formic acid (solvent A) and 0.05% formic acid in methanol (solvent B) used in gradient mode for the separation following the method of Wang et al. (2007). Samples (10 µl) were injected at a flow rate of 200 µL min–1 onto a Luna C18 column (250×2mm, 5 µm internal diameter; Phenomenex, Torrance, CA, USA) with selected reaction monitoring of compound-specific parent ions: JA=209, 5JA=214, SA=137, and 4SA=141.
Direct defence traits
To quantify how defences interact with photosynthesis TPIs and nicotine were assayed. TPI activity was determined by a radial diffusion assay (van Dam et al., 2001). Activity was normalized to protein content determined from an aliquot of ground leaf tissue extracted in 0.5ml of 100mM HEPES (titrated to pH 7.5 with NaOH) and assayed with a Pierce BCA protein kit (ThermoFisher Scientific, Rockford, IL, USA). Nicotine content was analysed by HPLC following a protocol modified from Keinanen et al. (2001). Aliquots of ground leaf samples were extracted with 1ml of 40% methanol with 0.5% acetic acid in H2O, combined with 0.3g of 1mm zircon/silica beads (Biospec Products, Bartlesville, OK, USA), and shaken in a Mini Beadbeater (Biospec Products) for 45 ss. Ground tissues were then vortexed for 2h, centrifuged at 13 000g for 10min, and 0.3ml was pipetted to HPLC vials for analysis.
The same HPLC-MS was used to measure TPIs and nicotine, although a mobile phase made up of 0.25% phosphoric acid in H2O (solvent A) and 100% acetonitrile (solvent B) was used for separation. Samples (10 µl) were injected at a flow rate of 200 µl min–1 onto a column (4.6×150mm, 3 µm internal diameter; Inertsil ODS3, GL Sciences, Torrance, CA, USA) and elution times and monitoring wavelengths were as described by Keinanen et al. (2001).
Data analysis
Data for plant hormones, TPIs, and nicotine were analysed with a mixed model analysis of variance (ANOVA) (Proc Mixed 9.2, SAS Institute, Cary, NC, USA) with genotype (wild-type or asLOX) and herbivore (+ or −) as fixed effects. When the initial ANOVA yielded a significant difference, post-hoc comparisons were made between least-squares means. Gas exchange (CO2 assimilation, stomatal conductance, intercellular CO2, and respiration) and chlorophyll fluorescence imaging parameters were analysed by ANOVA when measurements corresponded with destructively harvested leaves. Gas exchange measured over time was analysed by repeated-measures ANOVA with post-hoc comparisons as described above. Data describing the percentage of tissue where fluorescence was reduced relative to undamaged leaves were log-transformed prior to analysis by ANOVA. Defence metabolites were regressed against photosynthesis parameters using Proc Corr 9.2 (SAS Institute). All comparisons were made at P ≤0.05 unless otherwise specified.
Results
A 1 d bout of herbivory rapidly altered photosynthesis, defence signalling, and metabolites in N. attenuata, although the effects were mild (Table 1). A brief bout of herbivory caused a significant reduction in carbon assimilation but did not alter stomatal conductance (g s) (F=5.36; degrees of freedom (df)=1, 12; P=0.04; and F=2.37; df=1, 12; P=0.15, respectively). Defence-modified asLOX plants maintained slightly lower g s (F=4.65; df=1, 12; P=0.05) and lower respiration rates than wild-type plants (F=5.06, df=1, 12; P=0.04), but no interactions with herbivory were detected. Water flux did not differ between treatments during the day but tended to be higher in damaged leaves at pre-dawn (dark) measurements (F=3.61; df=1, 12; P=0.08). This diurnal effect indicated that evaporative flux was occurring from damaged tissues. Spatial patterns of leaf temperatures (not shown) supported these data and showed damaged leaf edge tissue was cooler and thereby evapotranspiring more water than the remaining undamaged leaf (F=9.98; df= 1, 4; P=0.03). Temperature profiles in undamaged leaves did not differ from undamaged tissues of damaged leaves (F=0.13; df=1, 12; P=0.7). Spatial patterns in chlorophyll fluorescence indicated that wild-type plants had more remaining leaf area damaged from feeding compared with asLOX plants (Table 1).
Table 1.
Chlorophyll fluorescence, leaf temperature, gas exchange, and defense parameters in N. attenuata immediately after 1 d of herbivory by M. sexta on wild-type and JA defense-suppressed (asLOX) plants. Unless otherwise indicated, different letters denote differences among all treatments (simple effects) at the P≤0.05 level of significance. Leaf temperature denotes tissues of the same leaf directly adjacent to where herbivores fed (+) or did not feed (−). *P≤0.07; **0.07< P≤0.1. FW, Fresh weight
| Wild type | asLOX | |||
|---|---|---|---|---|
| − herbivore | + herbivore | − herbivore | + herbivore | |
| Chlorophyll fluorescence | ||||
| Propagated damage to F v/F m | ||||
| % of remaining leaf | 2.9±0.2a | 2.3±0.3b* | ||
| Total area (mm2) | 66±5a | 52±7b* | ||
| Propagated damage to F q'/F m' | ||||
| % of remaining leaf | 9.0±1.9a | 4.8±0.7a | ||
| Total area (mm2) | 206±44a | 109±16a | ||
| Leaf temperature (°C) | 23.1±0.2a | 22.3±0.3b* | 22.8±0.3ab | 22.1±0.1c* |
| Gas exchange | ||||
| CO2 assimilation (µmol m–2s–1) | 8.4±0.1a | 8.0±0.2b** | 8.4±0.1a | 8.1±0.3ab |
| Stomatal conductance (mmol m–2s–1) | 0.233±0.007a | 0.222±0.016a | 0.214±0.015ab | 0.181±0.012b* |
| Intercellular CO2 (C i) | 295±2a | 298±3a | 292±4a | 281±3b* |
| Respiration (µmol m–2s–1) | -0.63±0.06a | -0.66±0.09a | -0.47±0.05b | -0.55±0.05ab |
| Defense metabolites | ||||
| JA (nmol mg FW–1) | 51±6a | 107±15b | 49±4a | 58±5a |
| TPI (nmol mg protein–1) | 1.3±0.1a | 4.4±0.4b | 0.4±0.1c | 0.4±0.1c |
| Nicotine (mg g FW–1) | 0.58±0.04a | 0.61±0.03a | 0.43±0.02b | 0.38±0.03b |
JA and TPI levels increased in wild-type plants compared with asLOX plants (F=13.03; df=1, 12; P=0.004; and F=111.2; df=1, 12; P <0.001, respectively), whereas nicotine content did not increase with 1 d herbivory and was lower overall in asLOX plants (Table 1). Carbon assimilation did not correlate with any defence compounds but correlated with propagated damage to dark-adapted electron transport (r=−0.46, P=0.04). This propagated damage also correlated with JA and TPIs but not nicotine levels (JA: r=0.65, P=0.002; TPIs: r=0.58, P=0.007; nicotine: r=0.03, P=0.9).
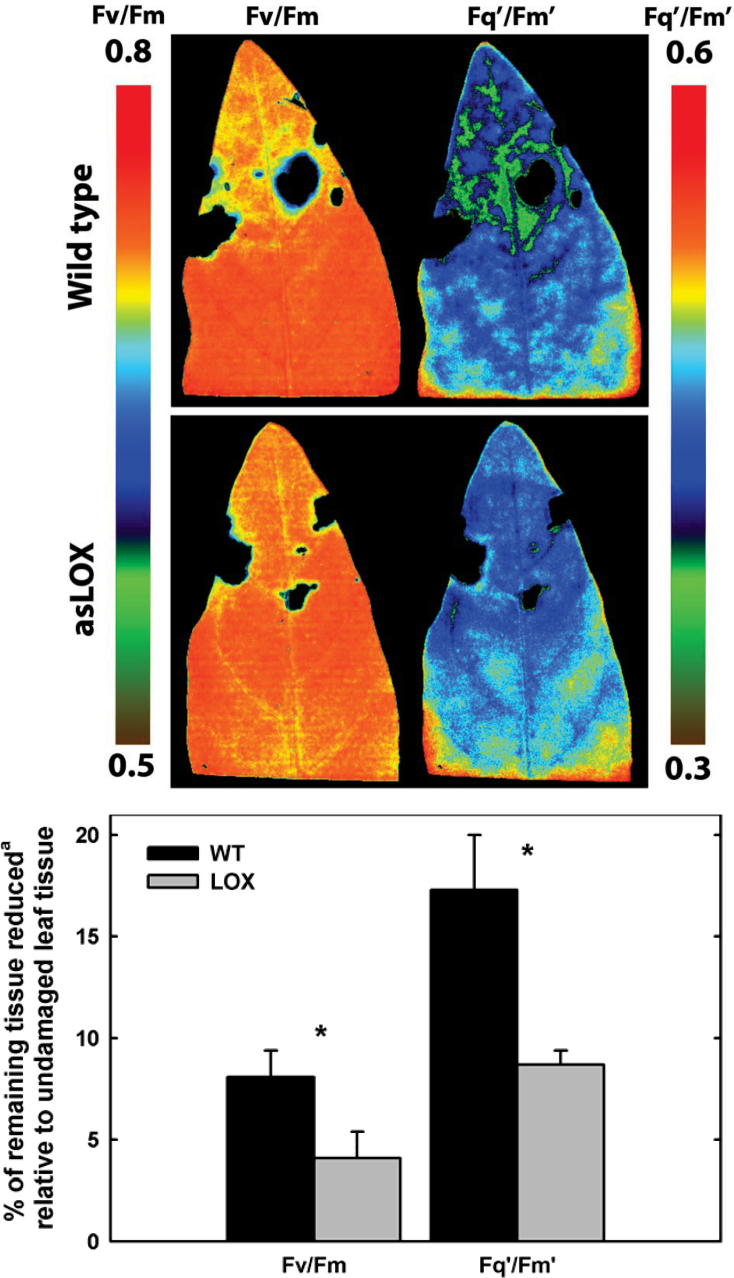
A longer 3 d bout of herbivory caused persistent reductions in photosynthesis and elicited a strong, sustained defence response. After 3 d of feeding, insects consumed 7% of total leaf area (~160mm2) and reduced maximal (F v/F m) and operating (F q’/F m’) efficiency in electron transport in remaining tissue adjacent to feeding sites. Reductions in fluorescence parameters were propagated further away from the cut edges in wild-type compared with asLOX plants (Fig. 1). This area of reduced fluorescence in wild-type plants decreased to the levels observed in asLOX plants after 2 d (F v/F m: wild type=3.7±0.2%, asLOX=3.7±1.1%; F q’/F m’: wild type=4.1±0.3%, asLOX=2.5±0.5%,). No main effects for herbivore or genotype occurred when assessed across the entire leaf.
Fig. 1.
Representative chlorophyll fluorescence images for maximal efficiency of PSII (F v/F m) and operating efficiency of PSII (F q’/F m’) for wild type and JA defence-modified (asLOX) N. attenuata leaves challenged by M. sexta herbivory for 3 d. The difference between genotypes in the percentage of remaining leaf tissue that was reduced by >5% for F v/F m and by >10% for F q’/F m’ is denoted by * at the P≤0.05 level of significance.
Spatial patterns in leaf temperatures (data not shown) after 3 d of feeding or with recovery (of 2 d) did not significantly reduce leaf temperatures adjacent to feeding sites (F=0.34; df=1, 4; P=0.6; and F=2.99; df=1, 4; P=0.2, respectively). Insects consumed equivalent amounts of leaf tissue across 3 d feeding experiments for both genotypes, indicating that the difference in percentage damage to remaining tissue was not related to the amount of leaf consumed.
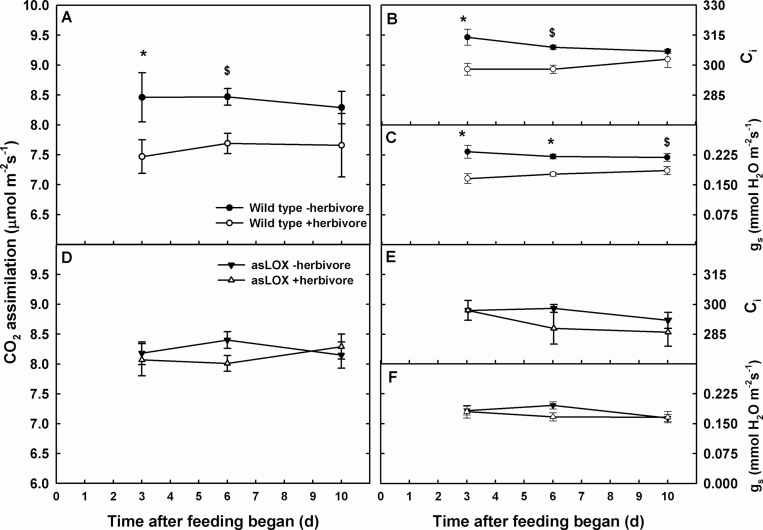
Herbivory reduced photosynthesis in both genotypes (F=6.92; df=1, 12; P=0.03) but interacted with genotype over time (F=4.73; df=1, 12; P=0.05). Photosynthesis declined in wild-type plants immediately following chewing damage and for up to 3 d after removal of insects, whereas photosynthesis remained similar to that in uninfested plants immediately after insect removal in asLOX plants (Fig. 2). Both g s and intercellular CO2 concentration (C i) decreased after 3 d of feeding in damaged wild-type plants, but only g s remained lower up to 3 d into recovery. Wild-type plants also maintained higher respiration rates over time (F=5.06; df=1, 12; P=0.04) with an immediate decrease in respiration in infested wild-type plants after feeding that attenuated with recovery. Gas exchange parameters for plants destructively harvested for measurement of metabolites were similar to those recorded in the infestation and recovery experiment (data not shown).
Fig. 2.
CO2 assimilation (A and D) of wild-type (A, B, C) and JA defence-modified asLOX (D, E, F) N. attenuata leaves challenged by M. sexta herbivory for 3 d. The corresponding intercellular CO2 concentration (C i) (B, E) and stomatal conductance (g s; C, F) are also shown. Asterisks denote treatment difference within a genotype at the P≤0.05 level of significance unless otherwise indicated; P≤0.1 is denoted by $.
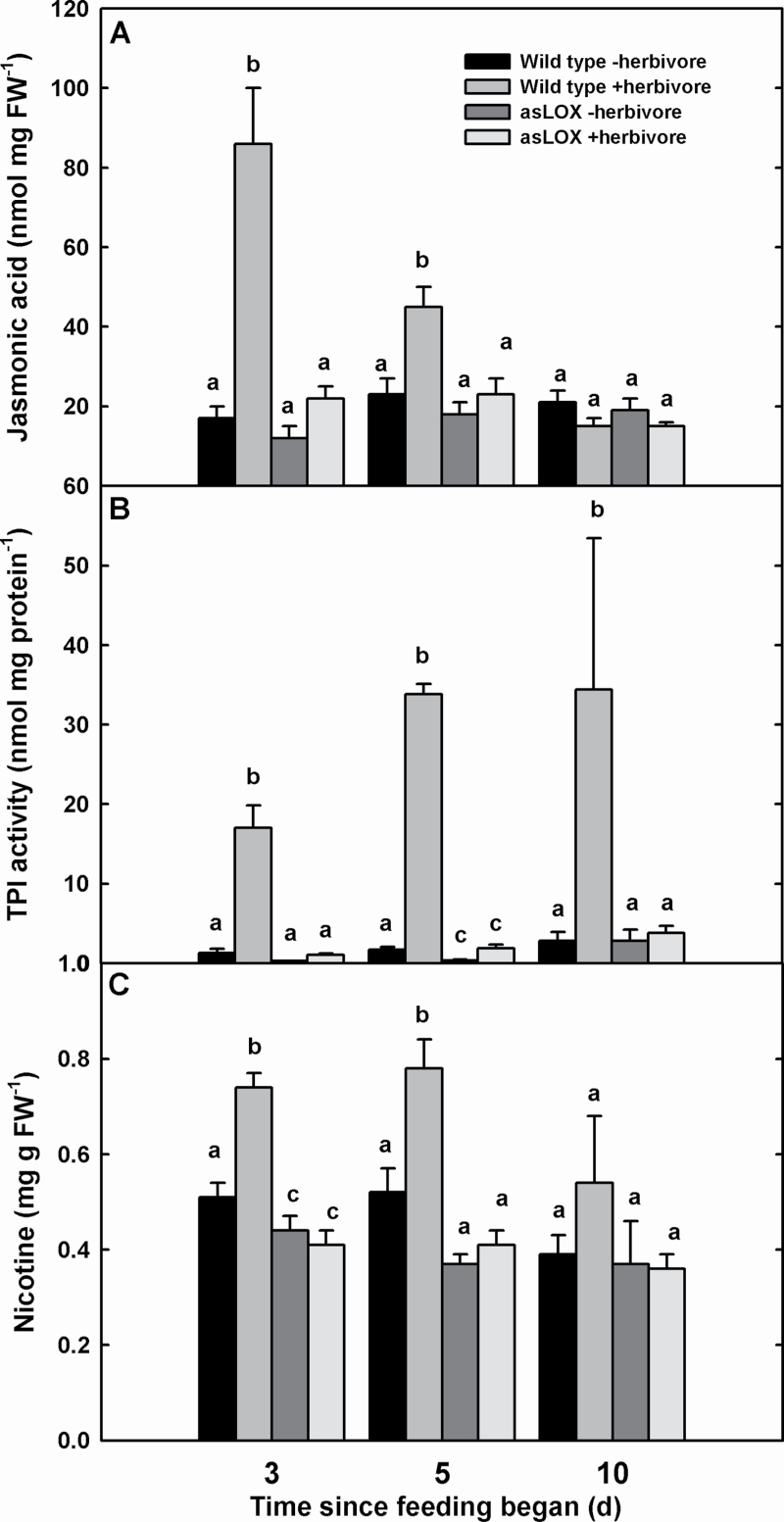
Changes in plant hormones and defensive chemicals following herbivory followed similar kinetics to changes in photosynthesis. JA levels increased immediately after 3 d feeding and remained elevated after a 2 d recovery time in wild-type plants, whereas asLOX plants maintained lower JA levels at each sample date until 7 d recovery (Fig. 3). TPI and nicotine levels also increased after feeding; however, the level of leaf TPIs remained elevated after a 7 d recovery. asLOX plants did not increase in defence metabolites with herbivory. SA levels did not differ among treatments throughout the experiment (data not shown).
Fig. 3.
JA, TPI, and nicotine content of wild-type and JA defence-modified asLOX N. attenuata leaves challenged by M. sexta herbivory. Insects were removed after 3 d feeding and tissues were harvested 3, 5, and 10 d after feeding began (i.e. 0, 2, and 7 d recovery time). Different letters denote differences among treatments at the P≤0.05 level of significance. FW, Fresh weight.
To determine how the defence response related to the suppression in photosynthesis, we correlated gas exchange and defence response variables (Table 2). The area consumed did not correlate with the propagated damage to F v/F m or F q’/F m’, thereby reducing the likelihood that physical damage determined the suppression in electron transport. There was a lack of consistency with how CO2 assimilation correlated with defence metabolites over time, but defence metabolites were strongly correlated with propagated damage to electron transport (Table 2). Carbon assimilation did not correlate with percentage reductions in electron transport, suggesting that reductions in gas exchange were not largely determined by limitations in electron transport. Stomatal conductance was negatively correlated with nicotine (r=−0.31, P=0.02) across all time points, but this relationship did not occur between nicotine and other gas exchange parameters.
Table 2.
Correlation coefficients (r) for the relationship of CO2 assimilation (PS) or the area of suppressed maximal electron transport efficiency (F v/F m) compared with defense metabolites JA, TPI, and nicotine for each time point assessed following 3 d of feeding Significant correlations are indicated as:***P ≤0.001, **P ≤0.01, *P ≤0.05 and $P ≤0.1.
| 3d | 5d | 7d | Summary | |||||
|---|---|---|---|---|---|---|---|---|
| PS | F v/F m | PS | F v/F m | PS | F v/F m | PS | F v/F m | |
| JA | −0.40$ | 0.67** | −0.54** | 0.54** | −0.13 | NA | 0.01 | 0.63*** |
| TPI | 0.58** | 0.58** | −0.50$ | 0.60* | 0.17 | NA | 0.09 | 0.52** |
| Nicotine | 0.44$ | 0.52* | −0.53** | 0.38$ | −0.60** | NA | −0.10 | 0.42** |
Discussion
Chewing damage to N. attenuata induced JA-dependent defences and this defence response immediately interrupted electron transport and reduced gas exchange for >6 d following the initial herbivore attack. Downregulation of photosynthesis and concurrent increases in respiration following herbivory occur in other plant species that synthesize biocidal compounds (e.g. terpenes: Zangerl et al., 2002; Gog et al., 2005). While we only observed a constitutive reduction in respiration in asLOX plants, there were strong interactions between JA defence signalling and electron transport, and between defence synthesis and CO2 assimilation. These data provide new insight into the proposed physiological link (Halitschke et al., 2011) between the herbivore-elicited downregulation of photosynthetic gene expression and the resulting reductions in fitness (Voelckel and Baldwin, 2004). Herbivore-mediated downregulation of photosynthesis was directly related to lipoxygenase signalling that interacted with electron transport. Because the induction of JA and lipoxygenase signalling varies with the type of herbivore damage (Heidel and Baldwin, 2004; Halitschke et al., 2011; Donovan et al., 2012), the link between these processes may help to explain the differential effects of herbivory on photosynthesis in the remaining leaf tissues (Nabity et al., 2009).
Because wound signalling is transduced by the oxidation of free α-linolenic acid within the chloroplast via electron-carrying lipoxygenases (LOX3; Halitschke and Baldwin, 2003) but results in downstream synthesis of JA (Gfeller et al ., 2010), it is difficult to assess which components of the signalling pathway interact directly with photosynthesis. We determined how suppressing the entire pathway altered photosynthesis and observed reductions in electron transport that were strongly correlated with JA levels and subsequently with the production of defence metabolites. Prior evidence indicated that wild-type N. attenuata has lower gene expression of light-harvesting complex (LHC) and PSII oxygen-evolving-complex polypeptides compared with asLOX plants when attacked by herbivores, whereas gene expression of the Rubisco small subunit and two peptides of PSII decreases more in asLOX than in wild-type plants (Halitschke and Baldwin, 2003). These expression patterns suggest that LOX3 signalling suppresses gene expression of transcripts related to electron transport proteins, whereas gene expression for the Calvin cycle and other electron transport proteins are uncoupled from LOX3-dependent signalling.
In comparison with inverted repeat JASMONATE RESISTANT (JAR4/6) plants that maintain functional LOX activity but reduce amino acid conjugation of JA, asLOX plants transcriptionally upregulate components of PSI (StPSI-I; PSI reaction centre subunit) and Rubisco (Wang et al., 2008). As a result, LOX3 signalling may interact with photosynthesis more directly than JA synthesis and the suppression of LOX3 reduces a potential physiological cost associated with defence signalling. Plants with reduced function of PsbS, a light-harvesting protein that facilitates the quenching of excess energy into dissipated heat, showed strong upregulation of the lipoxygenase signalling pathway (Frenkel et al., 2009), and this result reinforces the link between light reaction-associated electron transport and lipoxygenase signalling.
We observed reduced electron transport only under larval attack and not between undamaged genotypes, thereby indicating that an interaction with larval secretions may determine the degree of photosynthetic suppression upon signalling for defence. In the absence of herbivory, prolonged exposure (9 d) to exogenously applied JA concentrations may reduce electron transport for as long as JA levels remain elevated (Gao et al., 2012), and infiltration with the JA precursor 12-oxo-phytodienoic acid may transiently (<1 d) reduce electron transport (Berger et al., 2007). These data suggest that JA alone is not enough to suppress gas exchange but may, in part, contribute to reductions in electron transport. Our data support this because JA correlated with the area of suppressed electron transport across genotypes (Table 2). Larval saliva dephosphorylates constitutively phosphorylated lipoxygenase in Arabidopsis and reduces JA synthesis (Thivierge et al., 2010); thus, the lipoxygenase-dependent reduction in photosynthesis is probably herbivore mediated and, in part, supports the hypothesis that elicitors drive the suppression in photosynthesis (Halitschke et al., 2011). Given the suppressed oxidation that occurs constitutively in defence-silenced plants (Donovan et al., 2012) and the increase in oxidation that occurs upon herbivory, it is likely that LOX-mediated JA signalling regulates reactive oxygen species that in turn modulate LHCs of photochemistry and interrupt electron transfer. Barring any pleiotropic effects from silencing the LOX cascade, this hypothesis warrants additional testing.
Many forms of biotic attack including herbivory downregulate genes related to primary photochemistry and the Calvin cycle (Bilgin et al., 2010). The effect on translation into protein levels is less clear. Elicitation of JA defences both increases and decreases Calvin cycle enzymes in Citrus clementina (Hort. ex Tan.) (Maserti et al., 2010) and Arabidopsis thaliana (L.) Heynh. (Chen et al., 2011), but LHC proteins generally increase following damage. Larval attack in N. attenuata generates similar increases and decreases in gene expression and protein modification of Calvin cycle components, but LHC proteins decrease (Halitschke and Baldwin, 2003; Giri et al., 2006). Our data indicated a reduced capacity in the light-harvesting reactions of photochemistry, specifically when defence signalling is intact. Given that this effect occurred immediately after attack and was not sustained, it is possible that reduced synthesis of key photosynthetic proteins occurred simultaneously with attenuation of degradation pathways in an effort to optimize resource allocation towards defence without sacrificing vital components of photosynthesis.
Insect herbivory can reduce photosynthesis in remaining tissue (Zangerl et al., 2002; Nabity et al., 2009). We observed reduced electron transport efficiency in more remaining leaf tissue of wild-type than asLOX plants, but small reductions around chewed leaf edges in asLOX plants immediately after insect attack were evident. This result is consistent with previous data for N. attenuata (Halitschke et al., 2011) and surveys of chewing herbivore damage in other species (Aldea et al., 2006; Barron-Gafford et al., 2012; Nabity et al., 2012). Given that damage was propagated in both genotypes, feeding interrupted electron transport by physically disrupting tissues leading to desiccation, or by introducing electron scavengers through insect oral secretions or lysed cells (e.g. glucose oxidase, reactive oxygen species). Previously, 30% leaf area consumption was observed to reduce F q’/F m’ averaged across the remaining leaf area (Halitschke et al., 2011). We did not resolve this suppression at the leaf level when 7% leaf area was consumed and saw minimal suppression in chlorophyll fluorescence restricted to cut edges when plants were allowed to recover. Our data suggest that the greater reductions in F v/F m or F q’/F m’ were linked to defence but that the act of wounding produced transient effects (as observed by Halitschke et al., 2011) that immediately yet minimally contributed to downstream reductions in CO2 assimilation.
The manner of feeding influences the degree of propagated damage; younger larvae produce more cut edges per bite relative to older larvae, significantly compromising the function of remaining tissue (Tang et al., 2006). After 1 d of exposure to plants, neonates fed minimally (1.4% leaf area), yet doubled the propagated damage (2.9%) in wild-type plants relative to 3 d attack (by continuously growing neonates that developed into first instars) where leaf area was reduced by 6.3% but only suppressed 8.1% of the remaining tissue. Taken together, these results indicated that timeliness and the manner of the feeding damage interact to determine the degree of propagated damage in N. attenuata.
Gas exchange declined immediately after feeding in wild-type plants but not in asLOX plants. While this finding agrees, in part, with the results of Halitschke et al. (2011), we also observed stomatal limitations to CO2 assimilation and continued suppression in wild-type plants for days after the initial attack. With the onset of herbivory, both g s and C i decreased, indicating that stomata limited CO2 assimilation. Defoliation damage reduces g s in other species, often through desiccation and loss in turgor associated with severed vasculature (Sack and Holbrook, 2006). We also observed that electron transport decreased minimally in asLOX plants and transiently in both genotypes, indicating that the contribution of reduced electron transport to prolonged reductions in gas exchange was small. To this end, the sustained suppression in photosynthesis did not occur with altered C i, suggesting that neither electron transport nor stomatal limitations drove the long-term reduction in CO2 assimilation. Halitschke et al. (2011) also documented no decrease in g s or change in C i that, assuming transient reductions in F q’/F m’, links other defence-related components to suppressed photosynthesis.
Our 1 d feeding experiment showed that a combination of reduced electron transport and stomatal closure transiently reduced photosynthesis, but given 3 d feeding and full defence metabolite synthesis, photosynthetic suppression was sustained through time. We observed increased levels of JA, TPIs, and nicotine concurrent with reductions in gas exchange after 3 d of feeding. Although all defence compounds were positively correlated with the degree of propagated damage, only nicotine showed consistent negative correlations with gas exchange (Table 2). Exogenous nicotine application suppresses photosynthesis in solanaceous plants (Baldwin and Callahan, 1993), so it is possible that autotoxicity interfered with carbon uptake by closing stomata, a mechanism that warrants further attention. TPI synthesis also exacts fitness costs (Zavala et al., 2004), and these may be modulated through photosynthesis; however, reported reductions in Rubisco and other Calvin cycle enzymes may be stronger drivers for decreased CO2 assimilation (Giri et al., 2006).
Acknowledgements
We thank I. Baldwin of the Max Planck Institute for Chemical Ecology in Jena, Germany, for providing N. attenuata genotypes. We also thank J. Nardi of the University of Illinois at Urbana-Champaign for a continuous supply of M. sexta eggs, D. Bilgin for technical support in operating the HPLC-MS, and members of the DeLucia laboratory for editorial remarks on earlier drafts of this manuscript.
Glossary
Abbreviations:
- ANOVA
analysis of variance
- df
degrees of freedom
- gs
stomatal conductance
- HPLC-MS
high-performance liquid chromatography mass spectrometry
- JA
jasmonic acid
- LHC
light-harvesting complex
- SA
salicylic acid
- TPI
trypsin protease inhibitor.
References
- Aldea M, Hamilton JG, Resti JP, Zangerl AR, Berenbaum MR, DeLucia EH. 2005. Indirect effects of insect herbivory on leaf gas exchange in soybean. Plant, Cell and Environment 28, 402–411 [Google Scholar]
- Aldea M, Hamilton JG, Resti JP, Zangerl AR, Berenbaum MR, Frank TD, DeLucia EH. 2006. Comparison of photosynthetic damage from arthropod herbivory and pathogen infection in understory hardwood samplings. Oecologia 149, 221–232 [DOI] [PubMed] [Google Scholar]
- Baker NR. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo . Annual Review of Plant Biology 59, 89–113 [DOI] [PubMed] [Google Scholar]
- Baker NR, Harbinson J, Kramer DM. 2007. Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant, Cell & Environment 30, 1107–1125 [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Callahan P. 1993. Autotoxicity and chemical defense: nicotine accumulation and carbon gain in solanaceous plants. Oecologia 94, 534–541 [DOI] [PubMed] [Google Scholar]
- Barron-Gafford GA, Rasher U, Bronstein JL, Davidowitz G, Chaszar B, Huxman TE. 2012. Herbivory of wild Manduca sexta causes fast down-regulation of photosynthetic efficiency in Datura wrightii: an early signaling cascade visualized by chlorophyll fluorescence. Photosynthesis Research 113, 249–260 [DOI] [PubMed] [Google Scholar]
- Berger S, Benediktyova Z, Matous K, Bonfig K, Mueller MJ, Nedbal L, Roitsch T. 2007. Visualization of dynamics of plant–pathogen interaction by novel combination of chlorophyll fluorescence imaging and statistical analysis: differential effects of virulent and avirulent strains of P. syringae and of oxylipins on A. thaliana . Journal of Experimental Botany 58, 797–806 [DOI] [PubMed] [Google Scholar]
- Bilgin DD, Zavala JA, Zhu J, Clough SJ, Ort DR, DeLucia EH. 2010. Biotic stress globally down-regulates photosynthesis genes. Plant, Cell & Environment 33, 1597–1613 [DOI] [PubMed] [Google Scholar]
- Chen Y, Pang Q, Dai S, Wang Y, Chen S, Yan X. 2011. Proteomic identification of differentially expressed proteins in Arabidopsis in response to methyl jasmonate. Journal of Plant Physiology 168, 995–1008 [DOI] [PubMed] [Google Scholar]
- Cipollini D. 2007. Consequences of the overproduction of methyl jasmonate on seed production, tolerance to defoliation, and competitive effect and response of Arabidopsis thaliana . New Phytologist 173, 146–153 [DOI] [PubMed] [Google Scholar]
- Donovan MP, Nabity PD, DeLucia EH. 2012. Salicylic acid-mediated reductions in yield in Nicotiana attenuata challenged by aphid herbivory. Arthropod–Plant Interactions doi: 10.1007/s11829-012-9220-5 [Google Scholar]
- Frenkel M, Kulheim C, Jankanpaa HJ, Skogstrom O, Dall’Osto L, Agren J, Bassi R, Moritz T, Moen J, Jansson S. 2009. Improper excess light energy dissipation in Arabidopsis results in a metabolic reprogramming. BMC Plant Biology 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Meng C, Zhang X, Xu D, Zhao Y, Wang Y, Lv H, Yang L, Chen L, Ye N. 2012. Differential expression of carotenogenic genes, associated changes on astaxanthin production, and photosynthesis features induce by JA in H. pluvialis . PLoS One 7, e42243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfeller A, Dubugnon L, Liechti R, Farmer EE. 2010. Jasmonate biochemical pathway. Science Signaling 3, cm3. [DOI] [PubMed] [Google Scholar]
- Giri AP, Wunsche H, Mitra S, Zavala SJA, Muck A, Svatos A, Baldwin IT. 2006. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera: Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant’s proteome. Plant Physiology 142, 1621–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gog L, Berenbaum MR, DeLucia EH, Zangerl AR. 2005. Autotoxic effects of essential oils on photosynthesis in parsley, parsnip, and rough lemon. Chemoecology 15, 115–119 [Google Scholar]
- Halitschke R, Baldwin IT. 2003. Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata . The Plant Journal 36, 794–807 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Hamilton JG, Kessler A. 2011. Herbivore-specific elicitation of photosynthesis by mirid bug salivary secretions in the wild tobacco Nicotiana attenuata . New Phytologist 191, 528–535 [DOI] [PubMed] [Google Scholar]
- Heidel AJ, Baldwin IT. 2004. Microarray analysis of salicylic acid- and jasmonic acid-signaling in responses of Nicotiana attenuata to attack by insects from multiple feeding guilds. Plant, Cell & Environment 27, 1362–1373 [Google Scholar]
- Keinanen M, Oldham NJ, Baldwin IT. 2001. Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata . Journal of Agriculture and Food Chemistry 49, 3553–3558 [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Baldwin IT. 2004. Silencing the jasmonate cascade: induced plant defenses and insect populations. Science 305, 665–668 [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Poveda K. 2011. Herbivory-mediated pollinator limitation: negative impacts of induced volatiles on plant–pollinator interactions. Ecology 92, 1769–1780 [DOI] [PubMed] [Google Scholar]
- Kim EH, Kim YS, Park SH, Koo YJ, Choi YD, Chung YY, Lee IJ, Kim JK. 2009. Methyl jasmonate reduces grain yield by mediating stress signals to alter spikelet development in rice. Plant Physiology 149, 1751–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugel T, Lim M, Gase K, Halitschke R, Baldwin IT. 2002. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 12, 177–183 [Google Scholar]
- Longstaff BJ, Kildea T, Runcie JW, Cheshire A, Dennison WC, Hurd C, Kana T, Raven JA, Larkum AW. 2002. An in situ study of photosynthetic oxygen exchange and electron transport rate in the marine macroalga Ulva lactuca (Chlorophyta). Photosynthesis Research 74, 281–293 [DOI] [PubMed] [Google Scholar]
- Maserti BE, Del Carratore R, Della Croce CM, et al. 2010. Comparative analysis of proteome changes induced by the two-spotted spider mite Tetranychus urticae and methyl jasmonate in citrus leaves. Journal of Plant Physiology 168, 392–402 [DOI] [PubMed] [Google Scholar]
- McKey D. 1974. Adaptive patterns in alkaloid physiology. American Naturalist 108, 305–320 [Google Scholar]
- Nabity PD, Zavala JA, DeLucia EH. 2009. Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Annals of Botany 103, 655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabity PD, Hillstrom ML, Lindroth RL, DeLucia EH. 2012. Elevated CO2 interacts with herbivory to alter chlorophyll fluorescence and leaf temperature in Betula papyrifera and Populus tremuloides . Oecologia 169, 905–913 [DOI] [PubMed] [Google Scholar]
- Sack L, Holbrook NM. 2006. Leaf hydraulics. Annual Review of Plant Biology 57, 361–381 [DOI] [PubMed] [Google Scholar]
- Shi Q, Li C, Zhang F. 2006. Nicotine synthesis in Nicotiana tabacum L. induced by mechanical wounding is regulated by auxin. Journal of Experimental Botany 57, 2899–2907 [DOI] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. 2004. Nicotine’s defensive function in nature. PLoS Biology 2, 1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JY, Zielinski RE, Zangerl AR, Crofts AR, Berenbaum MR, DeLucia EH. 2006. The differential effects of herbivory by first and fourth instars of Trichoplusia ni (Lepidoptera: Noctuidae) on photosynthesis in Arabidopsis thaliana . Journal of Experimental Botany 57, 527–536 [DOI] [PubMed] [Google Scholar]
- Tang JY, Zielinski R, Aldea M, DeLucia EH. 2009. Spatial association of photosynthesis and chemical defense in Arabidopsis thaliana following herbivory by Trichoplusia ni . Physiologia Plantarum 137, 115–124 [DOI] [PubMed] [Google Scholar]
- Thivierge K, Prado A, Driscoll BT, Bonneil E, Thibault P, Bede JC. 2010. Caterpillar and salivary specific modification of plant proteins. Journal of Proteome Research 9, 5887–5895 [DOI] [PubMed] [Google Scholar]
- Van Dam NM, Horn M, Mares M, Baldwin IT. 2001. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata . Journal of Chemical Ecology 27, 547–568 [DOI] [PubMed] [Google Scholar]
- Voelckel C, Baldwin IT. 2004. Herbivore-induced plant vaccination. Part II. Array studies reveal the transience of herbivore-specific transcriptional imprints and a distinct imprint from stress combinations. The Plant Journal 38, 650–663 [DOI] [PubMed] [Google Scholar]
- Walling LL. 2000. The myriad plant responses to herbivores. Journal of Plant Growth and Regulation 19, 195–216 [DOI] [PubMed] [Google Scholar]
- Wang L, Halitschke R, Kang J, Berg A, Harnish F, Baldwin IT. 2007. Independently silencing two members of JAR family impairs trypsin protease inhibitors activities but not nicotine accumulations. Planta 226, 159–167 [DOI] [PubMed] [Google Scholar]
- Wang L, Allmann S, Wu J, Baldwin IT. 2008. Comparisons of LIPOXYGENASE3- and JASMONATE RESISTANT4/6-silenced plants reveal that jasmonic acid and jasmonic acid-amino acid conjugates play different roles in herbivore resistance of Nicotiana attenuata . Plant Physiology 146, 904–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter SC. 1989. Arthropod impact on plant gas exchange. In Bernays EA, ed. Insect–plant interactions. CRC Press: Boca Raton, FL, 135–151 [Google Scholar]
- Zangerl AR, Arntz AM, Berenbaum MR. 1997. Physiological price of an induced chemical defense: photosynthesis, respiration, biosynthesis, and growth. Oecologia 109, 433–441 [DOI] [PubMed] [Google Scholar]
- Zangerl AR, Hamilton JG, Miller TJ, Crofts AR, Oxborough K, Berenbaum MR, DeLucia EH. 2002. Impact of folivory on photosynthesis is greater than the sum of its holes. Proceedings of the National Academy of Sciences USA 99, 1088–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Baldwin IT. 2004. Fitness benefits of trypsin proteinase inhibitor expression in Nicotiana attenuata are greater than their costs when plants are attacked. BMC Ecology 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Baldwin IT. 2004. Constitutive and inducible trypsin proteinase inhibitor production incurs large fitness costs in Nicotiana attenuata . Proceedings of the National Academy of Sciences USA 101, 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]