Abstract
The type III secretion apparatus (T3SA), which is evolutionarily and structurally related to the bacterial flagellar hook basal body, is a key virulence factor used by many Gram-negative bacteria to inject effector proteins into host cells. A hollow extracellular needle forms the injection conduit of the T3SA. Its length is tightly controlled to match specific structures at the bacterial and host-cell surfaces but how this occurs remains incompletely understood. The needle is topped by a tip complex, which senses the host cell and inserts as a translocation pore in the host membrane when secretion is activated. The interaction of two conserved proteins, inner-membrane Spa40 and secreted Spa32, respectively, in Shigella, is proposed to regulate needle length and to flick a type III secretion substrate specificity switch from needle components/Spa32 to translocator/effector substrates. We found that, as in T3SAs from other species, substitution N257A within the conserved cytoplasmic NPTH region in Spa40 prevented its autocleavage and substrate specificity switching. Yet, the spa40N257A mutant made only slightly longer needles with a few needle tip complexes, although it could not form translocation pores. On the other hand, Δspa32, which makes extremely long needles and also formed only few tip complexes, could still form some translocation pores, indicating that it could switch substrate specificity to some extent. Therefore, loss of needle length control and defects in secretion specificity switching are not tightly coupled in either a Δspa32 mutant or a spa40N257A mutant.
Introduction
Shigella flexneri is the causative agent of shigellosis, which causes 1.1 million deaths each year, particularly among children under 5 years of age in developing countries (Kotloff et al., 1999). The type III secretion (T3S) system, a protein transport device used by many Gram-negative bacteria to inject effector proteins into the cytoplasm of eukaryotic cells, plays an important role in controlling host cell signalling, invasion and death during infection (Schroeder & Hilbi, 2008). The T3S system of Shigella is composed of approximately 50 proteins, including a specialized Mxi–Spa T3S apparatus (T3SA), four chaperones, three transcriptional activators, three translocators and approximately 25 effectors (Parsot, 2009). The Shigella T3SA consists of a cytoplasmic portion called ‘the bulb’, a basal body spanning the inner and outer membranes and a hollow needle protruding outside the bacterium (Blocker et al., 1999). The T3SA are evolutionarily and structurally related to the bacterial flagellar hook basal body (Minamino et al., 2008), their most conserved features being the inner-membrane protein export machinery and a sophisticated mechanism for control of needle or hook length (Cornelis, 2006).
The length of the flagellar hook is well regulated, although it differs somewhat from species to species (Hirano et al., 1994; Shibata et al., 2007). The length of the needle is tightly controlled to match specific structures at the bacterial and host-cell surfaces, ensuring efficient delivery of effectors into the host cell (Mota et al., 2005; West et al., 2005). It also varies between different bacterial species, for instance, 45 nm for S. flexneri (Tamano et al., 2002) and 58 nm for Yersinia enterocolitica E40 (Journet et al., 2003). Different mechanisms have been proposed to explain length control of flagellar hooks and virulence T3S needles. Two protein families, namely the FliK/YscP family and FlhB/YscU family, are always involved in these models. Together they regulate a substrate specificity switch, which leads to the arrest of hook/needle growth and hence determines hook/needle length (Botteaux et al., 2008; Cornelis, 2006; Erhardt et al., 2011; Ferris & Minamino, 2006; Ferris et al., 2005; Fraser et al., 2003; Journet et al., 2003; Makishima et al., 2001; Marlovits et al., 2006; Minamino & Macnab, 2000; Moriya et al., 2006; Wagner et al., 2010). What remains unclear is how these two components function at a mechanistic level.
The FliK/YscP family members are elongated, soluble proteins showing some structural disorder (Minamino et al., 2004; Mizuno et al., 2011) and carrying a more stably folded type III secretion substrate specificity switch domain (Agrain et al., 2005; Minamino et al., 2006). These proteins, including Spa32 in Shigella, may function as a molecular ruler or ‘tape measure’ that physically samples needle lengths as the proteins are secreted through the needle channel in low numbers and also to the more abundant needle subunits during needle growth (Botteaux et al., 2008; Erhardt et al., 2011; Journet et al., 2003; Magdalena et al., 2002; Moriya et al., 2006).
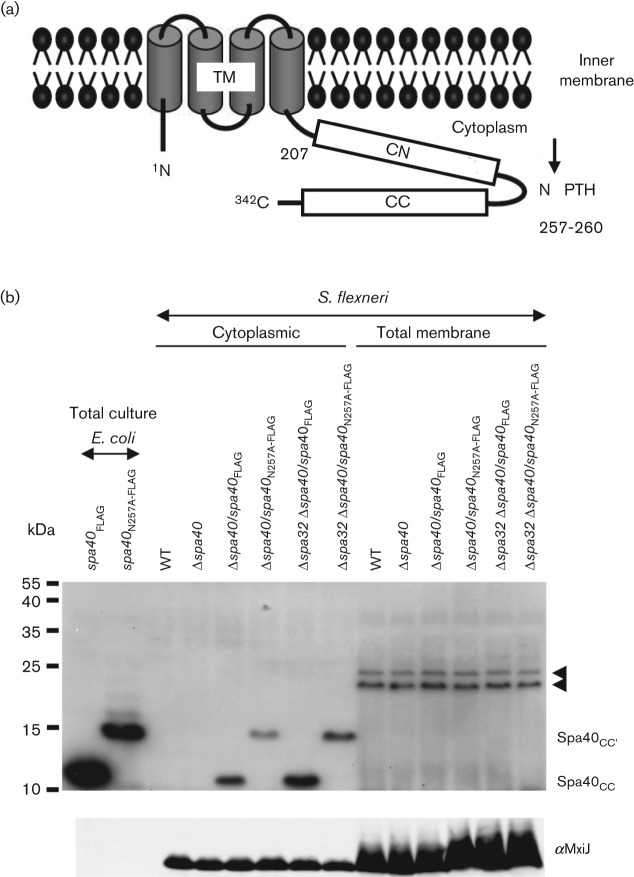
The FlhB/YscU protein family is one of the most highly conserved of all T3S protein families. In their N termini, these proteins carry four transmembrane regions, which position them in the inner bacterial membrane. The homologies amongst the C-terminal cytoplasmic domains of this protein family are particularly high (Allaoui et al., 1994). Aligning the protein sequence of the FlhB/YscU family reveals the presence of a conserved 4 amino acid sequence, NPTH, in the middle of their C-terminal cytoplasmic domains (Allaoui et al., 1994). The C-terminal cytoplasmic domain of the FlhB/YscU family undergoes autocleavage between the asparagine and proline residues within the NPTH sequence, leading to a small conformational change in the C-terminal domain, which may then interact with FliK/YscP family proteins via their type III secretion substrate specificity switch (T3S4) domain and contribute to the substrate specificity switch (Björnfot et al., 2009; Deane et al., 2008; Ferris et al., 2005; Lavander et al., 2002; Lorenz & Büttner, 2011; Lountos et al., 2009; Minamino & Macnab, 2000; Mizuno et al., 2011; Morris et al., 2010; Sorg et al., 2007; Wiesand et al., 2009; Zarivach et al., 2008). The FlhB/YscU homologue in S. flexneri is Spa40, a 342-residue polypeptide (Allaoui et al., 1994; Fig. 1a), which undergoes autoproteolytic cleavage before P258 resulting in two subdomains, N-terminal cytoplasmic Spa40CN and C-terminal cytoplasmic Spa40CC (Deane et al., 2008).
Fig. 1.
(a) Schematic representation of Spa40 based on previous studies (Deane et al., 2008). Letters indicate N terminus (N), C terminus (C), conserved NPTH sequence, transmembrane domain (TM, residues 27–204), N-terminal half of the cytoplasmic domain (CN, residues 207–257) and C-terminal half of the cytoplasmic domain (CC, residues 258–342). Numbers indicate amino acid positions in Spa40 of S. flexneri 5a (NCBI: NP_085319). The black arrow represents the cleavage site in the NPTH region. (b) Analysis of the cleavage of Spa40 protein. Overnight total cultures of E. coli expressing Spa40FLAG or Spa40N257A-FLAG and the cytoplasmic and total cell membrane fractions of S. flexneri were analysed by immunoblotting with anti-FLAG antibodies (top) and anti-MxiJ (bottom). Two different forms of Spa40 are indicated on the right side (top) as follows: Spa40CC and Spa40CC′. The latter results from cleavage at the alternative site. Non-Spa40-specific bands (black arrowheads) were also detected by the anti-FLAG antibody in Δspa40. The data shown here are representative of results from two independent assays.
T3S systems from animal pathogenic bacteria secrete at least three different sets of substrates, including (i) proteins involved in the assembly of the periplasmic and extracellular needle portions, MxiI (Blocker et al., 2001) and MxiH in Shigella, respectively, (Marlovits et al., 2004; Tamano et al., 2000) and Spa32, (ii) translocators, IpaD, IpaB and IpaC in Shigella, involved in the formation of the distal needle tip complex and then the translocon, the bacterial pore inserted into host membranes and used to translocate the protein effectors of virulence (Blocker et al., 1999; Ménard et al., 1993; Veenendaal et al., 2007), and (iii) effector proteins, including early effectors, such as IpgD of Shigella, which are involved in entry into polarized epithelial cells in the early stage of infection, and late effectors, which enable the bacteria to survive intracellularly, promote intra- and intercellular movement and modulate the host inflammatory response (Parsot, 2009). The secretion of needle components precedes translocators/early effector protein export (Magdalena et al., 2002). Our recent data suggest that upon T3S activation translocators and early effectors are secreted in the same overall group, but one class after the other (Kenjale et al., 2005; Martinez-Argudo & Blocker, 2010). Therefore, the T3S system of Shigella switches its substrate specificity over time from needle subunits and Spa32 (early substrates) to translocators and early effectors (here grouped as intermediate substrates). Late effector proteins (late substrates) are only synthesized after release of the intermediate substrate during activation (Parsot et al., 2005).
In this study, we investigated the function of Spa40 autocleavage and how it might affect or be affected by Spa32. We find that Spa32 is not required for the cleavage of Spa40 but that the presence of Spa40 (but not its normal cleavage) contributes to the expression/stability of Spa32. We also show that a spa40N257A mutant is severely impaired in the export of intermediate substrates but still exports early substrates. Accordingly, the spa40N257A mutant makes somewhat longer needles and assembles only a few tip complexes. It therefore fails to insert translocators into host membranes, as measured by contact haemolysis. However, we find that Δspa32, despite polymerizing very long needles, is better able to release intermediate substrates (although it cannot switch to their secretion efficiently) and causes weak haemolysis. Therefore, loss of needle length control and defects in secretion specificity switching are not tightly coupled either in a spa32 null mutant or in a spa40N257A mutant.
Methods
Bacterial strains and culture conditions.
Bacterial strains used in this study are listed in Table 1. S. flexneri strains were maintained and selected on Congo red (CR) agar plates (Meitert et al., 1991) and grown at 37 °C (except for the temperature shift experiments) in trypticase soy broth (Becton Dickinson) supplemented with antibiotics where appropriate (100 µg ampicillin ml−1, 50 µg kanamycin ml−1, 10 µg chloramphenicol ml−1 and 10 µg tetracycline ml−1).
Table 1. S. flexneri strains used in this study.
| Strain | Genotype [strain; plasmid(s)] | Reference |
| WT | Wild-type M90T, serotype 5a | Sansonetti et al. (1982) |
| WT (pRK2) | Wild-type M90T; pRK2mxiH | Kenjale et al. (2005) |
| Δspa40 | Wild-type M90T containing an in-frame deletion in spa40 ORF, corresponding to residues 6–338 | This study |
| Δspa40/spa40 | Δspa40; pUC19spa40 | This study |
| Δspa40/spa40N257A | Δspa40; pUC19spa40 | This study |
| Δspa40/spa40FLAG | Δspa40; pUC19spa40FLAG | This study |
| Δspa40/spa40N257A-FLAG | Δspa40; pUC19spa40N257A-FLAG | This study |
| Δspa32 | MJ321 | Magdalena et al. (2002) |
| Δspa32 Δspa40 | MJ321Δspa40 | This study |
| Δspa32 Δspa40/spa40FLAG | MJ321Δspa40; pUC19spa40FLAG | This study |
| Δspa32 Δspa40/spa40N257A-FLAG | MJ321Δspa40; pUC19spa40N257A-FLAG | This study |
| WT mxiH | Wild-type M90T; pACT3mxiH | Shen et al. (2010) |
| Δspa40/spa40FLAG mxiH | Δspa40; pUC19spa40FLAG, pACT3mxiH | This study |
| Δspa40/spa40N257A-FLAG mxiH | Δspa40; pUC19spa40N257A-FLAG, pACT3mxiH | This study |
| Δspa32 mxiH | MJ321; pACT3mxiH | This study |
| Δspa32 Δspa40 mxiH | MJ321Δspa40; pACT3mxiH | This study |
| Δspa32 Δspa40/spa40FLAG mxiH | MJ321Δspa40; pUC19spa40FLAG, pACT3mxiH | This study |
| Δspa32 Δspa40/spa40N257A-FLAG mxiH | MJ321Δspa40; pUC19spa40N257A-FLAG, pACT3mxiH | This study |
Molecular cloning
All primers used in this study are listed in Table S1 (available with the online version of this paper) and all constructs were verified by DNA sequencing.
Knockout of spa40.
In-frame deletion of amino acids 6–338 encoded by spa40 was carried out by using the λ Red system (Datsenko & Wanner, 2000). A kanamycin resistance cassette was amplified from plasmid pKD4 using the primers spa40-KO-kanF and spa40-KO-kanR and electroporated into S. flexneri wild-type carrying the Red recombinase to replace spa40, giving rise to Δspa40. A tetracycline resistance cassette, amplified from strain TH2788 (Frye et al., 2006) using the primers spa40-KO-tetF and spa40-KO-tetR, was used to replace spa40 in S. flexneri strain Δspa32 (Magdalena et al., 2002), giving rise to Δspa32 Δspa40.
Mutagenesis of spa40.
We generated a point mutation (N257A) in the NPTH sequence of Spa40 using a two-step PCR strategy. In the first step, 5′ and 3′ fragments of spa40 were amplified from pWR100 (Buchrieser et al., 2000) by using the primer pairs spa40-F/spa40N257A-R and spa40N257A-F/spa40-R, respectively. In the second step, using the primer pair spa40-F/spa40-R, the mixture of 5′ and 3′ fragments was used as the template to amplify spa40N257A, which was then cloned into pUC19 by ligation to the PstI and EcoRI sites of the polylinker. The resultant plasmid was transformed into Δspa40 to obtain Δspa40/spa40N257A (Table 1). The primers spa40-F and spa40-FLAG-R were used to obtain Δspa40/spa40N257A-FLAG.
Analysis of protein synthesis and secretion.
Total levels of protein expression, leakage and CR induction were determined as previously described (Martinez-Argudo & Blocker, 2010). For the leakage assay, TCA-precipitated supernatants from exponentially growing bacteria (OD600 approximately 1) were used.
Pulse–chase, time-course experiments.
Bacteria were grown overnight at either 37 or 30 °C and then diluted 1 : 50 and either they were grown at constant temperature or growth was shifted from 30 °C to 37 °C. At 45 min, 1.5 h, 3 h and 22 h, both cells and supernatants, corresponding to same quantity of bacteria based on the OD600, were collected by centrifugation. Supernatants were further precipitated by using TCA. Finally, whole cells and TCA-precipitated supernatants were resuspended into SDS-loading buffer.
Antibodies and Western blotting.
The antibodies include the mouse mAbs against IpaB H16 (Barzu et al., 1993), IpaC K24 (Phalipon et al., 1992), IpgD (Blocker et al., 1999) and FLAG M2 (Sigma) and the rabbit polyclonal sera against IpaD (Ménard et al., 1993), MxiC (Martinez-Argudo & Blocker, 2010), Spa32 (Magdalena et al., 2002) and MxiJ (Zenk et al., 2007). The anti-Spa40 polyclonal antibodies were raised against Spa40C (residues 207–342; Deane et al., 2008) in rabbits (Eurogentec) and were then purified by using immunoaffinity (Harlow & Lane, 1988) using a CNBr-activated Sepharose 4B column (GE Healthcare) where the beads were covalently coupled to Spa40C(N257A). Goat anti-mouse DyLight 800 (Fisher Scientific) or goat anti-rabbit Alexa 680 (Invitrogen) conjugates were used as secondary antibodies. The membranes were then visualized by using an Odyssey infrared imaging system (LI-COR Biosciences).
Preparation of total cell membranes.
To prepare total cell membranes, 500 ml exponentially growing bacteria (OD600 approximately 1) were harvested by centrifugation (20 min, 3170 g, 4 °C) and washed once with Tris buffer (20 mM Tris, 150 mM NaCl, pH 7.4). Bacteria were resuspended in 10 ml Tris buffer containing one tablet of protease inhibitor cocktail complete Mini, EDTA free (Roche) and 15 Kunitz units DNase I (Sigma) and disrupted twice by passage through a French press at 15 000 p.s.i. (103 500 kPa). After removal of unbroken cells by low-speed centrifugation (60 min, 6000 g, 4 °C; twice), the supernatants were passed through a 0.45 µm filter. About 9 ml clarified lysates was deposited on the surface of 3 ml 10 % (w/w) sucrose in an ultracentrifuge tube (SW 41 Ti rotor) and centrifuged at high speed (60 min, 178 305 g, 4 °C), the supernatants and the pellets were collected and an equivalent amount of protein from each was analysed by SDS-PAGE and Western blotting.
Electron microscopy.
To visualize needles at the cell surface of bacteria, ghost cells were obtained by osmotic shock treatment using glass beads as described previously (Kenjale et al., 2005). Samples were deposited onto 300-mesh, freshly glow-discharged, Formvar and carbon-coated copper grids and subsequently stained for 1 min with 1 % (w/v) phosphotungstic acid at pH 7. Bacteria were visualized in a Tecnai12 transmission electron microscope (FEI) fitted with an FEI Eagle 4k×4k CCD camera at ×20 000 magnification using FEI Tecnai Imaging Analysis (TIA) software. The length of the needles was measured using a ruler on a large computer screen.
Contact haemolysis.
These assays were performed as described previously (Blocker et al., 1999).
Red blood cell membrane isolation.
This assay was performed as described previously (Shen et al., 2010).
Needle purification.
To overexpress the needle protein MxiH, the mxiH gene was amplified by PCR using Shigella virulence plasmid pWR100 (Buchrieser et al., 2000) as template and primers mxiH-RBS and mxiH-HindIII. The PCR product was purified, digested with SacI and HindIII and cloned into the IPTG-inducible plasmid pACT3 (Dykxhoorn et al., 1996) giving rise to pACT3mxiH. Needles were purified as described previously (Veenendaal et al., 2007), but using 100 µM IPTG (isopropyl-β-d-thiogalactopyranoside) to induce mxiH expression from pACT3mxiH (Shen et al., 2010).
Results
Spa40N257A cleaves differently to wild-type Spa40
To analyse the phenotype of a non-cleavable spa40 mutant, we introduced a single point mutation in the NPTH sequence of Spa40 and expressed the resulting spa40N257A in trans in Escherichia coli DH5α and S. flexneri Δspa40. Using an affinity purified polyclonal anti-Spa40, we could easily detect Spa40 from E. coli B834 BL21(DE3) overexpressing Spa40C (Deane et al., 2008). However, we failed to detect full-length or cleaved Spa40 in either E. coli DH5α or S. flexneri expressing full-length Spa40 or Spa40N257A, from low-/high-copy-number plasmids or the virulence plasmid. Yet, in trans overexpression of the full-length wild-type protein did not inhibit bacterial growth and did functionally complement a Δspa40 mutant (Fig. S1). This suggests that natively encoded Spa40 is expressed or stable only at very low levels in Shigella and that our anti-Spa40 is not sensitive enough to detect it. Therefore, we constructed C-terminally FLAG-epitope-tagged full-length spa40FLAG and spa40N257A-FLAG and expressed these in trans in E. coli, S. flexneri Δspa40 or Δspa32 Δspa40.
Using an anti-FLAG antibody on total cultures of E. coli expressing Spa40FLAG, we detected a fragment of about 10 kDa, assignable to Spa40CC after cleavage in the NPTH region (Fig. 1b, top). In contrast, in E. coli expressing Spa40N257A-FLAG, a protein fragment of about 15 kDa (indicated as Spa40CC′) was observed. However, no Spa40 products were detectable in total culture extracts of S. flexneri expressing Spa40FLAG or Spa40N257A-FLAG (not shown).
As cleaved YscU from Y. enterocolitica was enriched in bacterial membrane fractions (Sorg et al., 2007), we prepared total cell membranes from S. flexneri and checked them using anti-FLAG antibodies. In both Δspa40 and Δspa32 Δspa40 expressing Spa40FLAG, a 10 kDa fragment corresponding to Spa40CC was clearly detectable from the cytoplasmic but not the total membrane fraction. In addition, in both Δspa40 and Δspa32 Δspa40 expressing Spa40N257A-FLAG, a 15 kDa fragment corresponding to Spa40CC′ was exclusively detectable from the cytoplasmic fraction. However, we never detected full-length Spa40FLAG, which has a predicted size of 40.8 kDa. Lack of detection of full-length Spa40 in both E. coli and S. flexneri suggests that complete autocleavage occurred under these experimental conditions.
To verify that Spa40CC was indeed enriched in the cytoplasmic fraction, we checked the fractionation of both MxiG and MxiJ, which are inner membrane components (Allaoui et al., 1992, 1995; Blocker et al., 2001) of the Shigella T3SA (Blocker et al., 2001; Kubori et al., 1998). Though we could always detect these proteins in the cytoplasmic fraction, there was a clear enrichment of MxiG (not shown) and MxiJ in the membrane fraction (Fig. 1b, bottom), supporting the notion that, when spa40 is expressed in trans in Shigella, the majority of Spa40CC genuinely partitions in the cytoplasmic fraction and any fraction that becomes membrane-associated is not detectable with our experimental set-up.
Together, these data indicate that, in Shigella (i) the conserved Asn within the NPTH region is essential for the cleavage of Spa40 at this site, (ii) the cleavage is complete, (iii) Spa32 is not necessary for cleavage of Spa40 and (iv) probably the majority of overexpressed and cleaved Spa40CC is not associated with bacterial membranes.
The spa40N257A mutant exports normal levels of Spa32 but more needle subunits and lower basal amounts of translocators/early effectors
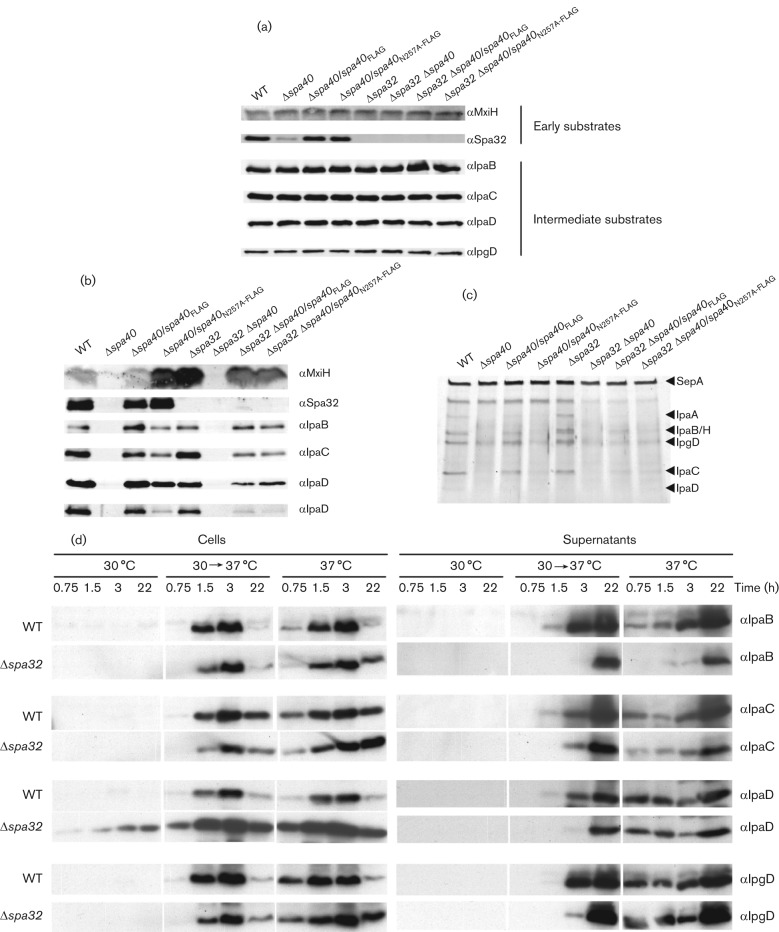
Next we tested whether secretion of translocators, early effectors and needle subunits were affected in the spa40N257A-FLAG mutant. To make sure that the phenotypes observed were not due to alterations in the expression levels or stability of these proteins, we first analysed their levels in total culture by immunoblotting. All strains expressed similar levels of translocators (IpaB, IpaC and IpaD), early effector (IpgD) and needle subunit (MxiH) (Fig. 2a). However, the expression level of Spa32 was dramatically lower in Δspa40 compared with wild-type Shigella and Δspa40 complemented with either spa40 or spa40N257A. This suggests that Spa40, but not its proper autocleavage, is required for intrabacterial expression/stability of Spa32.
Fig. 2.
Expression and secretion profiles of spa40 and spa32 mutants. Total cultures (a) and TCA-precipitated supernatants of exponentially grown bacteria were analysed by immunoblotting (b), with antibodies indicated on the right. Supernatants were also checked by silver staining (c); the positions of the major Ipa proteins detected are indicated on the right. (d) Expression and secretion of Ipa proteins in Δspa32, grown at 30 °C, 37 °C or shifted from 30 °C to 37 °C for 0.75, 1.5, 3 and 22 h, were analysed by immunoblotting. The wild-type (WT) was used as control and bacterial numbers were normalized by OD600. Each full set of WT and Δspa32 samples were run on separate gels but blotted in parallel. The antibodies used for the blots are indicated on the left. The data shown here are representative of results from two independent assays.
To analyse the effect of the spa40N257A mutation on the basal secretion of translocators, early effectors and needle subunits, the supernatants of bacteria grown to mid-exponential phase were analysed by silver staining and further verified by immunoblotting. As previously reported, the Δspa40 strain is defective in secretion, whereas complementation of Δspa40 with wild-type spa40 restores secretion to levels similar to that of the wild-type (Fig. 2b, c; Botteaux et al., 2010). In contrast, the spa40N257A mutant secreted fewer translocators (IpaB, IpaC and IpaD) and early effectors (IpgD), but more needle subunits (MxiH; Fig. 2b, c), supporting the notion that Spa40 is involved in switching the T3S substrate specificity from early substrates, i.e., MxiH and Spa32, to intermediate substrates, i.e., translocators and early effectors. Fig. 2(b, c) shows that the level of secreted translocators and of the early effector IpgD are similar between wild-type and the Δspa32 strain, suggesting that, interestingly, Δspa32 has no defect in switching the secretion specificity from early substrates to intermediate substrates. However, Δspa32 does secrete much more MxiH, confirming that Spa32 is required for arrest of needle growth (Botteaux et al., 2008; Magdalena et al., 2002; Tamano et al., 2002). Similar to Δspa40, the Δspa32 Δspa40 strain is also defective in secretion. This indicates that, as expected, the known requirement of Spa40 for export apparatus function is dominant over lack of Spa32. No obvious secretion differences were observed between Δspa32 Δspa40 complemented either by spa40 or by spa40N257A, and their basal secretion phenotype resembles that of Δspa40/spa40N257A more than that of Δspa32. This further supports the notion that normal Spa40 cleavage is a prerequisite for the action of Spa32 in T3S maturation.
Together, these data suggest that in Shigella, Spa40, rather than Spa32, is the protein primarily mediating the secretion specificity switch from early to intermediate substrates and that its action requires correct autocleavage at the NPTH site.
Δspa32 cannot switch to the secretion of translocators/early effectors efficiently
The Δspa32 and wild-type strains showed quite different phenotypes in terms of MxiH secretion (Fig. 2b) and needle length regulation (see later and Magdalena et al., 2002). Thus, it is surprising that they secreted similar basal levels of translocators (except IpaD, which was reduced in Δspa32) and of the early effector IpgD (Fig. 2b, c). We therefore asked whether the timing of Ipa ‘leakage’ is different between these two strains.
Since Ipas are expressed at 37 °C but not or less so at 30 °C (Le Gall et al., 2005; Maurelli et al., 1984), we tested for differences in secretion kinetics by shifting the cultures from 30 to 37 °C and performing a time-course analysis. Both cells and supernatants, corresponding to same quantity of bacteria, were collected and checked by Western blotting. At 30 °C, there was no expression of translocators (IpaBCD) or of the early effector IpgD in wild-type, as previously reported (Le Gall et al., 2005; Maurelli et al., 1984). However, we could easily detect the expression of IpaD from Δspa32 (Fig. 2d). Except for IpaD, the expression levels of translocators (IpaB and IpaC) and early effector (IpgD) were similar between wild-type and Δspa32. However, the secretion of translocators, especially IpaD and IpaB and early effector IpgD, was clearly delayed in Δspa32. These data suggest that Δspa32 has a delay, rather than an absolute defect, in switching the secretion from early substrates to intermediate substrates.
The needle termination defect in Δspa32 is probably not due to retention of a needle assembly cap
In the flagellum, ‘assembly cap’ proteins are always attached at the distal end of the growing structure, including rod, hook and filament, to promote the efficient assembly of each substructure (Chevance & Hughes, 2008; Minamino et al., 2008). Given the structural and mechanistic similarities between the flagellar hook and the T3S needle, it was expected that the needle would have an assembly cap too (Blocker et al., 2003; Cornelis, 2006), which would be different to the ‘tip complex’ later assembled atop needles. If the growing needle did have a cap, such a cap should be found at the top of the continuously growing needles (Magdalena et al., 2002) of Δspa32. In addition, it might also be found in the ΔipaD strain since IpaD is thought to be the first tip complex protein added (Veenendaal et al., 2007), analogous to the first hook-junction protein in the flagellum. Lack of this latter protein also leads to assembly cap retention at the hook tip (Ohnishi et al., 1994). Moreover, purified IpaD is able to halt needle growth in vitro (Poyraz et al., 2010). However, we did not find evidence for the existence of an assembly cap protein using mass spectrometry analysis of needles from these strains (see Supplementary Methods and Results, and Table S2).
spa40N257A regulates needle length better than Δspa32 but both are unable to form substantial numbers of tip complexes
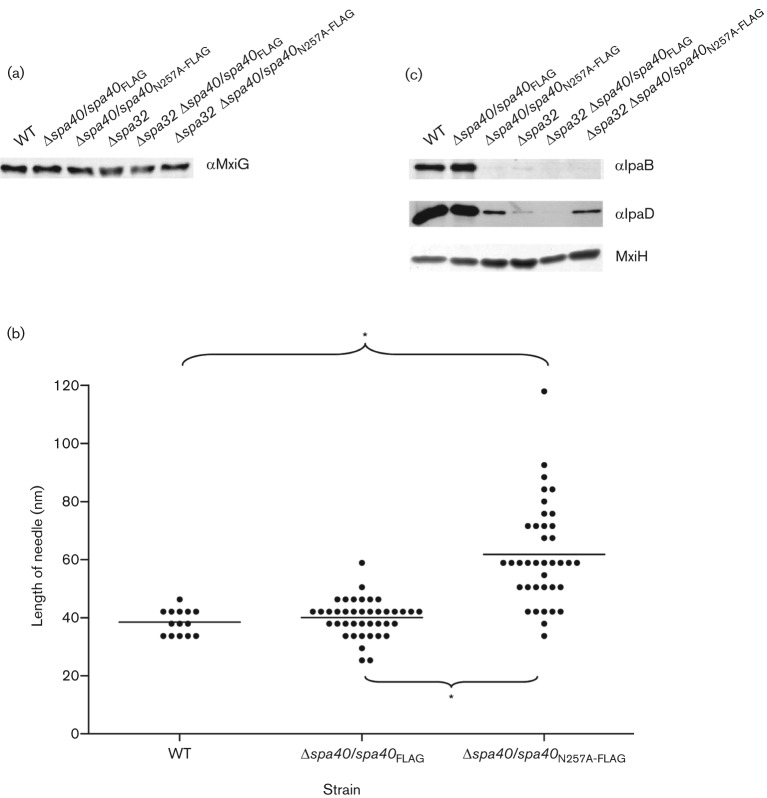
Loss of Spa32 causes hyperlong needles (Magdalena et al., 2002). To assess the effect of Spa40N257A on T3SA assembly, we first checked the expression level of MxiG, an inner membrane component of the needle complex. All strains showed similar levels of MxiG (Fig. 3a), suggesting that they have similar numbers of T3SA bases and hence needles. We next measured the length of needles from the wild-type and Δspa40 expressing Spa40 or Spa40N257A. Needles of the complemented Δspa40 strain had wild-type length, whereas needles from Δspa40 expressing Spa40N257A were 50 % longer than that of the wild-type or the Δspa40 complemented strains (Fig. 3b) and had a broader length distribution. These data reflect the fact that the spa40N257A mutant secreted as many or more needle subunits as Δspa32 (Fig. 2b). However, while Δspa32 and Δspa32 Δspa40 complemented with either spa40 or spa40N257A showed extraordinarily long needles up to 400–900 nm in length (Magdalena et al., 2002; data not shown), the spa40N257A mutant displayed only slightly extended needles. This indicates that Spa32 can still, albeit less efficiently, terminate needle length in the spa40N257A mutant. This demonstrates that in Shigella, flipping of the early secretion specificity switch and needle termination are not tightly coupled events.
Fig. 3.
Analysis of needle-associated proteins and determination of needle length. (a) Overnight total cultures from strains overexpressing MxiH were analysed by immunoblotting with anti-MxiG antibodies. (b) Needle length determination. Data were analysed using a Kruskal–Wallis test and then by Dunn’s multiple comparison test. Dots represent individual needles. Data were based on two independent experiments. *P<0.001. (c) Needles from overnight-grown strains overexpressing MxiH were purified, normalized for the amount of MxiH by silver-stained SDS-PAGE (bottom) and analysed by immunoblotting with antibodies as indicated on the right. The data shown are representative of two independent assays giving similar results.
We previously reported that both IpaD and IpaB localize to the tip of mature, quiescent needles (Veenendaal et al., 2007). Since the spa40N257A mutant essentially cannot switch the T3S substrate specificity from needle to intermediate substrates and Δspa32 cannot do it efficiently (Magdalena et al., 2002), we supposed that their needles might be immature and lack IpaD and/or IpaB. To facilitate examination of the Ipa composition of their needles, we overexpressed MxiH using the plasmid pACT3mxiH and prepared purified long needles as previously described (Shen et al., 2010; Veenendaal et al., 2007). The samples were normalized according to the amount of MxiH they contained and were then compared for Ipa composition by Western blotting (Fig. 3c). In needles derived from the complemented Δspa40 strain, IpaD and IpaB were found at levels similar to those found in wild-type needles. In contrast, very low IpaD and little IpaB were found in needles from spa40N257A and Δspa32, as well as Δspa32 Δspa40 complemented with either spa40 or spa40N257A. Together, these data suggest that neither Δspa40/spa40N257A nor Δspa32 can assemble substantial numbers of mature needle tips including normal amounts of IpaD and IpaB.
Both spa40N257A and Δspa32 are non-inducible; however, Δspa32 is weakly haemolytic
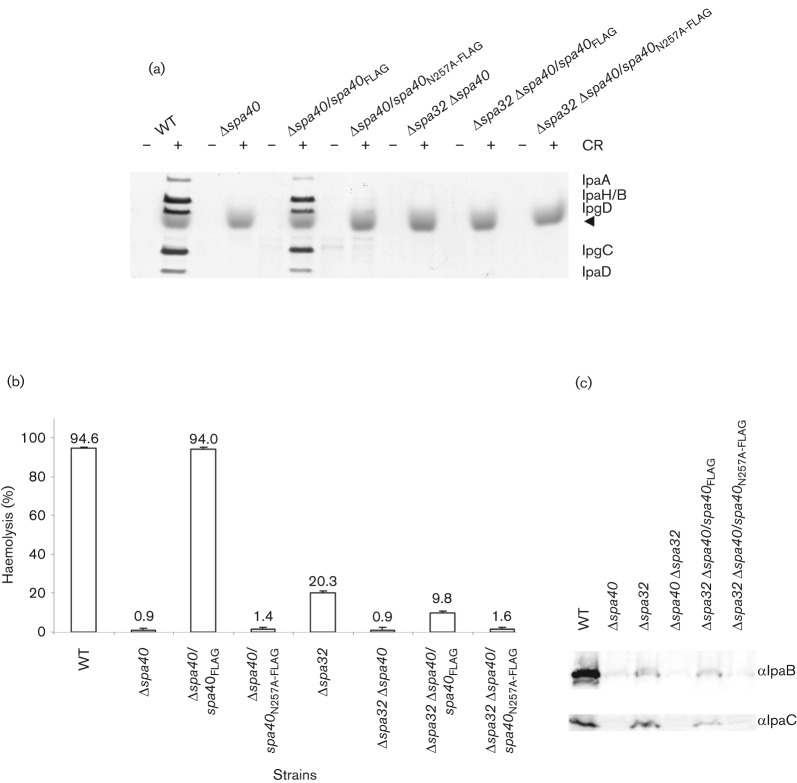
CR, a small amphipathic dye molecule, induces enhanced secretion of Ipa proteins in the wild-type Shigella (Bahrani et al., 1997). In contrast, the spa40N257A mutant was uninducible by CR (Fig. 4a). In addition, as previously reported, Δspa32 was also uninducible (Magdalena et al., 2002; data not shown).
Fig. 4.
CR induction, haemolysis and membrane insertion of spa40 mutants. (a) spa40N257A mutant is uninducible by CR. Induced secretion of Ipa proteins after the addition (+) or in the absence (−) of CR was analysed by silver staining. The positions of the major Ipa proteins detected are indicated on the right. The bacterial numbers were normalized by OD600 and the data shown here are representative of two independent experiments giving similar results. A trace of CR is indicated by a solid arrowhead. (b) The haemolysis percentages of sheep RBCs are means±sd of results from two independent experiments performed in triplicate. (c) Insertion of IpaB and IpaC into sheep RBC membranes after contact haemolysis. The antibodies used for the Western blots are indicated on the right and the blots shown are representative of two independent assays giving similar results.
Haemolysis is the ability of Shigella to lyse red blood cells (RBCs) and is due to the formation of a 25 Å (2.5 nm) pore by IpaB and IpaC within the RBC membrane following contact induced with them during centrifugation (Blocker et al., 1999). To study the effect of Spa40N257A and the lack of Spa32 on pore formation, we investigated the contact haemolytic activity of their respective mutants. As expected, the Δspa40 strain was non-haemolytic, whereas complementation of Δspa40 with wild-type spa40 restored haemolysis to levels similar to that of the wild-type (Fig. 4b). The spa40N257A mutant was also non-haemolytic. Surprisingly, Δspa32 and Δspa32 Δspa40/spa40 caused 20.3 and 9.8 % haemolysis, respectively (Fig. 4b), despite their greatly reduced number of needle tip complexes (Fig. 3c). To determine whether Δspa32 and Δspa32 Δspa40/spa40 could insert IpaB and IpaC within the RBC membrane, we examined the composition of the lysed RBC membranes isolated by floatation in a sucrose density gradient. As previously reported, IpaB and IpaC are present in these membranes when RBCs have been lysed by contact with wild-type bacteria (Fig. 4c; Blocker et al., 1999). However, no IpaB and IpaC were detected from Δspa40, Δspa32 Δspa40 and Δspa32 Δspa40/spa40N257A, which all fail to cause haemolysis (Fig. 4b). In contrast, a little IpaB and IpaC were detected from Δspa32 and Δspa32 Δspa40/spa40, which explains the observed 10–20 % haemolysis caused by these strains. Both Δspa32 and spa40N257A can form small numbers of needle tips (see above). However, as only Δspa32 is able to switch the secretion specificity with low efficiency, only it can perform weak haemolysis. This weak inducibility is, however, not detectable (or not replicated) in the CR-induction assay.
Discussion
The T3SA strongly resembles the hook basal body of the flagellar type III export apparatus in many respects and the two systems are assumed to share a mechanism for orchestrating T3S substrate specificity switching to control the length of the needles or hooks, respectively (Cornelis, 2006; Minamino et al., 2008). The T3S substrate specificity switch depends on interactions between inner-membrane proteins belonging to the FlhB/YscU family and the T3S4 proteins belonging to the FliK/YscP family (Botteaux et al., 2008; Ferris & Minamino, 2006; Minamino & Macnab, 2000). In this study, we investigated the role of the FlhB/YscU homologue Spa40 and the FliK/YscP homologue Spa32 of S. flexneri in the T3S substrate specificity switching and in the control of the needle length.
To understand the ordered export process, we introduced a point mutation into the NPTH cleavage site of Spa40. We found that the 40 kDa Spa40 is naturally cleaved and produces a 10 kDa CC fragment, as previously shown for FlhB (Minamino & Macnab, 2000) and for YscU (Lavander et al., 2002; Sorg et al., 2007). Though no 10 kDa CC fragment was detected from Spa40N257A, a band of 15 kDa was observed, suggesting that the spa40N257A mutant cleaved itself at an alternative site. Alternative cleavage has also been observed in Yersinia spp. YscU mutants (Lavander et al., 2002; Sorg et al., 2007) and in the Salmonella flhBN269A mutant, where it happens at D237/P238 (Fraser et al., 2003). Though D237/P238 is highly conserved among the YscU/FlhB family (Zarivach et al., 2008), the corresponding amino acids in S. flexneri are H225/F226. It seems unlikely that this could lead to the same cleavage mechanism (Zarivach et al., 2008). If the alternative cleavage did occur at H225/F226 in Spa40N257A, the resulting C-terminal plus FLAG sequence would have a predicted molecular mass of 14.6 kDa, which corresponds to what we observed (Fig. 1b, top). As flhBP238A, which does not undergo cleavage at the secondary site, is wild-type for motility, cleavage at D237/P238 probably has no physiological significance (Fraser et al., 2003).
In good agreement with the estimated stoichiometry of two FlhB molecules per flagellum (Zhu et al., 2002), Spa40CC could only be detected after enrichment. However, surprisingly, Spa40CC was found in the cytoplasmic fraction rather in than membrane fraction. Although Cornelis’ group observed that cleaved YscU was enriched in total bacterial membranes under denaturing conditions, they did not mention whether they checked the cytoplasmic fraction (Sorg et al., 2007). The enrichment of Spa40CC in the cytoplasm might suggest that Spa40CC is not tightly associated with the putatively membrane-bound N-terminal domain Spa40CN. Yet, the structures of the cytoplasmic domains of Spa40 (Deane et al., 2008), E. coli EscU and Salmonella typhimurium SpaS (Zarivach et al., 2008) highlight the tight association of the cleaved cytoplasmic subdomains. However, Deane et al. (2008) observed that the stable complex of Spa40CN–Spa40CC was only formed under native conditions, indicating that the folded state of these proteins is essential for their tight association. Therefore, the cytoplasmic enrichment of Spa40CC is most likely to be due to mistargetting and/or misfolding of the protein when expressed from a non-native promoter and/or at higher than native levels. Indeed, we did detect peptides corresponding to Spa40CC in purified needle complexes (Zenk et al., 2007; Cheung and other authors, unpublished data). As we know that FlhBC is rapidly cleaved (Minamino & Macnab, 2000) and the cleavage of FlhBC and its homologues is nearly complete (Fraser et al., 2003; Lavander et al., 2002; Lorenz & Büttner, 2011), this implies that, within the native T3SA base, the cleaved Spa40CC is still attached to the inner membrane via its interaction with Spa40CN.
Our data show that cleavage at the NPTH sequence is required to mediate the secretion specificity switch from early (MxiH, Spa32) to intermediate substrates (translocators/early effectors), as reflected in the fact that spa40N257A secreted more MxiH and made 50 % longer needles than the wild-type. This finding agrees with the previous report that flhBN269A bacteria fail to switch from early rod-/hook-type substrate export to late filament-type substrate export (Fraser et al., 2003). Though a yscUN263A mutant prevents the export of translocators (Sorg et al., 2007), it does not affect switching from needle subunits to Yop effectors, whereas spa40N257A, although it can still leak early effectors, is unable to induce their secretion. Therefore, from this point of view, either spa40N257A is different from yscUN263A or induction of effector secretion by CR in Shigella and by Ca2+ removal in Yersinia are not equivalent phenomena.
It was previously reported that ΔyscP Yersinia strain secreted dramatically lower levels of translocators/effectors relative to the wild-type strain (Edqvist et al., 2003). However, our data showed that Δspa32 leaks levels of translocators/early effectors similar to those of the wild-type although it fails to switch the substrate specificity as quickly as the wild-type. In addition, although Δspa32 makes superlong needles, in spa40N257A needle length is still approximately controlled by Spa32. The latter is reminiscent of the observation that the needle length in the yscUN263A mutant can still be controlled by YscP (Sorg et al., 2007), the only difference is that spa40N257A secrete normal levels of Spa32 while yscUN263A releases less YscP than wild-type bacteria do. This implies that (i) Δspa32, unlike spa40N257A, can still switch secretion specificity from early to intermediate substrates and (ii) the FliK/YscP protein family might interact with somewhat different efficiency with the uncleaved FlhB/YscU protein family in different bacteria.
Although both spa40N257A and Δspa32 present little IpaD and IpaB at their needle tips and are uninducible by CR, Δspa32 and Δspa32 Δspa40/spa40 cause 20.3 % and 9.8 % haemolysis, respectively. As haemolysis is due to the formation of a 25 Å (2.5 nm) pore by IpaB and IpaC within the RBC membrane, this suggests that Δspa32 does form some normal needle tip complexes but with very low efficiency. Weak haemolysis caused by Δspa32 also supports the observation that, unlike spa40N257A, Δspa32 has no absolute defect in switching the secretion specificity from MxiH to translocators. In fact, poor secretion of translocators, especially IpaC by spa40N257A (Fig. 2b) and relatively good secretion of translocators, especially IpaC by Δspa32 (Fig. 2b, d), mirrors the haemolytic difference observed between spa40N257A and Δspa32. Why would Δspa32 make so few functional tip complexes (Fig. 3c) given it only displays a delay in secretion of the translocators, particularly IpaD (Fig. 2b–d)? IpaD is the first component of the tip complex, without which IpaB cannot bind to make it fully functional (Veenendaal et al., 2007) in host-cell sensing. Therefore, a specifically greater reduction in IpaD release may enhance the kinetic block in tip complex assembly induced by the overall delay observed in translocator secretion in Δspa32.
Loss of needle length control and failure to secrete translocators/effectors is dissociable in Yersinia yscP internal deletion mutants (Agrain et al., 2005) but is tightly coupled in Shigella spa32 internal deletion mutants (Botteaux et al., 2008). Our observation is that loss of needle length control and defects in initial secretion of Ipas are not tightly coupled either in a spa32 null mutant or in a spa40N257A mutant. This agrees with the finding that Salmonella fliK deletion mutants are ‘leaky’ in that they produce approximately 90 % polyhooks and approximately 10 % polyhook-filaments (Patterson-Delafield et al., 1973; Suzuki & Iino, 1981). Furthermore, our data indicate that the correct cleavage of Spa40 is vital for the substrate specificity switch, while Spa32 is mainly responsible for needle length control.
Accordingly, our data agree with the molecular tape measure model, which itself is based on the ruler model (Botteaux et al., 2008; Journet et al., 2003; Moriya et al., 2006). The N-terminal end of the intermittently secreted FliK can bind to the hook cap strongly or to the wall of the growing hook, if for instance the hook is already too long to allow cap binding, with lower affinity (Minamino et al., 2009). Its C terminus would remain inside the bacterial cytoplasm and interact with FlhBC if the hook had reached a certain height, resulting in the switch of export specificity. As it has no assembly cap it can attach to, Spa32 would only bind to the wall of the growing needle and hence would still be able to regulate the length of the otherwise autonomously polymerizing needle. Lack of Spa32 would make needle length sensing and hence control impossible. However, in Δspa32, Spa40 seems still functional for switching, albeit with poor efficiency. Therefore, Spa32 may only act to enhance a conformational change that occurs anyway autonomously in Spa40C.
Mizuno et al. (2011) solved the NMR structure of the FliK T3S4 domain. Based on functional data obtained from deletion mutants within FliKC, they constructed a model of the interaction between FliKC and FlhBC where the autocleaved NPTH sequence in FlhB contacts loop 2 of FliKC, perhaps triggering the switching event. In their model, this contact is sterically prevented when NPTH is not cleaved. However, measuring the interaction between FliK and FlhB by optical biosensing methods, Morris et al. (2010) found that, while the affinity between the two components is in the micromolar range, FliK binds to both wild-type (autocleaved) and mutant (non-cleaved) FlhBs with similar strength. These latter data support our findings that the activities of Spa32 and Spa40 are not tightly linked. However, key questions remain. When and how does Spa40 switch the substrate specificity, in the presence and absence of Spa32? How can Spa32 alone roughly determine needle length and then terminate needle growth in spa40N257A?
Acknowledgements
We are indebted to Andreas Veenendaal (whilst at the Sir William Dunn School of Pathology, University of Oxford) and Claudia Danzer (trainee at the University of Bristol from the University of Erlangen) for preliminary work on the composition of Δspa32 and ΔipaD needles. We thank Benjamin Thomas for carrying out the mass spectrometry work at the Central Proteomics Facility and staff at the Computational Biology Research Group (both at the Sir William Dunn School of Pathology, University of Oxford) for assistance with analysis of the resulting data. We are grateful to Judith Mantell of the Wolfson Bioimaging Facility (Faculty of Medical Sciences, University of Bristol) for help with electron microscopy and to Dorothea Roehrich for critical comments on the manuscript. D. K. S. was funded by UK Medical Research Council grant G0701243 to A. J. B.. N. M. was supported by a Japanese Society for the Promotion of Science postdoctoral fellowship. I. M. A. was supported by Wellcome Trust project grant 088266 to I. M. A. and A. J. B.
Abbreviations:
- CR
Congo red
- RBC
red blood cell
- T3S
type III secretion
- T3S4
type III secretion substrate specificity switch
- T3SA
type III secretion apparatus
Edited by: V. J. Cid
A set of supplementary methods and results, a supplementary figure and two supplementary tables, with supplementary references, are available with the online version of this paper.
References
- Agrain C., Callebaut I., Journet L., Sorg I., Paroz C., Mota L. J., Cornelis G. R. (2005). Characterization of a Type III secretion substrate specificity switch (T3S4) domain in YscP from Yersinia enterocolitica. Mol Microbiol 56, 54–67. 10.1111/j.1365-2958.2005.04534.x [DOI] [PubMed] [Google Scholar]
- Allaoui A., Sansonetti P. J., Parsot C. (1992). MxiJ, a lipoprotein involved in secretion of Shigella Ipa invasins, is homologous to YscJ, a secretion factor of the Yersinia Yop proteins. J Bacteriol 174, 7661–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaoui A., Woestyn S., Sluiters C., Cornelis G. R. (1994). YscU, a Yersinia enterocolitica inner membrane protein involved in Yop secretion. J Bacteriol 176, 4534–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaoui A., Sansonetti P. J., Ménard R., Barzu S., Mounier J., Phalipon A., Parsot C. (1995). MxiG, a membrane protein required for secretion of Shigella spp. Ipa invasins: involvement in entry into epithelial cells and in intercellular dissemination. Mol Microbiol 17, 461–470. 10.1111/j.1365-2958.1995.mmi_17030461.x [DOI] [PubMed] [Google Scholar]
- Bahrani F. K., Sansonetti P. J., Parsot C. (1997). Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect Immun 65, 4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzu S., Nato F., Rouyre S., Mazie J. C., Sansonetti P., Phalipon A. (1993). Characterization of B-cell epitopes on IpaB, an invasion-associated antigen of Shigella flexneri: identification of an immunodominant domain recognized during natural infection. Infect Immun 61, 3825–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnfot A. C., Lavander M., Forsberg A., Wolf-Watz H. (2009). Autoproteolysis of YscU of Yersinia pseudotuberculosis is important for regulation of expression and secretion of Yop proteins. J Bacteriol 191, 4259–4267. 10.1128/JB.01730-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocker A., Gounon P., Larquet E., Niebuhr K., Cabiaux V., Parsot C., Sansonetti P. (1999). The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J Cell Biol 147, 683–693. 10.1083/jcb.147.3.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocker A., Jouihri N., Larquet E., Gounon P., Ebel F., Parsot C., Sansonetti P., Allaoui A. (2001). Structure and composition of the Shigella flexneri “needle complex”, a part of its type III secreton. Mol Microbiol 39, 652–663. 10.1046/j.1365-2958.2001.02200.x [DOI] [PubMed] [Google Scholar]
- Blocker A., Komoriya K., Aizawa S. (2003). Type III secretion systems and bacterial flagella: insights into their function from structural similarities. Proc Natl Acad Sci U S A 100, 3027–3030. 10.1073/pnas.0535335100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteaux A., Sani M., Kayath C. A., Boekema E. J., Allaoui A. (2008). Spa32 interaction with the inner-membrane Spa40 component of the type III secretion system of Shigella flexneri is required for the control of the needle length by a molecular tape measure mechanism. Mol Microbiol 70, 1515–1528. 10.1111/j.1365-2958.2008.06499.x [DOI] [PubMed] [Google Scholar]
- Botteaux A., Kayath C. A., Page A. L., Jouihri N., Sani M., Boekema E., Biskri L., Parsot C., Allaoui A. (2010). The 33 carboxyl-terminal residues of Spa40 orchestrate the multi-step assembly process of the type III secretion needle complex in Shigella flexneri. Microbiology 156, 2807–2817. 10.1099/mic.0.039651-0 [DOI] [PubMed] [Google Scholar]
- Buchrieser C., Glaser P., Rusniok C., Nedjari H., D’Hauteville H., Kunst F., Sansonetti P., Parsot C. (2000). The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol Microbiol 38, 760–771. 10.1046/j.1365-2958.2000.02179.x [DOI] [PubMed] [Google Scholar]
- Chevance F. F., Hughes K. T. (2008). Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol 6, 455–465. 10.1038/nrmicro1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G. R. (2006). The type III secretion injectisome. Nat Rev Microbiol 4, 811–825. 10.1038/nrmicro1526 [DOI] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97, 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane J. E., Graham S. C., Mitchell E. P., Flot D., Johnson S., Lea S. M. (2008). Crystal structure of Spa40, the specificity switch for the Shigella flexneri type III secretion system. Mol Microbiol 69, 267–276. 10.1111/j.1365-2958.2008.06293.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykxhoorn D. M., St Pierre R., Linn T. (1996). A set of compatible tac promoter expression vectors. Gene 177, 133–136. 10.1016/0378-1119(96)00289-2 [DOI] [PubMed] [Google Scholar]
- Edqvist P. J., Olsson J., Lavander M., Sundberg L., Forsberg A., Wolf-Watz H., Lloyd S. A. (2003). YscP and YscU regulate substrate specificity of the Yersinia type III secretion system. J Bacteriol 185, 2259–2266. 10.1128/JB.185.7.2259-2266.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt M., Singer H. M., Wee D. H., Keener J. P., Hughes K. T. (2011). An infrequent molecular ruler controls flagellar hook length in Salmonella enterica. EMBO J 30, 2948–2961. 10.1038/emboj.2011.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris H. U., Minamino T. (2006). Flipping the switch: bringing order to flagellar assembly. Trends Microbiol 14, 519–526. 10.1016/j.tim.2006.10.006 [DOI] [PubMed] [Google Scholar]
- Ferris H. U., Furukawa Y., Minamino T., Kroetz M. B., Kihara M., Namba K., Macnab R. M. (2005). FlhB regulates ordered export of flagellar components via autocleavage mechanism. J Biol Chem 280, 41236–41242. 10.1074/jbc.M509438200 [DOI] [PubMed] [Google Scholar]
- Fraser G. M., Hirano T., Ferris H. U., Devgan L. L., Kihara M., Macnab R. M. (2003). Substrate specificity of type III flagellar protein export in Salmonella is controlled by subdomain interactions in FlhB. Mol Microbiol 48, 1043–1057. 10.1046/j.1365-2958.2003.03487.x [DOI] [PubMed] [Google Scholar]
- Frye J., Karlinsey J. E., Felise H. R., Marzolf B., Dowidar N., McClelland M., Hughes K. T. (2006). Identification of new flagellar genes of Salmonella enterica serovar Typhimurium. J Bacteriol 188, 2233–2243. 10.1128/JB.188.6.2233-2243.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Lane D. (1988). Storing and purifying antibodies. Immunoaffinity purification of antibodies. In Antibodies: a Laboratory Manual, pp. 312–318. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- Hirano T., Yamaguchi S., Oosawa K., Aizawa S. (1994). Roles of FliK and FlhB in determination of flagellar hook length in Salmonella typhimurium. J Bacteriol 176, 5439–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet L., Agrain C., Broz P., Cornelis G. R. (2003). The needle length of bacterial injectisomes is determined by a molecular ruler. Science 302, 1757–1760. 10.1126/science.1091422 [DOI] [PubMed] [Google Scholar]
- Kenjale R., Wilson J., Zenk S. F., Saurya S., Picking W. L., Picking W. D., Blocker A. (2005). The needle component of the type III secreton of Shigella regulates the activity of the secretion apparatus. J Biol Chem 280, 42929–42937. 10.1074/jbc.M508377200 [DOI] [PubMed] [Google Scholar]
- Kotloff K. L., Winickoff J. P., Ivanoff B., Clemens J. D., Swerdlow D. L., Sansonetti P. J., Adak G. K., Levine M. M. (1999). Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ 77, 651–666. [PMC free article] [PubMed] [Google Scholar]
- Kubori T., Matsushima Y., Nakamura D., Uralil J., Lara-Tejero M., Sukhan A., Galán J. E., Aizawa S. I. (1998). Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280, 602–605. 10.1126/science.280.5363.602 [DOI] [PubMed] [Google Scholar]
- Lavander M., Sundberg L., Edqvist P. J., Lloyd S. A., Wolf-Watz H., Forsberg A. (2002). Proteolytic cleavage of the FlhB homologue YscU of Yersinia pseudotuberculosis is essential for bacterial survival but not for type III secretion. J Bacteriol 184, 4500–4509. 10.1128/JB.184.16.4500-4509.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall T., Mavris M., Martino M. C., Bernardini M. L., Denamur E., Parsot C. (2005). Analysis of virulence plasmid gene expression defines three classes of effectors in the type III secretion system of Shigella flexneri. Microbiology 151, 951–962. 10.1099/mic.0.27639-0 [DOI] [PubMed] [Google Scholar]
- Lorenz C., Büttner D. (2011). Secretion of early and late substrates of the type III secretion system from Xanthomonas is controlled by HpaC and the C-terminal domain of HrcU. Mol Microbiol 79, 447–467. 10.1111/j.1365-2958.2010.07461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lountos G. T., Austin B. P., Nallamsetty S., Waugh D. S. (2009). Atomic resolution structure of the cytoplasmic domain of Yersinia pestis YscU, a regulatory switch involved in type III secretion. Protein Sci 18, 467–474. 10.1002/pro.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdalena J., Hachani A., Chamekh M., Jouihri N., Gounon P., Blocker A., Allaoui A. (2002). Spa32 regulates a switch in substrate specificity of the type III secreton of Shigella flexneri from needle components to Ipa proteins. J Bacteriol 184, 3433–3441. 10.1128/JB.184.13.3433-3441.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makishima S., Komoriya K., Yamaguchi S., Aizawa S. I. (2001). Length of the flagellar hook and the capacity of the type III export apparatus. Science 291, 2411–2413. 10.1126/science.1058366 [DOI] [PubMed] [Google Scholar]
- Marlovits T. C., Kubori T., Sukhan A., Thomas D. R., Galán J. E., Unger V. M. (2004). Structural insights into the assembly of the type III secretion needle complex. Science 306, 1040–1042. 10.1126/science.1102610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlovits T. C., Kubori T., Lara-Tejero M., Thomas D., Unger V. M., Galán J. E. (2006). Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature 441, 637–640. 10.1038/nature04822 [DOI] [PubMed] [Google Scholar]
- Martinez-Argudo I., Blocker A. J. (2010). The Shigella T3SS needle transmits a signal for MxiC release, which controls secretion of effectors. Mol Microbiol 78, 1365–1378. 10.1111/j.1365-2958.2010.07413.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Blackmon B., Curtiss R., III (1984). Temperature-dependent expression of virulence genes in Shigella species. Infect Immun 43, 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitert T., Pencu E., Ciudin L., Tonciu M., Mihai I., Nicolescu S. (1991). Correlation between Congo red binding as virulence marker in Shigella species and Sereny test. Roum Arch Microbiol Immunol 50, 45–52. [PubMed] [Google Scholar]
- Ménard R., Sansonetti P. J., Parsot C. (1993). Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol 175, 5899–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T., Macnab R. M. (2000). Domain structure of Salmonella FlhB, a flagellar export component responsible for substrate specificity switching. J Bacteriol 182, 4906–4914. 10.1128/JB.182.17.4906-4914.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T., Saijo-Hamano Y., Furukawa Y., González-Pedrajo B., Macnab R. M., Namba K. (2004). Domain organization and function of Salmonella FliK, a flagellar hook-length control protein. J Mol Biol 341, 491–502. 10.1016/j.jmb.2004.06.012 [DOI] [PubMed] [Google Scholar]
- Minamino T., Ferris H. U., Moriya N., Kihara M., Namba K. (2006). Two parts of the T3S4 domain of the hook-length control protein FliK are essential for the substrate specificity switching of the flagellar type III export apparatus. J Mol Biol 362, 1148–1158. 10.1016/j.jmb.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Minamino T., Imada K., Namba K. (2008). Mechanisms of type III protein export for bacterial flagellar assembly. Mol Biosyst 4, 1105–1115. 10.1039/b808065h [DOI] [PubMed] [Google Scholar]
- Minamino T., Moriya N., Hirano T., Hughes K. T., Namba K. (2009). Interaction of FliK with the bacterial flagellar hook is required for efficient export specificity switching. Mol Microbiol 74, 239–251. 10.1111/j.1365-2958.2009.06871.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno S., Amida H., Kobayashi N., Aizawa S., Tate S. (2011). The NMR structure of FliK, the trigger for the switch of substrate specificity in the flagellar type III secretion apparatus. J Mol Biol 409, 558–573. 10.1016/j.jmb.2011.04.008 [DOI] [PubMed] [Google Scholar]
- Moriya N., Minamino T., Hughes K. T., Macnab R. M., Namba K. (2006). The type III flagellar export specificity switch is dependent on FliK ruler and a molecular clock. J Mol Biol 359, 466–477. 10.1016/j.jmb.2006.03.025 [DOI] [PubMed] [Google Scholar]
- Morris D. P., Roush E. D., Thompson J. W., Moseley M. A., Murphy J. W., McMurry J. L. (2010). Kinetic characterization of Salmonella FliK–FlhB interactions demonstrates complexity of the Type III secretion substrate-specificity switch. Biochemistry 49, 6386–6393. 10.1021/bi100487p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota L. J., Journet L., Sorg I., Agrain C., Cornelis G. R. (2005). Bacterial injectisomes: needle length does matter. Science 307, 1278. 10.1126/science.1107679 [DOI] [PubMed] [Google Scholar]
- Ohnishi K., Ohto Y., Aizawa S., Macnab R. M., Iino T. (1994). FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium. J Bacteriol 176, 2272–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsot C. (2009). Shigella type III secretion effectors: how, where, when, for what purposes? Curr Opin Microbiol 12, 110–116. 10.1016/j.mib.2008.12.002 [DOI] [PubMed] [Google Scholar]
- Parsot C., Ageron E., Penno C., Mavris M., Jamoussi K., d’Hauteville H., Sansonetti P., Demers B. (2005). A secreted anti-activator, OspD1, and its chaperone, Spa15, are involved in the control of transcription by the type III secretion apparatus activity in Shigella flexneri. Mol Microbiol 56, 1627–1635. 10.1111/j.1365-2958.2005.04645.x [DOI] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Stocker B. A., Yamaguchi S. (1973). A new fla gene in Salmonella typhimurium – flaR – and its mutant phenotype-superhooks. Arch Mikrobiol 90, 107–120. 10.1007/BF00414513 [DOI] [PubMed] [Google Scholar]
- Phalipon A., Arondel J., Nato F., Rouyre S., Mazie J. C., Sansonetti P. J. (1992). Identification and characterization of B-cell epitopes of IpaC, an invasion-associated protein of Shigella flexneri. Infect Immun 60, 1919–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyraz O., Schmidt H., Seidel K., Delissen F., Ader C., Tenenboim H., Goosmann C., Laube B., Thünemann A. F. & other authors (2010). Protein refolding is required for assembly of the type three secretion needle. Nat Struct Mol Biol 17, 788–792. 10.1038/nsmb.1822 [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. (1982). Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun 35, 852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder G. N., Hilbi H. (2008). Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev 21, 134–156. 10.1128/CMR.00032-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D. K., Saurya S., Wagner C., Nishioka H., Blocker A. J. (2010). Domains of the Shigella flexneri type III secretion system IpaB protein involved in secretion regulation. Infect Immun 78, 4999–5010. 10.1128/IAI.00470-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S., Takahashi N., Chevance F. F., Karlinsey J. E., Hughes K. T., Aizawa S. (2007). FliK regulates flagellar hook length as an internal ruler. Mol Microbiol 64, 1404–1415. 10.1111/j.1365-2958.2007.05750.x [DOI] [PubMed] [Google Scholar]
- Sorg I., Wagner S., Amstutz M., Müller S. A., Broz P., Lussi Y., Engel A., Cornelis G. R. (2007). YscU recognizes translocators as export substrates of the Yersinia injectisome. EMBO J 26, 3015–3024. 10.1038/sj.emboj.7601731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Iino T. (1981). Role of the flaR gene in flagellar hook formation in Salmonella spp. J Bacteriol 148, 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamano K., Aizawa S., Katayama E., Nonaka T., Imajoh-Ohmi S., Kuwae A., Nagai S., Sasakawa C. (2000). Supramolecular structure of the Shigella type III secretion machinery: the needle part is changeable in length and essential for delivery of effectors. EMBO J 19, 3876–3887. 10.1093/emboj/19.15.3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamano K., Katayama E., Toyotome T., Sasakawa C. (2002). Shigella Spa32 is an essential secretory protein for functional type III secretion machinery and uniformity of its needle length. J Bacteriol 184, 1244–1252. 10.1128/JB.184.5.1244-1252.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenendaal A. K., Hodgkinson J. L., Schwarzer L., Stabat D., Zenk S. F., Blocker A. J. (2007). The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol Microbiol 63, 1719–1730. 10.1111/j.1365-2958.2007.05620.x [DOI] [PubMed] [Google Scholar]
- Wagner S., Stenta M., Metzger L. C., Dal Peraro M., Cornelis G. R. (2010). Length control of the injectisome needle requires only one molecule of Yop secretion protein P (YscP). Proc Natl Acad Sci U S A 107, 13860–13865. 10.1073/pnas.1006985107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West N. P., Sansonetti P., Mounier J., Exley R. M., Parsot C., Guadagnini S., Prévost M. C., Prochnicka-Chalufour A., Delepierre M. & other authors (2005). Optimization of virulence functions through glucosylation of Shigella LPS. Science 307, 1313–1317. 10.1126/science.1108472 [DOI] [PubMed] [Google Scholar]
- Wiesand U., Sorg I., Amstutz M., Wagner S., van den Heuvel J., Lührs T., Cornelis G. R., Heinz D. W. (2009). Structure of the type III secretion recognition protein YscU from Yersinia enterocolitica. J Mol Biol 385, 854–866. 10.1016/j.jmb.2008.10.034 [DOI] [PubMed] [Google Scholar]
- Zarivach R., Deng W., Vuckovic M., Felise H. B., Nguyen H. V., Miller S. I., Finlay B. B., Strynadka N. C. (2008). Structural analysis of the essential self-cleaving type III secretion proteins EscU and SpaS. Nature 453, 124–127. 10.1038/nature06832 [DOI] [PubMed] [Google Scholar]
- Zenk S. F., Stabat D., Hodgkinson J. L., Veenendaal A. K., Johnson S., Blocker A. J. (2007). Identification of minor inner-membrane components of the Shigella type III secretion system ‘needle complex’. Microbiology 153, 2405–2415. 10.1099/mic.0.2007/007781-0 [DOI] [PubMed] [Google Scholar]
- Zhu K., González-Pedrajo B., Macnab R. M. (2002). Interactions among membrane and soluble components of the flagellar export apparatus of Salmonella. Biochemistry 41, 9516–9524. 10.1021/bi0203280 [DOI] [PubMed] [Google Scholar]