Abstract
Mycobacterium tuberculosis (Mtb) is thought to preferentially rely on fatty acid metabolism to both establish and maintain chronic infections. Its metabolic network, however, allows efficient co-catabolism of multiple carbon substrates. To gain insight into the importance of carbohydrate substrates for Mtb pathogenesis we evaluated the role of glucose phosphorylation, the first reaction in glycolysis. We discovered that Mtb expresses two functional glucokinases. Mtb required the polyphosphate glucokinase PPGK for normal growth on glucose, while its second glucokinase GLKA was dispensable. 13C-based metabolomic profiling revealed that both enzymes are capable of incorporating glucose into Mtb's central carbon metabolism, with PPGK serving as dominant glucokinase in wild type (wt) Mtb. When both glucokinase genes, ppgK and glkA, were deleted from its genome, Mtb was unable to use external glucose as substrate for growth or metabolism. Characterization of the glucokinase mutants in mouse infections demonstrated that glucose phosphorylation is dispensable for establishing infection in mice. Surprisingly, however, the glucokinase double mutant failed to persist normally in lungs, which suggests that Mtb has access to glucose in vivo and relies on glucose phosphorylation to survive during chronic mouse infections.
Author Summary
The development of new drugs targeting Mycobacterium tuberculosis (Mtb) will benefit from a better understanding of the mechanisms by which this pathogen establishes and maintains chronic infections. Mtb has to adapt its metabolic needs to the nutritional environment in the host. We investigated the role of glucose phosphorylation and discovered that Mtb expresses two functional glucokinases. Using 13C-tracing experiments we demonstrated that both enzymes are competent to incorporate glucose into central carbon metabolism. In agreement with the view that Mtb metabolizes fatty acids to grow in vivo, both enzymes were dispensable for Mtb replication in mouse lungs and spleens. Surprisingly, however, the glucokinase double mutant was attenuated during the chronic phase of mouse infections. These studies suggest that Mtb metabolizes glucose in vivo and that its survival in chronically infected mice depends on glucose phosphorylation.
Introduction
Mycobacterium tuberculosis (Mtb) is the causative agent of tuberculosis, a disease that killed 1.5 million people in 2010 worldwide [1]. The development of new and effective antimycobacterial drugs hinges on our ability to understand how Mtb establishes and maintains chronic infections. Mtb's metabolic adaptations are central to its pathogenicity and prevailing evidence has strongly implicated fatty acids and lipids for biomass and energy production during infection [2]–[6]. In contrast, the importance of other carbon sources including carbohydrates during in vivo growth and persistence of Mtb remains uncertain. Mtb possesses carbohydrate transporters and the enzymes required to metabolize sugars [7], [8], but it is unclear whether it utilizes these sugar metabolism pathways to support growth in vivo. Mtb is not thought to encounter a sugar-rich environment inside the host, given that expression of sugar catabolism genes was not induced during infection of macrophages and mice [9], [10]. However, sugar transport appears important in Mtb pathogenesis as mutants with transposon insertions in carbohydrate transport systems were attenuated in mouse spleens [11]. The LpqY-SugA-SugB-SugC carbohydrate transporter was confirmed to be indispensable for normal growth in mouse lungs and spleens [12]. This transporter was shown to be highly specific for uptake of the disaccharide trehalose. Trehalose is not present in mammals, but can be released by Mtb from trehalose-containing cell wall glycolipids and recycled [12]. The intracellular fate of recycled trehalose remains to be identified, and it is unknown which other sugars are metabolized by Mtb to maintain fitness during infection.
We sought to investigate the role of glucose metabolism for Mtb pathogenesis and approached this by studying glucokinase, the enzyme catalyzing the phosphorylation of glucose to produce glucose-6-phosphate. This phosphorylation reaction constitutes the first committed step in glucose metabolism activating glucose for oxidation through glycolysis. The Mtb genome contains ppgK (Rv2702) coding for a polyphosphate glucokinase [7]. Biochemical characterization showed that PPGK catalyzes the direct phosphorylation of glucose using a broad range of phosphoryl donors with a preference for polyphosphate (PolyP) and has a high affinity for the substrate glucose (Km = 0.28 mM) [13], [14]. We generated a ppgK deletion mutant in Mtb H37Rv and found that it was severely attenuated for growth with glucose. The mutant, however, retained the ability to grow slowly with glucose as sole carbon source and to incorporate glucose into central carbon metabolism. This led to the identification of a second glucokinase, GLKA, encoded by Rv0650. We demonstrate that both PPGK and GLKA are functional glucokinases that allow Mtb to metabolize glucose. Glucose-6-phosphate production by GLKA was, however, low and not sufficient for normal growth of Mtb on glucose. Thus, Mtb primarily relies on PPGK activity for growth on glucose as the sole carbon source in vitro. Mtb missing both PPGK and GLKA failed to persist normally in mouse lungs suggesting that glucose is available to Mtb in vivo and that Mtb depends on glucose phosphorylation to survive during chronic mouse infection.
Results
Mtb expresses two functional glucokinases but relies predominantly on PPGK for growth on glucose
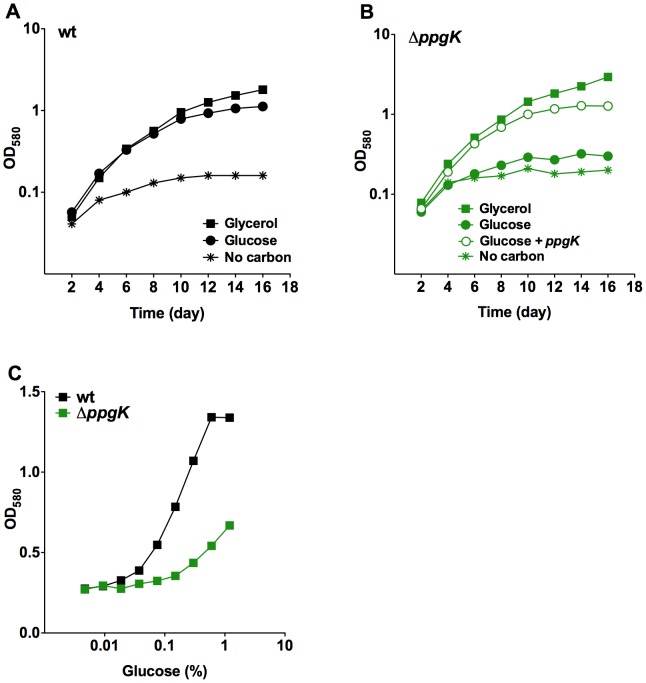
To investigate how PPGK contributes to the metabolism of glucose in Mtb we constructed a ppgK deletion mutant (Figure S1A,B) and measured its growth in carbon defined liquid media (Figure 1). Replication of the PPGK mutant (ΔppgK) was severely attenuated compared to wt with glucose as the sole carbon source (Figure 1A,B). The growth defect of ΔppgK in glucose containing medium was restored when a copy of ppgK was reintroduced into the genome of the mutant (Figure 1B). We noticed that ΔppgK displayed modest, yet reproducible, replication in glucose containing liquid medium above the no-carbon control. Growth of ΔppgK was also responsive to increasing glucose concentrations in the culture medium (Figure 1C) suggesting that a redundant enzyme mediated some glucose phosphorylation in the absence of PPGK.
Figure 1. PpgK is required for normal growth with glucose as sole carbon source.
Growth of wt Mtb (A) and ΔppgK (B) in carbon defined media with 0.4% glycerol (squares), 0.4% glucose (circles) or no carbon (asterisks). Open circles depict growth of the complemented mutant in glucose containing medium. (C) Growth of wt and ΔppgK in response to increasing glucose concentration. Data represent two to three independent experiments.
The Mtb genome contains a second possible sugar kinase gene, Rv0650 [7]. Rv0650's amino acid sequence is 71% identical to the GLKA homologue from Mycobacterium smegmatis, which was shown to phosphorylate glucose using ATP as the phosphoryl donor [15], but shares only 22% sequence identity with PPGK (Figure S2). To test whether Rv0650 can compensate for the lack of PPGK we expressed the gene from a multicopy plasmid using a strong promoter. This complemented the growth defect of the ppgK mutant (Figure 2A) suggesting that Rv0650 encodes a functional glucokinase that can phosphorylate glucose to support Mtb growth. Based on protein sequence homology to the M. smegmatis GLKA and the functional analysis presented in this study we have named the Rv0650 encoded protein GLKA. In wt Mtb glkA transcript levels were similar to those of ppgk (Figure S3), suggesting that a difference in mRNA expression does not explain why GLKA could not compensate for the lack of PPGK. We generated a glkA deletion mutant and ppgK/glkA double deletion mutant (Figure S1C,D). Growth of ΔglkA was indistinguishable from that of wt independently of the carbon source (Figure 2B,D) demonstrating that PPGK is sufficient for Mtb to utilize glucose as carbon source for growth.
Figure 2. Rv0650 (GLKA) can mediate growth on glucose.
(A) Growth of ΔppgK and ΔppgK complemented with ppgK on an integrative plasmid or with glkA on an episomal plasmid in carbon defined media with 0.4% glucose. (B, C) Growth of ΔglkA (B) and ΔppgKΔglkA (C) in carbon defined media with 0.4% glycerol (squares), 0.4% glucose (circles) or no carbon (asterisks). Open circles depict growth of the ΔppgKΔglkA mutant complemented with ppgK in glucose containing medium. (D) Glucose dose response in liquid media of wt, ΔglkA and ΔppgK ΔglkA. (E, F) Growth of wt (E) and ΔppgKΔglkA (F) in carbon defined media with 0.2% acetate and 0.2% acetate+0.2% glucose. Data represent two to three independent experiments.
Growth on glucose as sole carbon source was, however, completely abolished when both glucokinase orthologues ppgK and glkA were deleted from the Mtb genome (Figure 2C) and ΔppgKΔglkA did not respond to increasing glucose concentration (Figure 2D). Expression of ppgK from an integrative plasmid using its putative native promoter restored replication (Figure 2C). As expected, growth of ΔppgKΔglkA on carbon sources generating glucose-6-phosphate through gluconeogenesis was similar to that of wt Mtb (Figure 2E,F and data not shown). Addition of glucose increased the replication rate of wt Mtb on acetate as previously reported [16] (Figure 2E) but did not affect replication of ΔppgKΔglkA in acetate containing medium (Figure 2F). Together, these data indicate that Mtb expresses two functional glucokinases, but relies primarily on PPGK to produce energy and biomass from glucose. The carbon mixture growth experiment also revealed that glucose did not cause toxicity or interfere with the metabolism of a gluconeogenic carbon substrate when Mtb was missing both glucokinases.
PPGK and GLKA can incorporate glucose into Mtb's central carbon metabolism
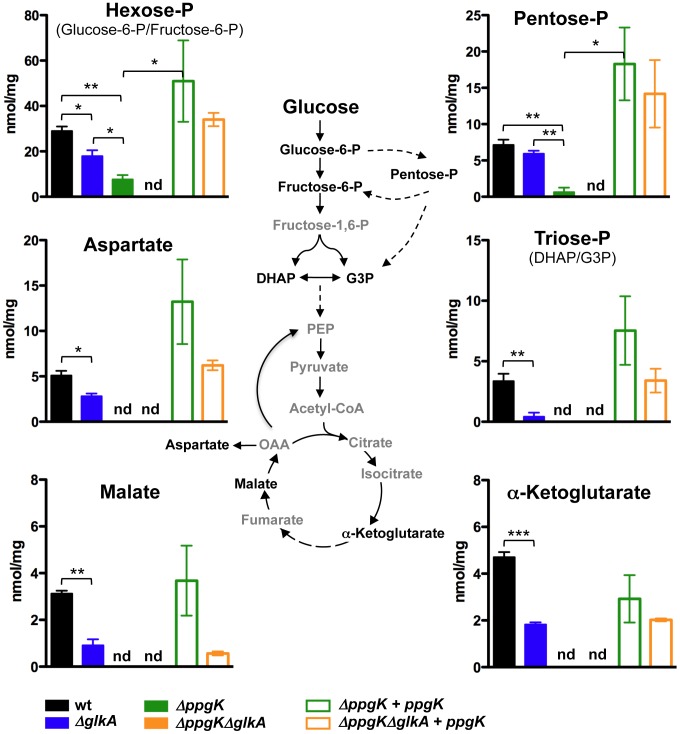
We studied the incorporation of glucose-derived carbon into central carbon metabolism to gain further insights into Mtb's glucokinases. We examined uniformly-(U)13C-labled glucose incorporation into metabolites representative of glycolysis (hexose-6-phosphate (P) and triose-P), pentose phosphate pathway (pentose-P) and the tricaboxylic acid (TCA) cycle (aspartate, malate and α-ketoglutarate). As previously described [16], wt Mtb incorporated U-13C-glucose-derived carbons into all the examined central carbon metabolism intermediates (Figure 3). Incorporation of U-13C glucose was abolished in ΔppgKΔglkA reflected by the lack of detectable incorporation of U-13C glucose-derived carbon into any of the examined central carbon metabolism intermediates (Figure 3). Expression of ppgK restored glucose metabolism in ΔppgKΔglkA. This confirmed that Mtb depends on PPGK and GLKA and has no alternative metabolic route for glucose assimilation.
Figure 3. Both PPGK and GLKA mediate glucose incorporation into central carbon metabolism.
Schematic illustration of the metabolic pathways studied using carbon tracing analysis and isotopic incorporation of U13C-glucose into the intracellular pool of selected metabolites. Isotopic labeling is indicated on the y-axis as nmol labeled/mg protein/16 h labeling interval. Each bar represents the mean of three sample replicates and error bars indicate standard deviation from the mean. nd = not detected. * P≤0.05, ** P≤0.01, *** P≤0.001. Data are representative of two independent experiments.
We examined the contribution of each glucokinase ortholog to glucose incorporation. Metabolism of U-13C glucose into intermediates of central carbon metabolism was reduced but not abolished in ΔglkA compared to wt (Figure 3). We observed a more pronounced decrease of U-13C glucose incorporation when Mtb was missing PPGK (Figure 3). Incorporation of U-13C-glucose-derived carbon into intermediates of glycolysis (hexose-P and triose-P) and the pentose phosphate pathway (pentose-P) was significantly reduced in Δppgk and we could not detect 13C incorporation into the TCA cycle metabolites α-ketoglutarate, malate or aspartate (Figure 3). Enzymatic quantification confirmed that both glucose-6-P and fructose-6-P pools were reduced in Δppgk after incubation with glucose as single carbon source (Table S1). Together, these data demonstrate that GLKA is enzymatically competent to phosphorylate glucose, but this activity is not sufficient to support efficient replication on glucose in the absence of PPGK. Hence, GLKA activity cannot meet the cell's demand for energy and biomass production when glucose is the sole carbon source. In conclusion, both PPGK and GLKA can phosphorylate glucose, but PPGK has a predominant role in metabolizing glucose.
The glucokinase double mutant is attenuated during the chronic phase of infection in mice
It is unknown whether Mtb has access and metabolizes glucose during growth or persistence in mice and the glucokinase mutants allowed investigating this question. Following low dose aerosol infection ΔppgKΔglkA replicated and established a bacterial load in mice lungs similar to wt Mtb (Figure 4A). However, between days 21 and 56 the ΔppgKΔglkA titer in the lung decreased 1.0 log and further declined 0.25 log between days 56 and 112, indicating that in the absence of PPGK and GLKA Mtb was unable to adapt like wt Mtb to the changes in the host environment associated with the onset of adaptive immunity. Restoring ppgK expression complemented the persistence defect of ΔppgKΔglkA. Deletion of either GLKA or PPGK did not impact growth or survival of Mtb in the mouse suggesting that the enzymes can compensate for each other to maintain Mtb fitness during the chronic phase of infection. None of the glucokinase mutants were attenuated in mouse spleens (Figure 4B). These observations revealed that Mtb relies on glucose phosphorylation for normal persistence in mouse lungs but not spleens.
Figure 4. Glucose phosphorylation is required for mycobacterial persistence in lungs of chronically infected mice.
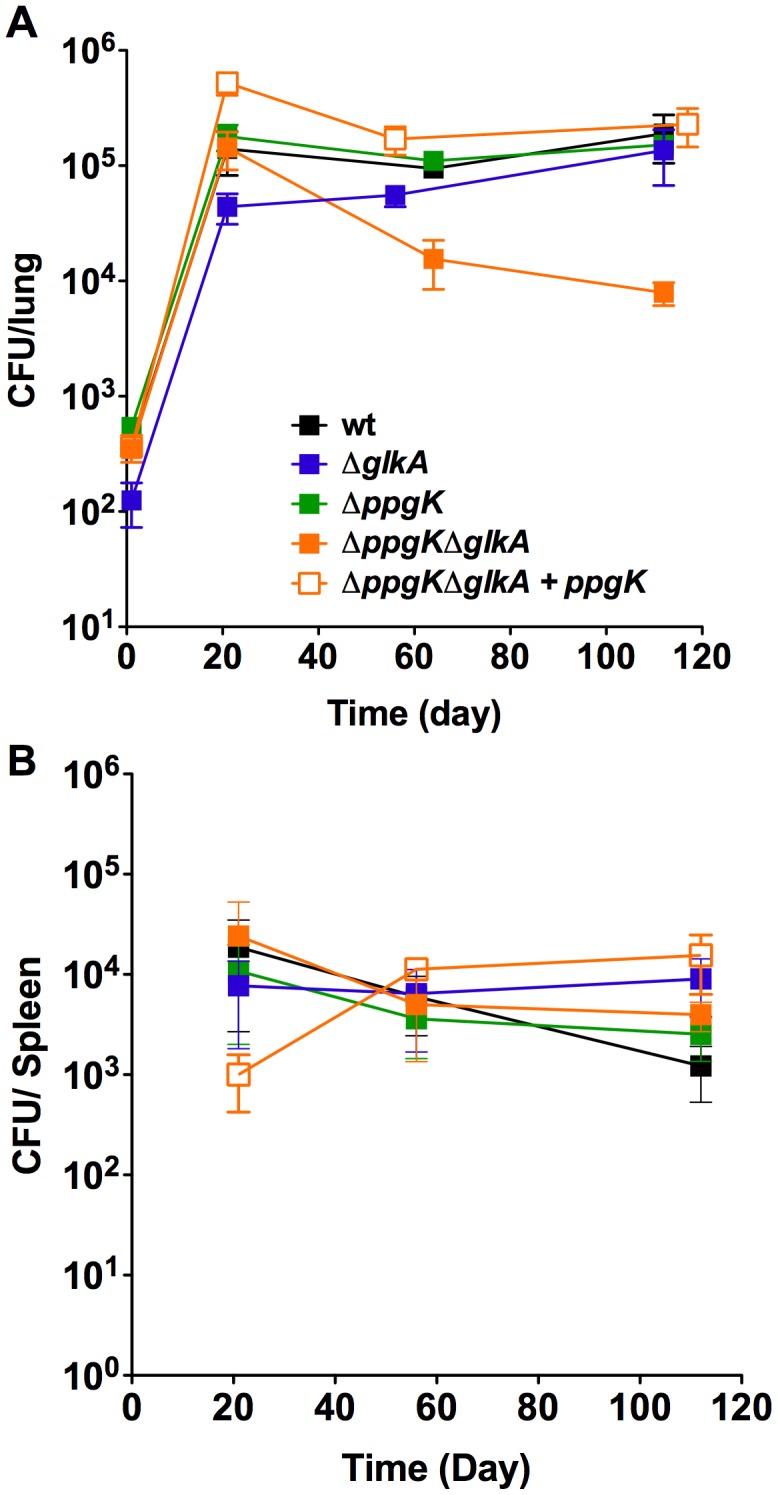
Bacterial titers in lungs (A) and spleens (B) from mice infected with wt, glucokinase mutants and complemented mutant. Data are means ± s.d. from four mice per time point per group and represent two independent experiments.
Loss of glucokinase activity does not impair trehalose metabolism or survival during nutrient starvation
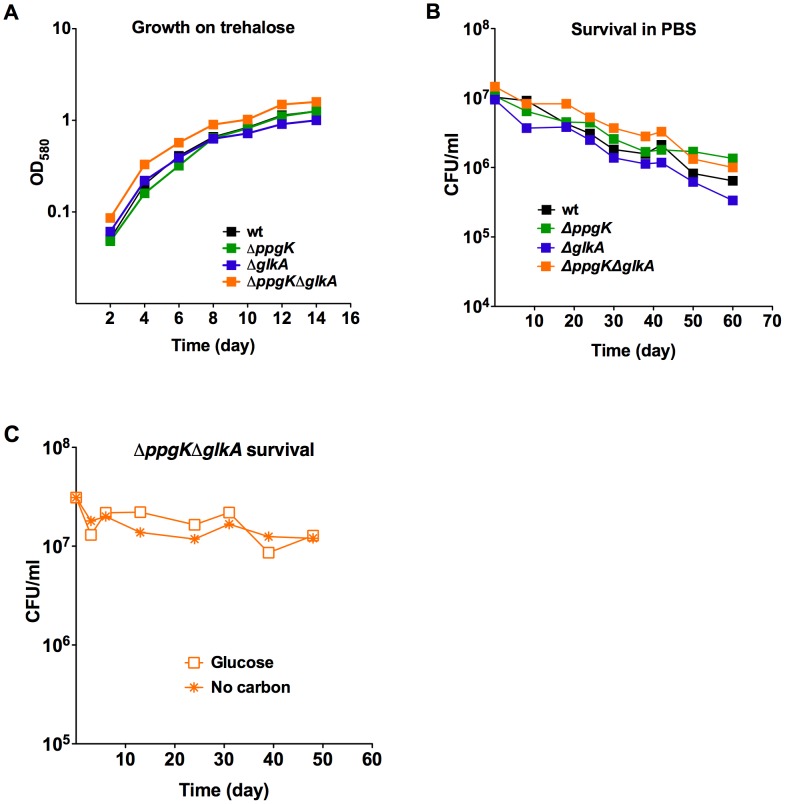
Retrograde transport of trehalose was shown to be necessary for Mtb virulence in mice [12]. The authors speculated that trehalose transport could fulfill several metabolic needs including supplying Mtb with a carbon source for growth. The disaccharide trehalose consists of two glucose units and can be converted to glucose by trehalase [17]. We tested whether loss of PPGK and GLKA impacted Mtb's ability to utilize trehalose for growth in vitro (Figure 5A). The single and double glucokinase mutants replicated like wt Mtb using trehalose as the sole carbon source demonstrating that Mtb metabolizes trehalose without the need for glucokinase activity. Thus, attenuation of the glucokinase double mutant in vivo was not due to an inability to utilize trehalose as a carbon source.
Figure 5. Gluokinases are dispensable for trehalose metabolism and survival during starvation.
(A) Growth in carbon defined media with 0.4% trehalose. (B) Survival of wt, ΔglkA, ΔppgK, and ΔppgKΔglkA in PBS. (C) Survival of ΔppgKΔglkA in carbon defined media containing 0.4% glucose or no carbon.
Mtb lacking the stringent response enzyme RelMtb was unable to survive prolonged periods of starvation in vitro and was attenuated in the chronic phase of infection in mice and guinea pigs [18]–[20]. This work highlighted that adaptation to starvation is an important aspect of Mtb's pathogenicity. We therefore investigated whether loss of glucokinase activity impaired Mtb's ability to adapt to conditions of nutrient starvation and determined whether the glucokinase double mutant was able to survive 60 days of incubation in nutrient free phosphate buffered saline (PBS). Survival of ΔppgKΔglkA in this starvation medium was indistinguishable from wt (Figure 5B) indicating that Mtb's ability to survive nutrient starvation does not depend on glucokinase activity. We also tested whether glucose caused toxicity in ΔppgKΔglkA, which cannot phosphorylate glucose. The glucokinase double mutant was fully viable during prolonged incubation in medium containing glucose as the sole carbon source (Figure 5C). Taken together these experiments revealed that Mtb does not employ glucose metabolism to adapt to starvation.
Increased susceptibility to oxidative stress is not responsible for the persistence defect of the glucokinase double mutant
Mtb persistence in chronically infected mice is achieved through resistance to a diverse array of stresses imposed by the host adaptive immune response [21], [22]. Glucose-6-P has been implicated in resistance of M. smegmatis to oxidative stress potentially by serving as a source of reducing power [23]. We investigated whether the Mtb glucokinase mutants were impaired in their ability to resist stresses encountered in the host, such as reactive oxygen species, reactive nitrogen species and low pH. The glucokinase mutants survived similar to wt after exposure to low pH and reactive nitrogen species (Figure 6A). However, Mtb lacking both glucokinases was killed 1 log more than wt after exposure to hydrogen peroxide (H2O2). This phenotype was fully complemented when ppgK expression was restored using an integrative plasmid indicating that the impaired survival after exposure to H2O2 was solely due to loss of glucokinase activity. This hypersusceptibility was not due inability to induce expression of genes involved resistance to oxidative stress (Table S2). Moreover, KatG and SodA protein amounts were similar in wt and ΔppgKΔglkA (Figure S4). Resistance to H2O2 mediated oxidative stress was previously shown to be important for Mtb virulence during the chronic phase of infection. Mtb lacking catalase KatG, was hypersuceptible to H2O2 and attenuated specifically in the chronic phase of infection [24]. In vivo attenuation was rescued in mice lacking NADPH oxidase (gp91Phox−/−). Thus, removal of oxidative species production by the host NADPH oxidase bypassed the need of catalase for Mtb pathogenesis. To determine if attenuation of ΔppgKΔglkA was dependent on the host respiratory burst we infected gp91Phox−/− mice. Similar to the phenotype in wt mice, viability of ΔppgKΔglkA decreased by 1 log between days 21 and 56 in gp91Phox−/− mouse lungs (Figure 6B) indicating that phagocyte oxidase is dispensable for killing ΔppgKΔglkA. These data suggest that the in vitro observed increased susceptibility to H2O2 does not contribute to the attenuation of Mtb lacking glucokinases pointing to a metabolic requirement for glucose-6-P during survival in chronically infected mice.
Figure 6. The glucokinase double mutant is hypersusceptible to hydrogen peroxide, but attenuated in phagocyte oxidase deficient mice.
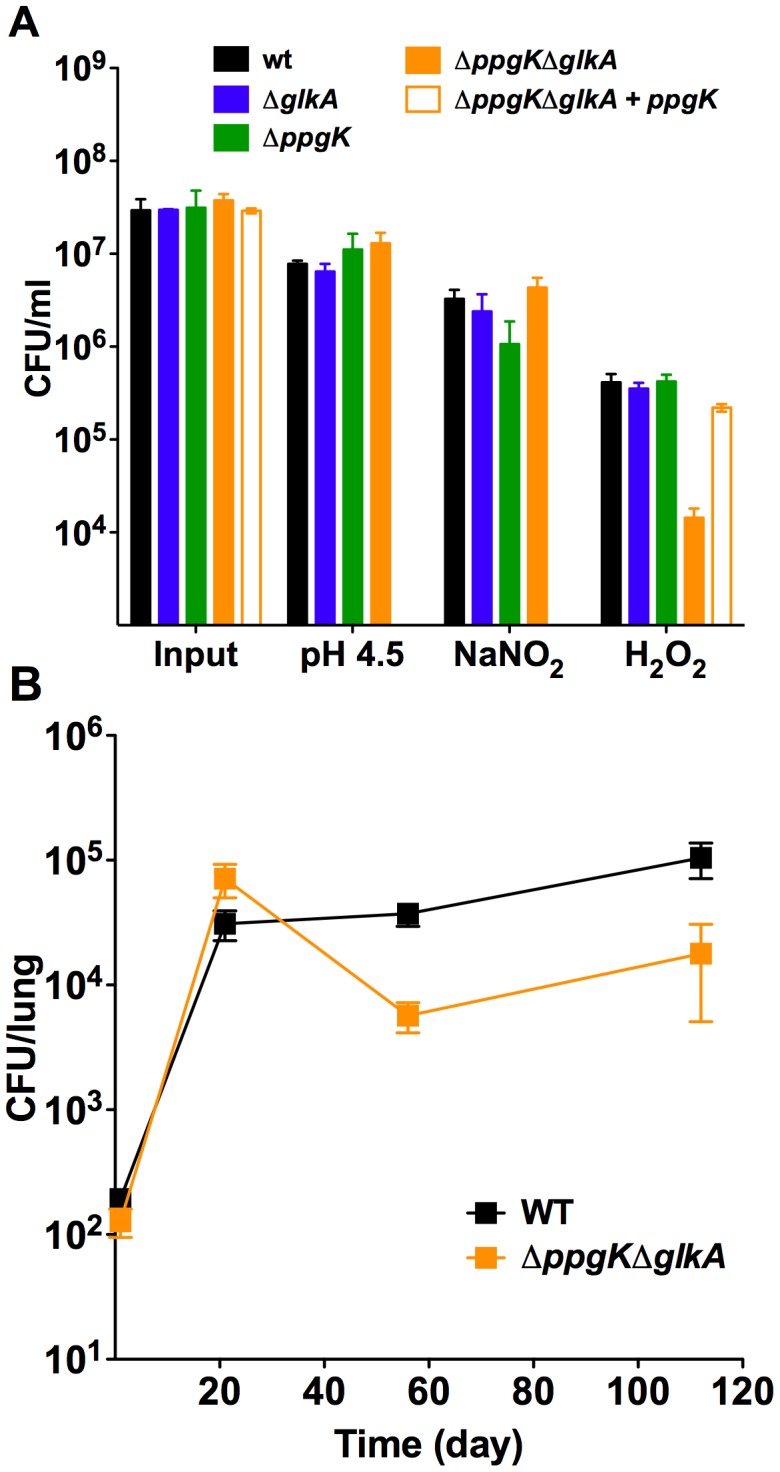
(A) Susceptibility to low pH, reactive nitrogen intermediates and hydrogen peroxide. Strains were exposed to pH 4.5 for 6 days, to 3 mM NaNO2 at pH 5.5 for 3 days and to 5 mM H2O2 for 4 hrs and bacterial survival was determined by plating CFU. Data are means ± s.d. of triplicate cultures. Hypersusceptibility of ΔppgKΔglkA to 5 mM H2O2 was demonstrated in three independent experiments. (B) Bacterial titers in lungs of gp96phox−/− mice infected with wt and ΔppgKΔglkA. Data are means ± s.d. from three mice per time point per group and representative of two independent experiments.
Discussion
That Mtb can metabolize glucose to support its growth in liquid culture has been established over 80 years ago [25]. Whether glucose metabolism is important for the generation of biomass during in vivo growth or required during chronic infections was unknown. Here, we investigated the role of Mtb's glucokinases catalyzing the generation of glucose-6-P, the first step in glycolysis and essential for glucose metabolism. We found that Mtb expresses two functional glucokinase ortholgues PPGK and Rv0650, which we named GLKA consistent with the M. smegmatis homolog [15]. Protein sequence analysis revealed that PPGK and GLKA belong to the ROK (repressor, ORF, kinase) family, which includes glucokinases and transcriptional repressors [26]. Bioinformatic analysis did not identify a canonical helix-turn-helix motif in PPGK or GLKA commonly found at the amino terminus of transcriptional repressors of the ROK family, suggesting that they do not act as transcriptional regulators. PPGK and GLKA have, however, conserved glucokinase features including the catalytic active aspartate residue, ATP binding domain, and glucose binding sites (Figure S2) [27]. PPGK contains five glucose binding sites and two ATP binding domains spanning amino acid residues 22–31 and 114–156 [13]. Similar to GLKA from M. smegmatis, Mtb GLKA contains only three of the five amino acid residues involved in glucose binding and only a single ATP binding domain spanning amino acids 4–17 [15]. This might explain why this enzyme is less efficient than PPGK in incorporating glucose into Mtb's central carbon metabolism and not sufficient to sustain robust growth on glucose in the absence of PPGK. Mtb lacking both PPGK and GLKA glucokinases could not metabolize glucose, which established that Mtb does not have any alternative pathway for glucose assimilation under the conditions tested.
Mtb has been shown to preferentially use host lipids as carbon sources for growth and persistence during infection [2], [4], [5], [9], [10]. In agreement with this, ΔppgK, while severely compromised for growth with glucose as sole carbon source in vitro, replicated and survived like wt Mtb in mice. Surprisingly, Mtb lacking both glucokinases PPGK and GLKA lost viability and failed to persist normally during the chronic phase of infection. This revealed that glucose phosphorylation is important for Mtb's fitness in chronically infected mice. The most pronounced decline in viability was observed between week three and eight post infection, while titers declined more slowly later on. This suggests that glucokinase activity helps Mtb adapt to changes in the host environment associated with the onset of adaptive immunity. The in vivo attenuation was tissue specific and only observed in lungs but not in spleens. This was reproducible in four independent mouse infections, two infections of wt and two of gp91phox−/− mice. Knowledge regarding differences with respect to the microenvironments Mtb is exposed to in lungs versus spleens is scarce, however it is conceivable that the metabolic constraints in the lung are different from those in the spleen especially following immune activation. The identification and characterization of mutants displaying tissue specific attenuation might help investigate this.
We assessed if the persistence defect was associated with increased susceptibility of ΔppgKΔglkA to stresses Mtb is likely exposed to in response to immune activation of the host [22]. Maintenance of a high glucose-6-P pool was proposed to be important for M. smegmatis ability to resist superoxide stress by serving as an electron store for detoxifying the superoxide generating agents plumbagin and menadione [23]. While ΔppgKΔglkA was hypersusceptible to hydrogen peroxide, its attenuation was not rescued in NADPH oxidase deficient mice arguing against the hypothesis that increased susceptibility to oxidative stress caused the in vivo phenotype. ΔppgKΔglkA was not hypersusceptible to plumbagin or menadione (data not shown) demonstrating that loss of glucokinases does not sensitize Mtb to all forms of oxidative stress. The reason for the specific hypersusceptibility to hydrogen peroxide is unclear.
We cannot exclude that Mtb's glucokinases phosphorylate an alternative substrate. Broad-specificity hexokinases have mostly been identified in Archea and eukaryotes and are less common in eubacteria [28]–[30]. The hexokinase RokA from Bacteroides fragilis has been shown to phosphorylate several substrates including glucose, mannose and N-acetyl-D-glucosamine (NAG) [30]. This unusual NAG kinase may act as activator for the synthesis of capsule components and be important for recycling of cell wall material [30]. To attribute the in vivo attenuation of ΔppgKΔglkA to loss of NAG kinase activity, both enzymes would need to be able to phosphorylate NAG, which seems unlikely, given the high affinity of PPGK for glucose [14]. Furthermore, none of the glucokinase mutants exhibited increased susceptibility to the antibiotic cycloserine, which targets peptidoglycan biosynthesis [31], [32] or to the detergent SDS (data not shown). Thus, we did not find evidence that deletion of ppgK and glkA affected permeability of the mycobacterial cell envelope.
Glucose-6-P has many important cellular functions such as energy production through glycolysis, production of reducing power through the pentose phosphate pathway, and it serves as an important precursor for anabolic processes, especially cell wall and nucleotide biosynthesis. Gluconeogenesis could potentially produce the necessary glucose-6-P to meet cellular demands. However, glucose-6-P generation directly from glucose via glucokinase consumes one molecule of ATP while its production through gluconeogenesis is more energetically costly. Exposure to diverse stresses associated with the host immune response might compromise ATP production in Mtb through the electron transport chain, such that ATP production through substrate level phosphorylation using glucose-6-P as a precursor might become important.
The glucokinase mutants replicated normally with trehalose as sole carbon source indicating that trehalose metabolism does not depend on trehalase. Trehalase hydrolyzes trehalose into two molecules of glucose, while trehalose phosphorylase catalyzes the phosphorolytic cleavage of trehalose into glucose1-P and glucose [33]. A phosphate dependent trehalase has been purified from M. smegmatis and Mtb contains a homolog [17]. Despite the requirement for phosphate, there was no evidence of phosphorolytic cleavage, suggesting that the enzyme produces two molecules of glucose, which require glucokinase activity for entry into glycolysis. Our data indicate that Mtb is able to bypass glucose phosphorylation when growing on trehalose.
How Mtb takes up glucose remains to be determined. In contrast to M. smegmatis which was predicted to encode 28 carbohydrate transporters, only five such transporters were identified in the Mtb genome [8]. Direct biochemical and structural analyses of these is lacking and glucose transport across the outer membrane of Mtb is even less well understood [34], [35].
Our work identified a previously unrecognized role for glucose phosphorylation in Mtb pathogenesis. Recent work showed that Mtb is metabolically flexible and capable of utilizing multiple carbon substrates simultaneously [16]. The in vivo attenuation of Mtb lacking both glucokinases, PPGK and GLKA, suggests that Mtb has access to glucose and relies on glucose phosphorylation to survive during chronic infection. While the physiological function of the generated glucose-6-P remains unclear, the impact of loss of glucokinase activity emphasizes that Mtb's metabolic flexibility is crucial to its pathogenesis and that the pathogen does not exclusively rely on metabolism of host lipids.
Materials and Methods
Ethics statement
All procedures including animal studies were conducted following the National Institutes of Health guidelines for housing and care of laboratory animals and performed in accordance with institutional regulations after protocol review and approval by the Institutional Animal Care and Use Committee of Weill Cornell Medical College (protocol # 0601-441A, Conditional Expression of Mycobacterial Genes).
Strains, media and culture conditions
M. tuberculosis (Erdman) strains were routinely grown at 37°C in liquid Middelbrook 7H9 medium (Difco) containing 0.2% glycerol, 0.5% bovine serum albumin, 0.2% dextrose, 0.085% NaCl, and 0.05% Tween 80 or on Middlebrook 7H10 agar plates containing 10% OADC supplement (Becton Dickinson) and 0.5% glycerol. For growth in carbon defined media, we used 7H9 with 0.05% Tyloxapol and a carbon substrate at the indicated concentration (% wt/vol). When required, hygromycin B was used at a concentration of 50 µg/ml and kanamycin at a concentration of 25 µg/ml.
Mutant strain and plasmid constructions
Mtb knockout strains were constructed via allelic exchange using the conditionally replicating mycobacteriophage method as described elsewhere [5], [36]. The double knockout glucokinase strain was constructed by first removing the hygromycin cassette from the ΔppgK genome by expressing the Cre recombinase from an unstable plasmid. The glkA gene was subsequently deleted in the ΔppgK strain that was cured of both the hygromycing cassette and the Cre expression plasmid. All Mtb knockout strains were confirmed using Southern Blot Analysis. Complementation plasmids were constructed using the Gateway Cloning Technology (Invitrogen) as described previously [37]. For complementation the ppgK gene was cloned containing its putative promoter (350 nucleotides upstream of the start codon) into a plasmid that integrates into the chromosomal attB site. To complement ΔppgK with glkA, the glkA gene was cloned into an episomal plasmid behind the strong mycobacterial promoter Psmyc [38]. Primer sequences are available upon request.
Liquid chromatography-mass spectrometry
Metabolites were separated on a Cogent Diamond Hydride Type C column (Microsolve Technologies). The mass spectrometer used was Agilent Accurate Mass 6220 TOF coupled to an Agilent 1200 LC system. Dynamic mass axis calibration was achieved by continuous infusion of a reference mass solution using an isocratic pump with a 100∶1 splitter. This configuration achieved mass errors of approximately 5 parts-per-million (ppm), mass resolution ranging from 10,000–25,000 (over m/z 121–955 amu), and 5 log10 dynamic range. Detected ions were deemed metabolites on the basis of unique accurate mass-retention time (AMRT) identifiers for masses exhibiting the expected distribution of accompanying isotopomers. Metabolite identities were established by querying against a pre-populated AMRT library of metabolite standards and demonstrating chromatographic co-elution of candidate metabolites with pure chemical standards spiked into representative biological samples.
Isotopomer data analysis
The extent of isotopic labeling for each metabolite was determined by dividing the summed peak height ion intensities of all labeled species by the ion intensity of both labeled and unlabeled species, expressed in percent. Label-specific ion counts were corrected for naturally occurring 13C species (i. e. [M+1] and [M+2]). The relative abundance of each isotopically labeled species was determined by dividing the peak height ion intensity of each isotopic form (corrected for naturally occurring 13C species as above) by the summed peak height ion intensity of all labeled species.
Mouse infections
Female 7–8 week old C57BL/6 and gp91phox−/− mice (Jackson Laboratory) were infected with Mtb by aerosol as described [39]. Bacterial numbers were determined by plating homogenized organs for CFU.
Stress assays
Starvation survival experiments were carried by inoculating ∼5×106 bacteria in PBS+0.5% Tyloxapol or in Sauton's base medium (0.5 g/L KH2PO4, 0.5 g/L MgSO4, 2.0 g/L citric acid, 0.05 g ferric ammonium citrate, 0.5 g/L ammonium sulfate, 0.05% Tyloxapol, pH 7.4) supplemented with 0.4% glucose or no carbon. Bacterial survival in these media was determined by periodically plating serial dilutions on 7H10 plates to estimate CFU/ml. Susceptibility to nitrosative, oxidative stress and low pH was determined as described previously [39], [40].
Supporting Information
Southern blot analysis of glucokinase mutants. (A) Schematic representation of the ppgK genomic region in wt, ΔppgK and ΔppgKΔglkA, in which the hygromycin cassette was removed. (B) Southern blot of chromosomal DNA digested with PvuI+PvuII from wt (lane1), ΔppgK (lane 2) and ΔppgKΔglkA (lane 3). (C) Schematic representation of glkA genomic region in wt and ΔglkA. (D) Southern blot of chromosomal DNA digested with SacI from wt (lane 1), ΔglkA (lane 2) and ΔppgKΔglkA (lane 3).
(PDF)
Comparison of Mtb's glucokinases, GLKA and PPGK. (A) Amino acid sequence alignment of PPGK and GLKA. The overall sequence identity is 22%. (B) Schematic representation of PPGK and GLKA. Residues involved in glucose binding are indicated in red with corresponding residue number shown below. Grey boxes represent ATP binding domains and the catalytic aspartate residues are marked with an asterisk.
(PDF)
Quantification of ppgK and glkA ( Rv0650 ) transcript levels in wt Mtb and mutants. Absolute mRNA amounts were determined by quantitative real time PCR. Serial dilutions of defined copy numbers of the Mtb chromosome were included for each real-time PCR to generate standard curves, which were used to calculate the absolute copy number of each gene. Data are means from 3 independent replicates ± SD.
(TIFF)
KatG and SodA amounts in cell extracts. KatG and SodA were detected by immunoblotting in lysates from wt, ΔppgK, ΔglkA and ΔppgKΔglkA.
(TIFF)
Enzymatic quantification of glucose-6-P and fructose-6-P metabolite pools in cell extracts from wt and glucokinase mutants. Metabolite amounts were measured as described in Materials and Methods. Data represent the mean of three independent replicates from two independent experiments ± SEM. * P≤0.05 compared to wt.
(PDF)
Expression of antioxidant genes in wt and ΔppgKΔglkA . mRNA amounts were quantified by quantitative real time PCR before and after exposure to 5 mM H2O2 for 30 min and normalized to sigA. Data represent the mean of three experimental replicates ± SD.
(PDF)
Acknowledgments
We thank Christopher Sassetti for helpful discussions and Dirk Schnappinger for critical reading of the manuscript.
Funding Statement
This work was supported by grants from the National Institutes of Health (AI063446 to SE, AI081094 to KYR) and a Burroughs Wellcome Career Award in the Biomedical Sciences to KYR. The Department of Microbiology & Immunology acknowledges support from the William Randolph Hearst Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO (2011) Global Tuberculosis Control. http://www.who.int/tb/publications/global_report/en/index.html.
- 2. Muñoz-Elías E, McKinney J (2005) Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat Med 11: 638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muñoz Elías E, McKinney J (2006) Carbon metabolism of intracellular bacteria. Cell Microbiol 8: 10–22. [DOI] [PubMed] [Google Scholar]
- 4. Pandey A, Sassetti C (2008) Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci USA 105: 4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marrero J, Rhee KY, Schnappinger D, Pethe K, Ehrt S (2010) Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc Natl Acad Sci USA 107: 9819–9824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhee KY, de Carvalho LPS, Bryk R, Ehrt S, Marrero J, et al. (2011) Central carbon metabolism in Mycobacterium tuberculosis: an unexpected frontier. Trends Microbiol 19: 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, et al. (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393: 537–544. [DOI] [PubMed] [Google Scholar]
- 8. Titgemeyer F, Amon J, Parche S, Mahfoud M, Bail J, et al. (2007) A genomic view of sugar transport in Mycobacterium smegmatis and Mycobacterium tuberculosis . J Bacteriol 189: 5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, et al. (2003) Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J Exp Med 198: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Timm J, Post FA, Bekker L-G, Walther GB, Wainwright HC, et al. (2003) Differential expression of iron-, carbon-, and oxygen-responsive mycobacterial genes in the lungs of chronically infected mice and tuberculosis patients. Proc Natl Acad Sci USA 100: 14321–14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sassetti CM, Rubin EJ (2003) Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci USA 100: 12989–12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalscheuer R, Weinrick B, Veeraraghavan U, Besra GS, Jacobs WR (2010) Trehalose-recycling ABC transporter LpqY-SugA-SugB-SugC is essential for virulence of Mycobacterium tuberculosis . Proc Natl Acad Sci USA 107: 21761–21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsieh PC, Shenoy BC, Samols D, Phillips NF (1996) Cloning, expression, and characterization of polyphosphate glucokinase from Mycobacterium tuberculosis . J Biol Chem 271: 4909–4915. [DOI] [PubMed] [Google Scholar]
- 14. Hsieh PC, Kowalczyk TH, Phillips NF (1996) Kinetic mechanisms of polyphosphate glucokinase from Mycobacterium tuberculosis . Biochemistry 35: 9772–9781. [DOI] [PubMed] [Google Scholar]
- 15. Pimentel-Schmitt EANF, Thomae AW, Amon J, Klieber MA, Roth H-M, et al. (2007) A Glucose Kinase from Mycobacterium smegmatis . J Mol Microbiol Biotechnol 12: 75–81. [DOI] [PubMed] [Google Scholar]
- 16. de Carvalho LPS, Fischer SM, Marrero J, Nathan C, Ehrt S, et al. (2010) Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chemistry & Biology 17: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 17. Carroll JD, Pastuszak I, Edavana VK, Pan YT, Elbein AD (2007) A novel trehalase from Mycobacterium smegmatis – purification, properties, requirements. FEBS Journal 274: 1701–1714. [DOI] [PubMed] [Google Scholar]
- 18. Primm TP, Andersen SJ, Mizrahi V, Avarbock D, Rubin H, et al. (2000) The Stringent Response of Mycobacterium tuberculosis Is Required for Long-Term Survival. J Bacteriol 182: 4889–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dahl JL, Kraus CN, Boshoff HIM, Doan B, Foley K, et al. (2003) The role of RelMtb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc Natl Acad Sci USA 100: 10026–10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klinkenberg LG, Lee JH, Bishai WR, Karakousis PC (2010) The Stringent Response Is Required for Full Virulence of Mycobacterium tuberculosis in Guinea Pigs. J Infect Dis 202: 1397–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ehrt S, Schnappinger D (2009) Mycobacterial survival strategies in the phagosome: defence against host stresses. Cell Microbiol 11: 1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stallings CL, Glickman MS (2010) Is Mycobacterium tuberculosis stressed out? A critical assessment of the genetic evidence. Microbes Infect 12: 1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hasan MR, Rahman M, Jaques S, Purwantini E, Daniels L (2010) Glucose 6-phosphate accumulation in mycobacteria: implications for a novel F420-dependent anti-oxidant defense system. J Biol Chem 285: 19135–19144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ng VH, Cox JS, Sousa AO, MacMicking JD, McKinney JD (2004) Role of KatG catalase-peroxidase in mycobacterial pathogenesis: countering the phagocyte oxidative burst. Mol Microbiol 52: 1291–1302. [DOI] [PubMed] [Google Scholar]
- 25. Merrill MH (1930) Carbohydrate metabolism of organisms of the genus mycobacterium. J Bacteriol 20: 235–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Titgemeyer F, Reizer J, Reizer A, Saier MH (1994) Evolutionary relationships between sugar kinases and transcriptional repressors in bacteria. Microbiology 140: 2349–2354. [DOI] [PubMed] [Google Scholar]
- 27. Conejo MS, Thompson SM, Miller BG (2010) Evolutionary Bases of Carbohydrate Recognition and Substrate Discrimination in the ROK Protein Family. J Mol Evol 70: 545–556. [DOI] [PubMed] [Google Scholar]
- 28. Cárdenas ML, Cornish-Bowden A, Ureta T (1998) Evolution and regulatory role of the hexokinases. Biochim Biophys Acta 1401: 242–264. [DOI] [PubMed] [Google Scholar]
- 29. Hansen T, Reichstein B, Schmid R, Schonheit P (2002) The First Archaeal ATP-Dependent Glucokinase, from the Hyperthermophilic Crenarchaeon Aeropyrum pernix, Represents a Monomeric, Extremely Thermophilic ROK Glucokinase with Broad Hexose Specificity. J Bacteriol 184: 5955–5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brigham CJ, Malamy MH (2005) Characterization of the RokA and HexA Broad-Substrate-Specificity Hexokinases from Bacteroides fragilis and Their Role in Hexose and N-Acetylglucosamine Utilization. J Bacteriol 187: 890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halouska S, Chacon O, Fenton RJ, Zinniel DK, Barletta RG, et al. (2007) Use of NMR Metabolomics To Analyze the Targets of d-Cycloserine in Mycobacteria: Role of d-Alanine Racemase. J Proteome Res 6: 4608–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bruning JB, Murillo AC, Chacon O, Barletta RG, Sacchettini JC (2010) Structure of the Mycobacterium tuberculosis D-Alanine:D-Alanine Ligase, a Target of the Antituberculosis Drug D-Cycloserine. Antimicrobial Agents and Chemotherapy 55: 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Argüelles JC (2000) Physiological roles of trehalose in bacteria and yeasts: a comparative analysis. Archives of Microbiology 174: 217–224. [DOI] [PubMed] [Google Scholar]
- 34. Niederweis M (2008) Nutrient acquisition by mycobacteria. Microbiology 154: 679–692. [DOI] [PubMed] [Google Scholar]
- 35. Niederweis M, Danilchanka O, Huff J, Hoffmann C, Engelhardt H (2010) Mycobacterial outer membranes: in search of proteins. Trends Microbiol 18: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bardarov S, Kriakov J, Carriere C, Yu S, Vaamonde C, et al. (1997) Conditionally replicating mycobacteriophages: a system for transposon delivery to Mycobacterium tuberculosis . Proc Natl Acad Sci USA 94: 10961–10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blumenthal A, Trujillo C, Ehrt S, Schnappinger D (2010) Simultaneous analysis of multiple Mycobacterium tuberculosis knockdown mutants in vitro and in vivo. PLoS ONE 5: e15667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ehrt S, Guo XV, Hickey CM, Ryou M, Monteleone M, et al. (2005) Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res 33: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vandal OH, Roberts JA, Odaira T, Schnappinger D, Nathan CF, et al. (2009) Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J Bacteriol 191: 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vandal OH, Pierini LM, Schnappinger D, Nathan CF, Ehrt S (2008) A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis . Nat Med 14: 849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Southern blot analysis of glucokinase mutants. (A) Schematic representation of the ppgK genomic region in wt, ΔppgK and ΔppgKΔglkA, in which the hygromycin cassette was removed. (B) Southern blot of chromosomal DNA digested with PvuI+PvuII from wt (lane1), ΔppgK (lane 2) and ΔppgKΔglkA (lane 3). (C) Schematic representation of glkA genomic region in wt and ΔglkA. (D) Southern blot of chromosomal DNA digested with SacI from wt (lane 1), ΔglkA (lane 2) and ΔppgKΔglkA (lane 3).
(PDF)
Comparison of Mtb's glucokinases, GLKA and PPGK. (A) Amino acid sequence alignment of PPGK and GLKA. The overall sequence identity is 22%. (B) Schematic representation of PPGK and GLKA. Residues involved in glucose binding are indicated in red with corresponding residue number shown below. Grey boxes represent ATP binding domains and the catalytic aspartate residues are marked with an asterisk.
(PDF)
Quantification of ppgK and glkA ( Rv0650 ) transcript levels in wt Mtb and mutants. Absolute mRNA amounts were determined by quantitative real time PCR. Serial dilutions of defined copy numbers of the Mtb chromosome were included for each real-time PCR to generate standard curves, which were used to calculate the absolute copy number of each gene. Data are means from 3 independent replicates ± SD.
(TIFF)
KatG and SodA amounts in cell extracts. KatG and SodA were detected by immunoblotting in lysates from wt, ΔppgK, ΔglkA and ΔppgKΔglkA.
(TIFF)
Enzymatic quantification of glucose-6-P and fructose-6-P metabolite pools in cell extracts from wt and glucokinase mutants. Metabolite amounts were measured as described in Materials and Methods. Data represent the mean of three independent replicates from two independent experiments ± SEM. * P≤0.05 compared to wt.
(PDF)
Expression of antioxidant genes in wt and ΔppgKΔglkA . mRNA amounts were quantified by quantitative real time PCR before and after exposure to 5 mM H2O2 for 30 min and normalized to sigA. Data represent the mean of three experimental replicates ± SD.
(PDF)