Abstract
A novel genetic approach for the control of virus replication was used for the design of a conditionally replicating human immunodeficiency virus (HIV) variant, HIV-rtTA. HIV-rtTA gene expression and virus replication are strictly dependent on the presence of a non-toxic effector molecule, doxycycline (dox), and thus can be turned on and off at will in a graded and reversible manner. The in vivo replication capacity, pathogenicity and genetic stability of this HIV-rtTA variant were evaluated in a humanized mouse model of haematopoiesis that harbours lymphoid and myeloid components of the human immune system (HIS). Infection of dox-fed BALB Rag/γc HIS (BRG-HIS) mice with HIV-rtTA led to the establishment of a productive infection without CD4+ T-cell depletion. The virus did not show any sign of escape from dox control for up to 10 weeks after the onset of infection. No reversion towards a functional Tat–transactivating responsive (TAR) RNA element axis was observed, confirming the genetic stability of the HIV-rtTA variant in vivo. These results demonstrate the proof of concept that HIV-rtTA replicates efficiently in vivo. HIV-rtTA is a promising tool for fundamental research to study virus–host interactions in vivo in a controlled fashion.
Introduction
We recently developed a conditionally replicating human immunodeficiency virus type 1 (HIV-1) variant, HIV-rtTA, containing a reverse tetracycline transactivator (rtTA). This variant represents a unique viral tool, as replication of this drug-dependent variant can be turned on and off at will by simple addition/withdrawal of doxycycline (dox), a tetracycline analogue (Marzio et al., 2001, 2002; Verhoef et al., 2001). In wild-type HIV-1, transcription of the viral DNA genome is enhanced by the viral Tat protein, which binds to the viral transactivating responsive (TAR) RNA element. In HIV-rtTA, the nef gene has been deleted, and Tat and TAR have been inactivated by mutations and functionally replaced by components of the Escherichia coli-derived tetracycline-inducible (Tet-On) gene expression system (i.e. rtTA- and tetO-binding sites). Such a drug-controllable variant is a powerful tool to address several biological questions (Centlivre et al., 2008; Das et al., 2007). For instance, this virus tool may facilitate mechanistic studies on HIV-1 proviral latency (Jeeninga et al., 2008). It may also allow the study of the role of residual replication under combinatorial drug pressure, and it may help in defining the exact correlates of vaccine protection, as observed for live-attenuated simian immunodeficiency virus strains in the macaque model (Klausner et al., 2003).
We have studied the virus replication characteristics and genetic stability of HIV-rtTA extensively in vitro using various cell-culture systems (Berkhout et al., 2001; Das et al., 2004a, b; Zhou et al., 2006a). These studies resulted in an optimized HIV-rtTA variant that replicates efficiently in PBMCs following dox addition, as well as in human tonsil lymphoid tissue ex vivo (Kiselyeva et al., 2004). However, the in vivo replication properties and pathogenesis of HIV-rtTA still need to be evaluated. To address these questions, we used a humanized mouse model that offers a unique human-specific experimental set-up. BALB Rag/γc human immune system (BRG-HIS) mice, which harbour components of the HIS, are generated by injecting human haematopoietic progenitor cells (hHPCs) into immunodeficient [BALB/c RAG2−/− interleukin (IL)-2Rγc−/−] newborn mice (Gimeno et al., 2004; Legrand et al., 2011; Traggiai et al., 2004). All major subsets of the human innate and adaptive immune system are found in the reconstituted BRG-HIS mice, including T- and B-cells, conventional and plasmacytoid dendritic cells (cDCs and pDCs), macrophages and natural killer cells. Importantly, productive infection of HIV-1 (both X4- and R5-tropic strains) in BRG-HIS mice has been demonstrated, with infection parameters being reminiscent of those observed in humans (An et al., 2007; Baenziger et al., 2006; Berges et al., 2006; Gorantla et al., 2007; Zhang et al., 2007). The BRG-HIS mouse model therefore represents an attractive tool to analyse HIV-1 pathogenesis and to test the potential of new antiviral compounds in an in vivo setting.
In this study, we evaluated the in vivo replicative capacity of the conditionally replicating HIV-rtTA variant in BRG-HIS mice. Infection of BRG-HIS mice with HIV-rtTA in the presence of dox led to the establishment of a productive infection without inducing human CD4+ T-cell depletion. During the 10 weeks of infection follow-up, the virus did not show signs of escape from dox control. Overall, HIV-rtTA is a promising tool to study in vivo virus–host interactions in a controlled fashion.
Results
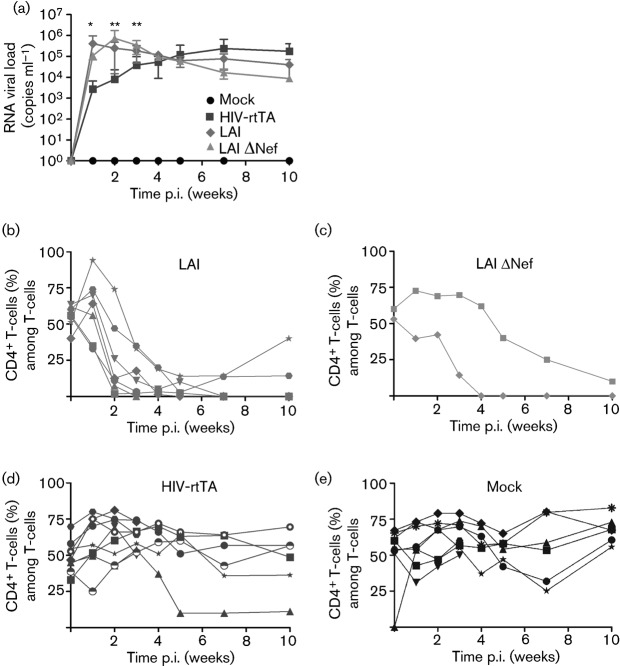
HIV-rtTA does not induce CD4+ T-cell depletion in blood, despite active replication in the presence of dox
BRG-HIS mice (12–15 weeks old) were infected intraperitoneally with 5×104 TCID50 of the HIV-rtTA variant (n = 9), parental HIV-1 LAI strain (n = 7) or HIV-1 LAI-ΔNef strain (n = 2) (Fig. 1), or were mock infected (n = 8). To ensure optimal virus replication on the day of virus injection, a dox diet (2 g kg−1) was initiated 3 days prior to infection (Centlivre et al., 2010; Kleibeuker et al., 2009). All animals were treated equally with a dox diet. We monitored plasma RNA viral load as well as the frequency of human CD4+ and CD8+ T-cells in the blood of the animals throughout the time course of the experiment (10 weeks; Fig. 2).
Fig. 1.
Schematic of the HIV-rtTA genome compared with the parental HIV-1 LAI virus and HIV-1 LAI-ΔNef variant. (a) Schematic of the HIV-1 LAI and HIV-1 LAI-ΔNef proviral DNA genomes. The out-of-frame, 106 bp deletion in the nef gene of the HIV-1 LAI-ΔNef variant is indicated. (b) Schematic of the HIV-rtTA proviral DNA genome. In HIV-rtTA, the Tat–TAR axis of transcription regulation has been inactivated by mutations in both Tat (Y26A) and TAR (five point mutations). Transcription and replication of the virus was made dox dependent by the introduction of tetO elements in the U3 promoter region and by replacing the nef gene with the rtTA gene.
Fig. 2.
HIV-rtTA does not induce CD4+ T-cell depletion in blood, despite active replication in the presence of dox. (a) BRG-HIS mice were bled before and regularly after HIV-1 infection. Virus replication was determined by quantification of viral RNA in the plasma of the animals and is expressed as means±sd. Significant differences in plasma RNA viral load between HIV-1 LAI- and HIV-rtTA-infected BRG-HIS mice are indicated (*P<0.05; **P<0.01). (b–e) PBMCs were stained for human T-cell markers and analysed by flow cytometry. The frequency of human CD4+ T-cells (among CD3+ T-cells) was determined for mice infected with HIV-1 LAI (b), HIV-1 LAI-ΔNef (c), HIV-rtTA (d) and the mock-infected BRG-HIS mice (e). Each symbol corresponds to an individual animal. The results were pooled from two independent experiments.
In the HIV-1 LAI-infected BRG-HIS mice, a rapid increase in plasma RNA viral load was observed, peaking at 1 week post-infection (p.i.) at ~106 copies ml−1 (Fig. 2a). In parallel with the RNA viral load increase, we observed a dramatic reduction in the percentage of blood human CD4+ T-cells as early as 2 weeks p.i. (Fig. 2b), concomitant with an increase in the frequency of human CD8+ T-cells (Fig. S1a, available in JGV Online). In the HIV-1 LAI-ΔNef-infected BRG-HIS mice, plasma RNA viral load increased to a peak as high as that observed for the parental HIV-1 LAI but with a 1–2-week delay. The percentages of human CD4+ T-cells in blood were severely reduced, whereas those of human CD8+ T-cells were increased in these HIV-1 LAI-ΔNef-infected animals but also with 1–2-week delay compared with the HIV-1 LAI-infected group (Fig. 2c and Fig. S1b).
In the HIV-rtTA group, the increase in plasma RNA viral load was delayed compared with the parental HIV-1 LAI group and reached a plateau at 5–7 weeks p.i. at ~105 copies ml−1 (Fig. 2a). Furthermore the frequency of human CD4+ and CD8+ T-cells remained stable in the blood of the HIV-rtTA group (Fig. 2d and Fig. S1c), similar to what was observed in mock-infected animals (Fig. 2e and Fig. S1d), even when the HIV-rtTA viraemia reached a plateau of ~105 copies ml−1. One HIV-rtTA-infected BRG-HIS mouse exhibited a reduced CD4+ T-cell percentage at 5 weeks p.i., but this animal also exhibited a severe reduction in the frequency of human haematopoietic (CD45+) cells. This reduction was already apparent at the time of virus injection and probably reflects an HIV-independent phenomenon. Indeed, the stability of the human xenograft in BRG-HIS mice decreases over time, especially when the animals reach 4–6 months of age (Lepus et al., 2009; Strowig et al., 2011; Traggiai et al., 2004). Overall, we concluded that a steady accumulation of plasma RNA viral load can be observed in HIV-rtTA-infected BRG-HIS mice without human CD4+ T-cell depletion in the blood.
HIV-rtTA disseminates to lymphoid organs without depleting CD4+ T-cells
We next analysed dissemination of infection to the lymphoid organs of the animals. BRG-HIS mice were sacrificed early and late after infection, at 3 and 10 weeks p.i., respectively. We harvested the bone marrow, thymus, spleen and liver of the animals and analysed their content for various human haematopoietic cell populations, in particular human CD4+ T-cells. We observed a profound reduction in human CD4+ T-cell numbers in the thymus, spleen, liver and, to a lesser extent, bone marrow of the HIV-1 LAI-infected BRG-HIS mice at 3 weeks p.i. compared with the mock-infected group (Table 1). Human CD4+ T-cell depletion persisted at 10 weeks p.i., with a more pronounced CD4+ T-cell reduction in the bone marrow (Table 2).
Table 1. Human CD4+ T-cell numbers at 3 weeks p.i. in the organs of mock-, HIV-1 LAI- and HIV-rtTA-infected BRG-HIS mice.
The total number of human CD4+ T-cells (×103) is indicated for each organ analysed (mean±sd).
| Organ | Mock (n = 2) | HIV-1 LAI (n = 2) | HIV-rtTA (n = 3) |
| Bone marrow | 167±50.2 | 124±170 | 605±522 |
| Thymus | 123±29.1 | 0.83±0.91 | 171±25.3 |
| Spleen | 717±117 | 0.09±0.12 | 2120±1060 |
| Liver | 121±147 | 0.64±0.45 | 201±243 |
Table 2. Human CD4+ T-cell numbers at 10 weeks p.i. in the organs of mock-, HIV-1 LAI- and HIV-rtTA-infected BRG-HIS mice.
The total number of human CD4+ T-cells (×103) is indicated for each organ analysed (mean±sd).
| Organ | Mock (n = 6) | HIV-1 LAI (n = 5) | HIV-rtTA (n = 6) |
| Bone marrow | 2260±3780 | 4.60±8.62* | 460±637 |
| Thymus | 36.9±15 | 1.10±0.95* | 44.6±49.9 |
| Spleen | 284±459 | 0.37±0.64* | 964±1640 |
| Liver | 16.9±16.9 | 0.05±0.06 | 32.7±31.3 |
Significantly different from mock-infected cells at P<0.05.
Similar to what we observed in the blood, HIV-rtTA-infected BRG-HIS mice did not exhibit human CD4+ T-cell depletion in the analysed organs at 3 and 10 weeks p.i. (Tables 1 and 2). Depletion of other human haematopoietic cell subsets was also not observed in the HIV-rtTA group at 10 weeks p.i. (Tables S1–S4). Interestingly, the thymus was significantly affected by HIV-1 LAI infection with a reduction in total human CD45+ cells and human CD8+ T-cells compared with the mock-infected group (P<0.01 and P<0.05, respectively; Table S2). The human pDC subset was also significantly depleted in the spleen of the HIV-1 LAI-infected BRG-HIS mice (P<0.05; Table S3), in contrast to previous data obtained at 2 weeks p.i. with a dual-tropic strain (Zhang et al., 2011). The numbers of human CD4+ and CD8+ T-cells in the spleen of HIV-rtTA-infected animals showed a trend towards an increase, but this was not statistically significant compared with the mock-infected control group at 10 weeks p.i. (Tables 2 and S3).
To look for productive viral infection of human CD4+ T-cells in the HIV-rtTA group, we performed intracellular staining with a mAb against the viral capsid p24 protein (CA-p24) on the splenocytes of BRG-HIS mice. We detected a significant fraction of human CA-p24+ cells in the spleen of HIV-rtTA-infected BRG-HIS mice (Fig. 3a), which were mostly (>95 %) human CD4+ T-cells. The percentage of human CA-p24+ cells increased at least tenfold from 3 to 10 weeks p.i. (Fig. 3b), in accordance with the higher RNA viral load measured in the plasma of the animals. Of note, one HIV-rtTA-infected BRG-HIS mouse did not exhibit CA-p24+ splenocytes at 10 weeks p.i. This animal had no detectable RNA viral load at this time, but viral DNA was detected in the splenocytes.
Fig. 3.
Viral capsid protein is expressed in human T-cells from the spleen of HIV-rtTA-infected BRG-HIS mice. Splenocytes of mock- and HIV-rtTA-infected BRG-HIS mice were isolated, stained and analysed by fluorescence-activated cell sorting (FACS). (a) The flow cytometry dot plots show the percentage of human T-cells expressing the viral CA-p24 protein. The values in the upper right quadrant indicate the percentage of human CA-p24+ CD4+ T-cells. (b) The percentage of human CA-p24+ T-cells from the spleen of mock- and HIV-rtTA-infected BRG-HIS mice are presented at 3 weeks p.i. (upper graph) and 10 weeks p.i. (lower graph). Each dot represents one animal. Horizontal bars represent the mean values. *Significantly different at P<0.05.
To confirm dissemination of HIV-rtTA infection in the lymphoid organs and production of new virions by the infected human CD4+ T-cells, we co-cultured BRG-HIS mice splenocytes ex vivo with activated human PBMCs harvested from healthy individuals in the presence of dox. We observed a steady accumulation of CA-p24 in the supernatant of the splenocyte co-cultures of HIV-1 LAI- and HIV-rtTA-infected animals in samples at both 3 and 10 weeks p.i. (Fig. 4a, b). These results demonstrated that HIV-rtTA integrated as provirus in the genome of human CD4+ T-cells from the spleen was able to productively infect new human cells and initiate a spreading infection. Consistent with the more aggressive profile of HIV-1 LAI, co-cultures of splenocytes from HIV-1 LAI-infected animals produced higher CA-p24 levels than those from HIV-rtTA-infected BRG-HIS mice. Co-cultures performed with the splenocytes of all HIV-1 LAI- and HIV-rtTA-infected animals exhibited detectable and increasing CA-p24 levels. When co-cultures were performed with cells isolated from the bone marrow of HIV-1 LAI- and HIV-rtTA-infected animals, we also observed virus replication (data not shown). Moreover, we performed these splenocyte co-cultures with and without dox for the animals sacrificed at 10 weeks p.i., and HIV-rtTA replication was observed exclusively in the presence of dox (Fig. 4c), demonstrating that the HIV-rtTA variant had remained strictly dox dependent during the 10-week period of in vivo replication. Replication of HIV-1 LAI in the splenocyte co-cultures was not affected by the presence of dox (data not shown).
Fig. 4.

HIV-rtTA-infected human splenocytes isolated from HIV-rtTA-infected BRG-HIS mice can propagate and productively infect new human PBMCs in vitro in a dox-dependent manner. (a, b) Splenocytes of mock-, HIV-1 LAI- and HIV-rtTA-infected BRG-HIS mice obtained at 3 weeks p.i. (a) and 10 weeks p.i. (b) were co-cultured with activated human PBMCs in the presence of 1000 ng ml−1 dox. (c) Splenocytes of HIV-rtTA-infected BRG-HIS mice obtained at 10 weeks p.i. were co-cultured with activated human PBMCs with or without 1000 ng dox ml−1. Virus replication was monitored during the 14 days of co-culture by determination of the CA-p24 levels in the co-culture supernatants.
The absence of CD4+ T-cell depletion with HIV-rtTA does not correlate with the presence of an anti-HIV-1 B-cell immune response
T-cell responses against pathogens (including HIV antigens) have not been reported in BRG-HIS mice (Baenziger et al., 2006), whereas specific antibody responses to tetanus toxoid, hepatitis B surface antigen and ovalbumin can be generated in these animals following repeated vaccination (Becker et al., 2010; Huntington et al., 2011; Strowig et al., 2011; Traggiai et al., 2004). However, previous studies have shown that when BRG-HIS mice are infected by HIV-1, only one of 25 mice was able to mount a detectable HIV-1-specific IgG response (Baenziger et al., 2006). We wondered whether we would be able to detect an antibody immune response in HIV-rtTA-infected BRG-HIS mice because of the absence of human CD4+ T-cell depletion. To detect the presence of HIV-specific Igs (IgM, IgG and IgA), we performed an ELISA on plasma of the three groups of BRG-HIS mice (mock, HIV-1 LAI and HIV-rtTA) at 10 weeks p.i. No HIV-1-specific Igs were detected in the HIV-1-infected BRG-HIS mice (HIV-1 LAI and HIV-rtTA) (Fig. 5). This result was confirmed by a Western blot assay, which did not detect specific Igs against HIV-1 protein in the three groups of BRG-HIS mice (Fig. S2).
Fig. 5.

HIV-rtTA infection does not trigger the production of HIV-1-specific antibodies. Plasma from mock-, HIV-1 LAI- and HIV-rtTA-infected BRG-HIS mice at 10 weeks p.i. was tested for HIV-1-specific antibodies by ELISA. The negative (−) and positive (+) bars correspond to negative and positive human serum controls provided in the Genscreen HIV-1/HIV-2 kit, respectively. Results are presented as means±sd.
In vivo evolution of HIV-rtTA
Next, we monitored the in vivo evolution of HIV-rtTA. By sequencing three regions of the viral genome [long-terminal repeat (LTR) with the TAR motif, tat and rtTA genes], we screened for mutations potentially leading to reduced dox dependency or restoration of the Tat–TAR axis. Viral RNA sequences and proviral DNA sequences were amplified respectively from plasma and splenocyte samples at 10 weeks p.i., and eight to ten TA clones (see Methods) were sequenced per animal. From the plasma of HIV-1 LAI-infected BRG-HIS mice, we were only successful in amplifying the Tat region. In two HIV-rtTA-infected BRG-HIS mice, we observed a single clone with a G→A hypermutated sequence, probably due to the action of the enzyme apolipoprotein B mRNA-editing, enzyme-catalytic, polypeptide-like 3G (APOBEC3G; Sato et al., 2010).
We observed several point mutations across the LTR region in sequences from plasma and splenocyte samples of HIV-rtTA-infected BRG-HIS mice at 10 weeks p.i. (between zero and seven point mutations per plasma sequence, and zero and four per splenocyte sample). However, none of the acquired mutations was present in both plasma and splenocyte samples from the same animal, which argues against outgrowth of these variants. Furthermore, no specific common mutation was found across the different mice analysed. Some of these LTR point mutations depicted in Fig. S3 have previously been observed in long-term in vitro replication cultures and have no impact on the dox-dependent phenotype (unpublished data). We also did not observe point mutations in the TAR region that would restore the binding capacity of Tat (Fig. S4). We did observe a correlation between the number of mutations acquired in the LTR region of plasma virus and the RNA viral load: the higher the RNA viral load, the more mutations had accumulated.
We sequenced the rtTA gene in plasma and splenocyte samples at 10 weeks p.i. and found several point mutations, but none of these changes has been associated with loss of dox control (Zhou et al., 2006a, c). This is in accordance with the co-culture results, where no HIV-rtTA replication was scored in the absence of dox. As in the LTR analysis, we did not observe any dominant rtTA mutations in multiple clones of the plasma and splenocyte samples of each HIV-rtTA-infected BRG-HIS mouse, nor across the different HIV-rtTA-infected BRG-HIS mice.
We sequenced the tat gene and, in four of the six HIV-rtTA-infected BRG-HIS mice at 10 weeks p.i., we observed several clones with the same G→A mutation at nt 182 in this gene. This point mutation leads to an amino acid substitution in both Tat (G61D) and Rev (A15T) located, respectively, in the C-terminal Tat domain and the Rev nuclear inhibitory sequence. This point mutation was observed in the plasma and splenocyte samples of HIV-rtTA-infected BRG-HIS mice, and was also observed in a single plasma clone of a single HIV-1 LAI-infected mouse (Table 3). It should be noted that this point mutation was observed in animals from two independent experiments. No other specific mutations were detected in the HIV-rtTA- or HIV-1 LAI- infected BRG-HIS mice. In the original HIV-rtTA variant, Tat encodes the Y26A substitution, which abolishes the activity of Tat, but no reversion at this position was observed in HIV-rtTA-infected BRG-HIS mice.
Table 3. Frequency of clones bearing the G→A mutation at nt 182 in the Tat sequence from plasma and splenocyte samples of HIV-1 LAI- and HIV-rtTA-infected BRG-HIS mice.
nd, Not determined: the plasma RNA viral load was undetectable for mouse 9004-1 and no spleen cells were collected for mouse 9004-2.
| Mouse no. | Virus | Plasma | Splenocytes |
| 8106-6 | HIV-rtTA | 3/8 | 4/18 |
| 8106-8 | HIV-rtTA | 1/8 | 1/16 |
| 9004-1 | HIV-rtTA | nd | 7/8 |
| 9004-2 | HIV-1 LAI | 1/7 | nd |
| 9004-6 | HIV-rtTA | 2/8 | 2/8 |
Discussion
In this study, we analysed the in vivo replicative capacity of the conditionally replicating HIV-rtTA variant. Because the major cellular targets of HIV replication are found among human haematopoietic cell subsets, the BRG-HIS mouse model is well suited to evaluate the in vivo replication capacity and genetic stability of HIV-rtTA. Overall, we found that HIV-rtTA was able to replicate in vivo in the presence of dox, as accumulating plasma RNA viral load was detected throughout the 10 weeks of infection follow-up. Moreover, the virus disseminated to lymphoid organs of the infected animals, with a remarkable number of detectable CA-p24+ human CD4+ T-cells (around 4 % at 10 weeks p.i.). Nevertheless, infection with HIV-rtTA induces a pathogenicity profile that is much less severe than that of the parental X4-tropic HIV-1 LAI strain, as replication of HIV-rtTA took place without human CD4+ T-cell depletion in blood and lymphoid tissues.
Although HIV-rtTA infection of BRG-HIS mice resulted in a plasma RNA viral load plateau similar to that obtained for HIV-1 LAI and HIV-1 LAI-ΔNef at 4–6 weeks p.i., it is not clear why HIV-rtTA did not induce CD4+ T-cell depletion, despite a high frequency of infected (CA-p24+) cells. One could speculate that limited cytopathogenicity might be linked to the reduced replication capacity of HIV-rtTA, which was demonstrated previously in vitro (Berkhout et al., 2001; Das et al., 2004a, b; Zhou et al., 2006a) and which is also apparent from the delayed kinetics of viral plasma RNA accumulation in vivo. This reduced replication capacity may result in a slower rate of human CD4+ T-cell elimination, either via direct (virus-induced) or indirect (cytotoxic lymphocyte-mediated) cell killing, and therefore allows constant repopulation of the human CD4+ T-cell compartment of BRG-HIS mice in a steady-state fashion. It also cannot be excluded that reduced virus replication kinetics contributes to the establishment of a cellular immune response that is able to control the virus – at least partially – as suggested by the trend towards enhanced numbers of human CD4+ and CD8+ T-cells that we observed in the spleen of HIV-rtTA-infected BRG-HIS mice. Considering the rare occurrence of human T-cell responses in BRG-HIS mice studies reported so far, as well as the lack of a B-cell response against HIV-rtTA, the latter possibility remains less likely.
It should be noted that HIV-rtTA is a Nef-deficient virus, as the rtTA gene was inserted in place of nef in the viral genome. This may explain the non-pathogenic phenotype of HIV-rtTA. However, when infections of BRG-HIS mice were performed with an HIV-1 LAI ΔNef virus (bearing an out-of-frame 106 bp deletion), pathogenesis was close to that of the parental X4-tropic HIV-1 LAI strain, ruling out a major Nef effect. Mechanisms other than Nef deletion should thus be responsible for the attenuation of HIV-rtTA pathogenesis. One possibility is that the numerous modifications introduced in the HIV-rtTA genome (tetO-binding sites/rtTA; mutations of Tat/TAR) hamper viral gene expression in specific cellular subsets. For instance, HIV-rtTA gene expression will depend on cellular uptake of dox and a specific pool of transcription factors, which may in fact differ for HIV-1 versus HIV-rtTA. In addition, the viral gene expression rate may be altered because HIV-rtTA mRNAs are less stable than the wild-type HIV-1 mRNAs. Such alterations in gene expression pattern were observed in BRG-HIS mice for a lentiviral vector in which GFP expression was dox dependent (Centlivre et al., 2010): the frequency of GFP-expressing cells was higher in B-cells and conventional DC subsets compared with T-cells and pDCs cells, whereas the GFP mean fluorescence intensity was lower in GFP+ B-cells and T-cells than in the pCDs and cDCs. It is unlikely that a limiting dox level in the BRG-HIS mice was restricting HIV-rtTA replication, as the in vivo dox levels measured in the plasma of the BRG-HIS mice (>4000 ng dox ml−1; Centlivre et al., 2010 and data not shown) were superior to those required for optimal replication in vitro of this highly active and dox-sensitive HIV-rtTA-V14 variant (10–100 ng dox ml−1; Zhou et al., 2006b). Furthermore, when a dox-inducible lentiviral vector was tested in the BRG-HIS mice, we observed reliable gene expression in response to dox in the blood and in diverse organs (bone marrow, thymus, spleen and liver) (Centlivre et al., 2010), supporting the notion that dox diffusion is not a limiting factor in this setting.
We investigated the genetic stability of HIV-rtTA in vivo. Interestingly, we did not observe mutations in the rtTA gene that may reduce dox control, which was confirmed by the absence of detectable CA-p24 in the supernatant of splenocyte co-cultures in the absence of dox. The introduced Tet-On axis (i.e. the rtTA- and tetO-binding sites) did not show fixation of any specific mutation in the 10-week time frame. However, a G→A mutation at nt 182 of tat was observed in four of the six HIV-rtTA-infected BRG-HIS mice. This mutation is not silent and affects the encoded Tat and Rev proteins by amino acid substitutions G61D and A15T, respectively. For Rev, the observation that HIV isolates exhibit natural variation at this position, with both A15 and T15 frequently observed, suggests that this mutation does not affect Rev function (Kubota & Pomerantz, 1998; HIV sequence compendium/Los Alamos database: http://www.hiv.lanl.gov/). For Tat, the G61D amino acid change has been observed previously in the ACH2 model for HIV latency (Emiliani et al., 1996). However, HIVACH2 Tat is fully functional and exhibits a similar transactivation activity to HIV-1 LAI Tat (Emiliani et al., 1996). Moreover, in isolates from HIV-infected individuals, a certain degree of flexibility is present at aa 61 (Los Alamos database), with D61 frequently observed. Tat D61 has been observed in HIV-infected individuals presenting with various pathogenicity profiles, i.e. long-term non-progressors and progressors (Yamada & Iwamoto, 2000), and in superinfected HIV-1 individuals in both the primary and superinfecting strain (van der Kuyl et al., 2010). All these data suggest that there is no association of the D61 substitution with a change in Tat activity. Alternatively, introduction of the inactivating Y26A mutation in Tat of HIV-rtTA may have affected other Tat functions needed by the virus for efficient virus replication and dissemination, and G61D may represent a compensatory mutation. Another interesting possibility is that this Tat/Rev substitution results from virus evolution in a new in vivo context, e.g. BRG-HIS mice. Even though the innate and adaptive immune responses are suboptimal in BRG-HIS mice, several pieces of evidence indicate that some immune responses can occur in humanized mice (Denton & García, 2011; Gorantla et al., 2010; Jiang et al., 2008). In particular, virus evolution driven by immune responses has been observed in the Env protein, with some features recapitulating virus evolution observed in humans (Ince et al., 2010). Consequently, the G61D mutation could be driven by immune pressure on Tat and/or Rev epitopes. As this mutation was also observed in a single clone of a single HIV-1 LAI-infected BRG-HIS mouse, it is likely that the immune pressure imposed on Tat/Rev is a general phenomenon and is not restricted to the HIV-rtTA virus, e.g. influenced by the presence of the Y26A mutation or the exogenous rtTA gene, although this cannot be excluded.
B-cell responses are suboptimal in BRG-HIS mice (Becker et al., 2010; Traggiai et al., 2004; Vuyyuru et al., 2011), although some specific Igs can be observed and isolated (Becker et al., 2010; Gorantla et al., 2007; Traggiai et al., 2004). In the case of HIV infection, HIV-specific Igs were detected in only one of 25 mice at 6 weeks p.i. by Baenziger et al. (2006), in none of 17 mice by Gorantla et al. (2007) and in none of 16 mice by Berges & Rowan (2011). Depletion of the helper CD4+ T-cells may provide an explanation for this inefficient generation of an HIV-specific antibody response. However, the absence of HIV-specific Igs in HIV-rtTA-infected mice seems to show that the lack of an HIV-specific humoral response is not due to the absence of signalling through helper CD4+ T-cells but rather to an intrinsic suboptimal response in the BRG-HIS mice (Vuyyuru et al., 2011). Alternatively, the general lack of detectable HIV-specific Igs in HIV-rtTA-infected BRG-HIS mice may relate to the low viral load in the spleen. It has been reported previously that a strong antibody response against HIV-1 gp120 correlates with a high viral load in the spleen of HIV-1 strain JRCSF-infected animals (Sango et al., 2010). This suboptimal generation of an antibody response and, specifically, anti-HIV antibodies in BRG-HIS mice, may be overcome by the development of new optimized mouse models for HIV infection studies. For example, optimization of human cell reconstitution was demonstrated after restoration of a functional CD47/SIRPα interaction, either by genetic engineering of the hHPCs prior to injection in the animals or by generating new genetically modified BALB/c RAG2−/− IL-2Rγc−/− mouse strains (Legrand et al., 2011; Strowig et al., 2011). In these models, the number of engrafted human B-cells and the total plasma IgM and IgG concentrations are increased. Supplementation or replacement of several mouse cytokine genes, such as IL-7, IL-15, TPO and MCSF/IL-3, by their human equivalents might also have beneficial effects on the functional properties of the human immune cells generated in the HIS mouse models (Chen et al., 2009; Huntington et al., 2011; O’Connell et al., 2010; Rongvaux et al., 2011; van Lent et al., 2009; Willinger et al., 2011). Overall, such new optimized humanized mouse models will be of interest to study HIV-rtTA replication properties and genetic stability over time, especially in settings where animals are successively exposed to and subsequently deprived of dox.
We have demonstrated the proof of concept of the ability of HIV-rtTA to replicate in vivo. This viral tool will be useful for fundamental research in vivo to study the impact of virus replication on different aspects of the HIV-1 infection process, such as immune activation or virus latency. Moreover, this drug-dependent virus is a powerful reagent that can provide essential information on the exact correlates of protection against HIV-1, which remain a major challenge in the HIV-1 vaccine field.
Methods
Generation of BRG-HIS mice.
BALB/c (H-2d) RAG2−/− IL-2Rγc−/− mice (Weijer et al., 2002) were bred and maintained in isolators and fed autoclaved food and water. Human fetal liver was obtained from elective abortions, with the gestational age ranging from 14 to 18 weeks. The use of this tissue was approved by the Medical Ethical Committee of the AMC-UvA and was contingent on informed consent. BRG-HIS mice were generated with 5×104–10×104 sorted CD34+ CD38− hHPCs, as described previously (Gimeno et al., 2004; ter Brake et al., 2009; Traggiai et al., 2004; van Lent et al., 2010). All manipulations of BRG-HIS mice were performed under laminar flow. All animal experiments were approved by the Animal Ethical Committee of the AMC-UvA.
Viruses and cell culture.
SupT1 cells (6×106) were infected with 20 ng CA-p24 of HIV-1 LAI or HIV-rtTA-V14 (Zhou et al., 2006b) and cultured for 4–5 days with 1000 ng dox ml−1. Culture supernatants were harvested, filtered (0.45 µm) and frozen at −80 °C. Virus production was determined by CA-p24 ELISA and TCID50 determination. Culture supernatants of uninfected SupT1 cultured in the presence of 1000 ng dox ml−1 were also harvested, filtered and frozen and used as the mock control.
SupT1 cells were grown at 37 °C and 5 % CO2 in RPMI 1640 (Gibco-BRL) supplemented with 10 % FCS, 100 U penicillin ml−1 and 100 µg streptomycin ml−1. Human PBMCs were isolated from healthy individuals, activated with 2 µg phytohaemagglutinin ml−1 for 2 days and maintained with 100 U recombinant IL-2 ml−1 in RPMI 1640 (Gibco-BRL) supplemented with 10 % FCS, 100 U penicillin ml−1 and 100 µg streptomycin ml−1.
HIV-1 infection.
BRG-HIS mice (12–15 weeks old), reconstituted with a percentage superior to 15 % of human CD45+ cells in the blood at 8 weeks post-hHPC injection, were injected intraperitoneally with 0.3 ml HIV-1 LAI (n = 7) or HIV-rtTA (n = 9) at a dose of 5×104 TCID50 or were mock infected (n = 8). Infection was performed in a Biosafety Level 3 laboratory in accordance with the AMC-UvA animal facility guidelines. To allow in vivo HIV-rtTA-V14 production, dox was administered to the BRG-HIS mice in the food diet (2 g kg−1; Bioserv), starting 3 days prior to HIV-1 injection, and the food was refreshed once every 1–2 weeks during the course of the experiment. Two independent experiments were performed and the results were grouped. Taking into account the fact that the stability of the human xenograft in BRG-HIS mice decreases over time, especially when the animals reach 4–6 months of age (Lepus et al., 2009; Strowig et al., 2011; Traggiai et al., 2004), the animals were sacrificed up to 10 weeks after onset of infection to ensure that optimal haematopoietic reconstitution was still obtained at time of sacrifice.
Plasma HIV-1 RNA load and CD4+/CD8+ T-cell frequency.
At each blood sampling of the animals, plasma HIV-1 RNA loads were determined by quantitative RT-PCR, as described previously (Pasternak et al., 2008), and blood cells were labelled with anti-human mAbS targeting the following cell-surface markers: CD45 (clone 2D1), CD3 (clone SK7), CD4 (clone SK3) and CD8 (clone RPA-T8) (all from BD Biosciences), allowing determination of the frequency of blood CD4+ and CD8+ T-cells.
ELISA and Western blotting.
Plasma samples collected from the BRG-HIS mice were analysed for HIV-1-specific human antibodies by ELISA using a Genscreen HIV-1/HIV-2 kit (Bio-Rad Laboratories) and by Western blotting with an HIV Blot 2.2 (MP Diagnostics) according to the manufacturer’s instructions.
Flow cytometry analysis for cell-surface markers.
Cell suspensions of the BRG-HIS mouse organs were prepared in RPMI 1640 supplemented with 2 % FCS. The stained cells were fixed with 1 % paraformaldehyde for at least 30 min prior to FACS analysis. Cell suspensions were labelled with anti-human mAb targeting the following cell-surface markers: CD45 (clone 2D1), CD3 (clone SK7), CD4 (clone SK3), CD8 (clone RPA-T8), CD19 (clone SJ25C1), HLA-DR (clone G46-6) (all from BD Biosciences) and BDCA2 (clone AC144; Miltenyi Biotech). All washings and reagent dilutions were performed with PBS containing 2 % FCS and 0.02 % sodium azide. All data acquisitions were performed with an LSR-II (BD Bioscience) flow cytometer interfaced with the FACS-Diva software system.
Co-culture assay.
Splenocytes from the BRG-HIS mice were co-cultured with phytohaemagglutinin-activated human PBMCs isolated from healthy individuals in the presence of IL-2 (100 U ml−1), without or with dox (1000 ng ml−1). Virus replication was monitored in the co-cultures by CA-p24 ELISA.
Virus sequencing.
Splenocytes of the BRG-HIS mice were lysed by incubation with 200 µg proteinase K ml−1 at 56 °C for 1 h, followed by 95 °C for 10 min. Proviral DNA sequences were amplified by conventional nested PCR from total cellular DNA. For the LTR amplification, the primers AD-Gag (5′-ATGGATCCGTTCTAGCTCCCTGCTTGCCC-3′) and U3-Xba-Not (5′-ACGTCTAGAGCGGCCGCACTGGAAGGGCTAATTCACTC-3′) were used for the first PCR, and MO21 (5′-CCCTGGCCTTAACCGAATTTT-3′) and U3-Xba-Not for the nested PCR. For Tat amplification, FGS009 (5′-ACCTTTGCCTAGTGTTACGAAACT-3′) and Tat-intron-1 (5′-CTATGATTACTATGGACCACACA-3′) were used for the first PCR, and FGS009 and Tat-splice-1 (5′-GGGAGGTGGGTTGCTTTGATAGAGAAACTTGATG-3′) for the nested PCR. For rtTA amplification, tTA2 (5′-GTTATAGAAGTAGTACAAGGAGCT-3′) and tTArev1 (5′-GTCAAACCTCCACTCTAACACT-3′) were used for the first PCR, and tTA2 and tTArev2 (5′-GATCAAGGATATCTTGTCTTCGT-3′) for the nested PCR.
Viral RNA was extracted from the plasma of BRG-HIS mice according to the isolation method of Boom et al. (1990). Reverse transcription was performed using either random hexamers as primers or an equimolar mixture of four specific primers [tTArev1, Tat-intron-1, AD-Gag and scani (5′-GAAGCACTCAAGGCAAGCTTTA-3′)] for the genes of interest and SuperScript III (Invitrogen) at 42 °C for 60 min according to the manufacturer’s instructions, after which the reverse transcriptase was heat inactivated for 10 min at 70 °C. The same sets of primers as used for proviral DNA amplification were used for viral cDNA amplification, except for the LTR region, which was amplified with scani and tTA3 (5′-CTGTGTCAGCAAGGCTTCTC-3′) for the first PCR, and scani and tTA4 (5′-ACGCACTGTACGCTCTGTC-3′) for the nested PCR.
Amplified products were purified on gels, extracted with a Bioké Extraction kit and cloned using a TOPO TA cloning kit (Invitrogen). An average of eight to ten colonies were sequenced with a BigDye Terminator cycle sequencing kit (Applied Biosystems) and aligned by CodonCode Aligner.
Statistical analyses.
GraphPad Prism 5.01 was used for statistical analysis. Data were subjected to a Mann–Whitney U test analysis for comparisons of two groups. Multiple groups were subjected to a Kruskall–Wallis test adjusted for multiple comparisons. The obtained P values were considered significant at P<0.05 or P<0.01.
Acknowledgements
This research was sponsored by the Dutch AIDS Foundation (AIDS Fonds, grant 2007025), the NIH/NIAID (R21-AI073136) and the ZonMW Translational Gene Therapy Program. M. C. was supported by Marie Curie Intra-European fellowship (MEIF-CT-2007-039689). N. L. was supported by the Bill and Melinda Gates Foundation (Grand Challenges in Global Health program – GC4 ‘Human Vaccine Consortium’). We thank the staff of the ABSL-3 unit of the Animal Research Institute Amsterdam (ARIA) of the AMC-UvA for excellent care of the animals. We also thank Berend Hooibrink for expertise in cell sorting and maintenance of the flow cytometry facility, Stephan Heynen for assistance with the CA-p24 ELISA, and Petra Blom and Suzanne Jurriaans for assistance with anti-HIV Western blot. Lastly, we are grateful to the Bloemenhove Clinic (Heemstede, The Netherlands) for providing fetal tissues.
Footnotes
Four supplementary figures and four supplementary tables are available with the online version of this paper.
References
- An D. S., Poon B., Fang R. H. T., Weijer K., Blom B., Spits H., Chen I. S. Y., Uittenbogaart C. H. (2007). Use of a novel chimeric mouse model with a functionally active human immune system to study human immunodeficiency virus type 1 infection. Clin Vaccine Immunol 14, 391–396 10.1128/CVI.00403-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger S., Tussiwand R., Schlaepfer E., Mazzucchelli L., Heikenwalder M., Kurrer M. O., Behnke S., Frey J., Oxenius A. & other authors (2006). Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−γ c−/− mice. Proc Natl Acad Sci U S A 103, 15951–15956 10.1073/pnas.0604493103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker P. D., Legrand N., van Geelen C. M., Noerder M., Huntington N. D., Lim A., Yasuda E., Diehl S. A., Scheeren F. A. & other authors (2010). Generation of human antigen-specific monoclonal IgM antibodies using vaccinated “human immune system” mice. PLoS ONE 5, e13137 10.1371/journal.pone.0013137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berges B. K., Rowan M. R. (2011). The utility of the new generation of humanized mice to study HIV-1 infection: transmission, prevention, pathogenesis, and treatment. Retrovirology 8, 65 10.1186/1742-4690-8-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berges B. K., Wheat W. H., Palmer B. E., Connick E., Akkina R. (2006). HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−γc−/− (RAG-hu) mouse model. Retrovirology 3, 76 10.1186/1742-4690-3-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhout B., Marzio G., Verhoef K. (2001). Control over HIV-1 replication by an antibiotic; a novel vaccination strategy with a drug-dependent virus. Virus Res 82, 103–108 10.1016/S0168-1702(01)00399-9 [DOI] [PubMed] [Google Scholar]
- Boom R., Sol C. J., Salimans M. M., Jansen C. L., Wertheim-van Dillen P. M., van der Noordaa J. (1990). Rapid and simple method for purification of nucleic acids. J Clin Microbiol 28, 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centlivre M., Klaver B., Berkhout B., Das A. T. (2008). Functional analysis of the complex trans-activating response element RNA structure in simian immunodeficiency virus. J Virol 82, 9171–9178 10.1128/JVI.00530-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centlivre M., Zhou X., Pouw S. M., Weijer K., Kleibeuker W., Das A. T., Blom B., Seppen J., Berkhout B., Legrand N. (2010). Autoregulatory lentiviral vectors allow multiple cycles of doxycycline-inducible gene expression in human hematopoietic cells in vivo. Gene Ther 17, 14–25 10.1038/gt.2009.109 [DOI] [PubMed] [Google Scholar]
- Chen Q., Khoury M., Chen J. (2009). Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci U S A 106, 21783–21788 10.1073/pnas.0912274106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A. T., Verhoef K., Berkhout B. (2004a). A conditionally replicating virus as a novel approach toward an HIV vaccine. Methods Enzymol 388, 359–379 10.1016/S0076-6879(04)88028-5 [DOI] [PubMed] [Google Scholar]
- Das A. T., Zhou X., Vink M., Klaver B., Verhoef K., Marzio G., Berkhout B. (2004b). Viral evolution as a tool to improve the tetracycline-regulated gene expression system. J Biol Chem 279, 18776–18782 10.1074/jbc.M313895200 [DOI] [PubMed] [Google Scholar]
- Das A. T., Harwig A., Vrolijk M. M., Berkhout B. (2007). The TAR hairpin of human immunodeficiency virus type 1 can be deleted when not required for Tat-mediated activation of transcription. J Virol 81, 7742–7748 10.1128/JVI.00392-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton P. W., García J. V. (2011). Humanized mouse models of HIV infection. AIDS Rev 13, 135–148 [PMC free article] [PubMed] [Google Scholar]
- Emiliani S., Van Lint C., Fischle W., Paras P., Jr, Ott M., Brady J., Verdin E. (1996). A point mutation in the HIV-1 Tat responsive element is associated with postintegration latency. Proc Natl Acad Sci U S A 93, 6377–6381 10.1073/pnas.93.13.6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno R., Weijer K., Voordouw A., Uittenbogaart C. H., Legrand N., Alves N. L., Wijnands E., Blom B., Spits H. (2004). Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− γc−/− mice: functional inactivation of p53 in developing T cells. Blood 104, 3886–3893 10.1182/blood-2004-02-0656 [DOI] [PubMed] [Google Scholar]
- Gorantla S., Sneller H., Walters L., Sharp J. G., Pirruccello S. J., West J. T., Wood C., Dewhurst S., Gendelman H. E., Poluektova L. (2007). Human immunodeficiency virus type 1 pathobiology studied in humanized BALB/c-Rag2−/−γc−/− mice. J Virol 81, 2700–2712 10.1128/JVI.02010-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla S., Makarov E., Finke-Dwyer J., Gebhart C. L., Domm W., Dewhurst S., Gendelman H. E., Poluektova L. Y. (2010). CD8+ cell depletion accelerates HIV-1 immunopathology in humanized mice. J Immunol 184, 7082–7091 10.4049/jimmunol.1000438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington N. D., Alves N. L., Legrand N., Lim A., Strick-Marchand H., Mention J. J., Plet A., Weijer K., Jacques Y. & other authors (2011). IL-15 transpresentation promotes both human T-cell reconstitution and T-cell-dependent antibody responses in vivo. Proc Natl Acad Sci U S A 108, 6217–6222 10.1073/pnas.1019167108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince W. L., Zhang L., Jiang Q., Arrildt K., Su L., Swanstrom R. (2010). Evolution of the HIV-1 env gene in the Rag2−/− γC−/− humanized mouse model. J Virol 84, 2740–2752 10.1128/JVI.02180-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeeninga R. E., Westerhout E. M., van Gerven M. L., Berkhout B. (2008). HIV-1 latency in actively dividing human T cell lines. Retrovirology 5, 37 10.1186/1742-4690-5-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Zhang L., Wang R., Jeffrey J., Washburn M. L., Brouwer D., Barbour S., Kovalev G. I., Unutmaz D., Su L. (2008). FoxP3+CD4+ regulatory T cells play an important role in acute HIV-1 infection in humanized Rag2−/−γC−/− mice in vivo. Blood 112, 2858–2868 10.1182/blood-2008-03-145946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyeva Y., Ito Y., Lima R. G., Grivel J.-C., Das A. T., Berkhout B., Margolis L. B. (2004). Depletion of CD4 T lymphocytes in human lymphoid tissue infected ex vivo with doxycycline-dependent HIV-1. Virology 328, 1–6 10.1016/j.virol.2004.07.014 [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Fauci A. S., Corey L., Nabel G. J., Gayle H., Berkley S., Haynes B. F., Baltimore D., Collins C. & other authors (2003). The need for a global HIV vaccine enterprise. Science 300, 2036–2039 10.1126/science.1086916 [DOI] [PubMed] [Google Scholar]
- Kleibeuker W., Zhou X., Centlivre M., Legrand N., Page M., Almond N., Berkhout B., Das A. T. (2009). A sensitive cell-based assay to measure the doxycycline concentration in biological samples. Hum Gene Ther 20, 524–530 10.1089/hum.2008.182 [DOI] [PubMed] [Google Scholar]
- Kubota S., Pomerantz R. J. (1998). A cis-acting peptide signal in human immunodeficiency virus type I Rev which inhibits nuclear entry of small proteins. Oncogene 16, 1851–1861 10.1038/sj.onc.1201738 [DOI] [PubMed] [Google Scholar]
- Legrand N., Huntington N. D., Nagasawa M., Bakker A. Q., Schotte R., Strick-Marchand H., de Geus S. J., Pouw S. M., Böhne M. & other authors (2011). Functional CD47/signal regulatory protein alpha (SIRPα) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proc Natl Acad Sci U S A 108, 13224–13229 10.1073/pnas.1101398108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepus C. M., Gibson T. F., Gerber S. A., Kawikova I., Szczepanik M., Hossain J., Ablamunits V., Kirkiles-Smith N., Herold K. C. & other authors (2009). Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/γc−/−, Balb/c-Rag1−/−γc−/−, and C.B-17-scid/bg immunodeficient mice. Hum Immunol 70, 790–802 10.1016/j.humimm.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzio G., Verhoef K., Vink M., Berkhout B. (2001). In vitro evolution of a highly replicating, doxycycline-dependent HIV for applications in vaccine studies. Proc Natl Acad Sci U S A 98, 6342–6347 10.1073/pnas.111031498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzio G., Vink M., Verhoef K., de Ronde A., Berkhout B. (2002). Efficient human immunodeficiency virus replication requires a fine-tuned level of transcription. J Virol 76, 3084–3088 10.1128/JVI.76.6.3084-3088.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell R. M., Balazs A. B., Rao D. S., Kivork C., Yang L., Baltimore D. (2010). Lentiviral vector delivery of human interleukin-7 (hIL-7) to human immune system (HIS) mice expands T lymphocyte populations. PLoS ONE 5, e12009 10.1371/journal.pone.0012009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak A. O., Adema K. W., Bakker M., Jurriaans S., Berkhout B., Cornelissen M., Lukashov V. V. (2008). Highly sensitive methods based on seminested real-time reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 unspliced and multiply spliced RNA and proviral DNA. J Clin Microbiol 46, 2206–2211 10.1128/JCM.00055-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A., Willinger T., Takizawa H., Rathinam C., Auerbach W., Murphy A. J., Valenzuela D. M., Yancopoulos G. D., Eynon E. E. & other authors (2011). Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci U S A 108, 2378–2383 10.1073/pnas.1019524108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sango K., Joseph A., Patel M., Osiecki K., Dutta M., Goldstein H. (2010). Highly active antiretroviral therapy potently suppresses HIV infection in humanized Rag2−/−γc−/− mice. AIDS Res Hum Retroviruses 26, 735–746 10.1089/aid.2009.0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Izumi T., Misawa N., Kobayashi T., Yamashita Y., Ohmichi M., Ito M., Takaori-Kondo A., Koyanagi Y. (2010). Remarkable lethal G-to-A mutations in vif-proficient HIV-1 provirus by individual APOBEC3 proteins in humanized mice. J Virol 84, 9546–9556 10.1128/JVI.00823-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T., Rongvaux A., Rathinam C., Takizawa H., Borsotti C., Philbrick W., Eynon E. E., Manz M. G., Flavell R. A. (2011). Transgenic expression of human signal regulatory protein alpha in Rag2−/−γc−/− mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A 108, 13218–13223 10.1073/pnas.1109769108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Brake O., Legrand N., von Eije K. J., Centlivre M., Spits H., Weijer K., Blom B., Berkhout B. (2009). Evaluation of safety and efficacy of RNAi against HIV-1 in the human immune system (Rag-2−/−γc−/−) mouse model. Gene Ther 16, 148–153 10.1038/gt.2008.124 [DOI] [PubMed] [Google Scholar]
- Traggiai E., Chicha L., Mazzucchelli L., Bronz L., Piffaretti J. C., Lanzavecchia A., Manz M. G. (2004). Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304, 104–107 10.1126/science.1093933 [DOI] [PubMed] [Google Scholar]
- van der Kuyl A. C., Kozaczynska K., Ariën K. K., Gali Y., Balázs V. R., Dekker S. J., Zorgdrager F., Vanham G., Berkhout B., Cornelissen M. (2010). Analysis of infectious virus clones from two HIV-1 superinfection cases suggests that the primary strains have lower fitness. Retrovirology 7, 60 10.1186/1742-4690-7-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lent A. U., Dontje W., Nagasawa M., Siamari R., Bakker A. Q., Pouw S. M., Maijoor K. A., Weijer K., Cornelissen J. J. & other authors (2009). IL-7 enhances thymic human T cell development in “human immune system” Rag2−/−IL-2Rγc−/− mice without affecting peripheral T cell homeostasis. J Immunol 183, 7645–7655 10.4049/jimmunol.0902019 [DOI] [PubMed] [Google Scholar]
- van Lent A. U., Centlivre M., Nagasawa M., Karrich J. J., Pouw S. M., Weijer K., Spits H., Blom B., Legrand N. (2010). In vivo modulation of gene expression by lentiviral transduction in “human immune system” Rag2−/−γc−/− mice. Methods Mol Biol 595, 87–115 10.1007/978-1-60761-421-0_6 [DOI] [PubMed] [Google Scholar]
- Verhoef K., Marzio G., Hillen W., Bujard H., Berkhout B. (2001). Strict control of human immunodeficiency virus type 1 replication by a genetic switch: Tet for Tat. J Virol 75, 979–987 10.1128/JVI.75.2.979-987.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuyyuru R., Patton J., Manser T. (2011). Human immune system mice: current potential and limitations for translational research on human antibody responses. Immunol Res 51, 257–266 10.1007/s12026-011-8243-9 [DOI] [PubMed] [Google Scholar]
- Weijer K., Uittenbogaart C. H., Voordouw A., Couwenberg F., Seppen J., Blom B., Vyth-Dreese F. A., Spits H. (2002). Intrathymic and extrathymic development of human plasmacytoid dendritic cell precursors in vivo. Blood 99, 2752–2759 10.1182/blood.V99.8.2752 [DOI] [PubMed] [Google Scholar]
- Willinger T., Rongvaux A., Strowig T., Manz M. G., Flavell R. A. (2011). Improving human hemato-lymphoid-system mice by cytokine knock-in gene replacement. Trends Immunol 32, 321–327 10.1016/j.it.2011.04.005 [DOI] [PubMed] [Google Scholar]
- Yamada T., Iwamoto A. (2000). Comparison of proviral accessory genes between long-term nonprogressors and progressors of human immunodeficiency virus type 1 infection. Arch Virol 145, 1021–1027 10.1007/s007050050692 [DOI] [PubMed] [Google Scholar]
- Zhang L., Kovalev G. I., Su L. (2007). HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood 109, 2978–2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Jiang Q., Li G., Jeffrey J., Kovalev G. I., Su L. (2011). Efficient infection, activation, and impairment of pDCs in the BM and peripheral lymphoid organs during early HIV-1 infection in humanized rag2−/−γ C−/− mice in vivo. Blood 117, 6184–6192 10.1182/blood-2011-01-331173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Vink M., Berkhout B., Das A. T. (2006a). Modification of the Tet-On regulatory system prevents the conditional-live HIV-1 variant from losing doxycycline-control. Retrovirology 3, 82 10.1186/1742-4690-3-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Vink M., Klaver B., Berkhout B., Das A. T. (2006b). Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene Ther 13, 1382–1390 10.1038/sj.gt.3302780 [DOI] [PubMed] [Google Scholar]
- Zhou X., Vink M., Klaver B., Verhoef K., Marzio G., Das A. T., Berkhout B. (2006c). The genetic stability of a conditional live HIV-1 variant can be improved by mutations in the Tet-On regulatory system that restrain evolution. J Biol Chem 281, 17084–17091 10.1074/jbc.M513400200 [DOI] [PubMed] [Google Scholar]