Abstract
Common cancer is an age-related disease. Slow aging is associated with reduced and delayed carcinogenesis. Calorie restriction (CR), the most studied anti-aging intervention, prevents cancer by slowing down the aging process. Evidence is emerging that CR decelerates aging by deactivating MTOR (Target of Rapamycin). Rapamycin and other rapalogs suppress cellular senescence, slow down aging and postpone age-related diseases including cancer. At the same time, rapalogs are approved for certain cancer treatments. Can cancer prevention be explained by direct targeting of cancer cells? Or does rapamycin prevent cancer indirectly through slowing down the aging process? Increasing evidence points to the latter scenario.
Keywords: aging, senescence, geroconversion, gerosuppression, rapamycin, diseases, mTOR
Cancer is an Age-Related Disease
Common cancers such as lung, breast, prostate, colon, gastric, esophageal, pancreatic, thyroid, brain and certain leukemias, are age-related diseases, meaning that their incidence is dramatically increased with aging and about one third of elderly people die from cancer. In rapidly aging mammals such as mice, cancer develops by the age of one to two years. In humans, common cancers are delayed and occur toward the end of the life, too. Centenarians, people who live more than 100 years, are especially protected from cancer.1-3 In long-lived mice, cancer is delayed.4 In slow-aging naked mole-rats, cancer is uncommon despite high levels of oxidative damage.5 Also CR slows down aging and delays cancer.6-14 In contrast, overeating, high caloric food and obesity accelerate cancer.15
mTOR in Organismal Aging
So why do overeating, obesity and nutrients accelerate aging? One explanation is that nutrients and insulin activate the nutrient-sensing mTOR pathway. This pathway drives cellular mass growth.16-19 Growth factors, insulin and nutrients activate nutrient-sensing- and growth-promoting pathways, which in turn drive developmental growth. Later in life the same pathway drives aging, which is a continuation of developmental growth.20-22 In agreement, calorie restriction, which deactivates the TOR pathway, slows growth early in life and delays aging later in life.10,11 This is simple and straightforward. This predicts that rapamycin would slow down aging and extend life span.23 In fact, rapamycin extends life span in yeast,24 Drosophila25,26 and mice.27-33 Also, genetic manipulations that decrease the activity of TOR in turn increase life span in diverse species.34-41
mTOR and Cellular Aging (Geroconversion)
In proliferating cells, growth factors (GFs) activate both the growth-promoting mTOR pathway and the cell cycle. Therefore cellular growth is balanced by cell division. When the cell cycle is blocked, yet mTOR is active, cells undergo gerogenic conversion or geroconversion.42 At first, the arrested cell is not senescent. Overtime, the mTOR pathway converts arrest into senescence.42-44 Also activation of mTOR converts quiescence into senescence, when the cell cycle is still locked.45-47 This type of geroconversion may imitate physiological aging of post-mitotic cells in the organism. Aged cells are hypertrophic and hyper-functional.48,49 In turn, aging cells due to their hyper-functions cause diseases of aging such as obesity, pro-inflammatory syndrome, atherosclerosis, hypertension, neurodegeneration and osteoporosis.50-55It is very important to emphasize that geroconversion is not a transition from proliferation to arrest. Geroconversion is a transition from arrest and quiescence to senescence. In the young organism, post-mitotic cells are quiescent, becoming senescent over time. In theory, a proliferating cell may also undergo pro-gerogenic conversion. This condition may be manifested as cancer.
mTOR in Cancer
The PI3K/mTOR is almost universally activated in cancer56-62 and is a promising therapeutic target.63-78 The similarity between cancer and aging is not coincidental. Aging can be viewed as “twisted growth,” when actual growth is precluded.43 Cancer is actual growth and proliferation of pro-gerogenic cells (it is sufficient to ensure cell cycle arrest and then gerogenic conversion will occur). Oncogenic proteins such as growth factor-receptors, activated Ras, tyrosine (Src) and serine/threonine kinases, such as Raf, MEK, PI3K, Akt, all activate mTOR. Inactivation of tumor suppressors such as PTEN, NF-1, TSC2 activate the mTOR pathway.56-62 Inactivation of some tumor suppressors (Rb, p53 and p16) overcomes cell cycle arrest and prevents the senescent phenotype. P53 inhibits mTOR.79-81 Here it is important to emphasize that p53 not only causes arrest but also can suppress geroconversion (conversion from arrest to senescence), ensuring instead quiescence.45,82-93 Therefore, p53 is so often inactivated in cancer cells, which can be viewed as proliferating senescent cells. In aging, the mTOR pathway is activated by signals from other cells and by feedback loops.94 In cancer, the mTOR pathway is activated by mutations and other stable alterations in upstream oncoproteins/tumor suppressors. Multiple rounds of cellular proliferation and selection transform initially random mutations into non-random activation of the mTOR pathway. Growth-limiting conditions and senescence of normal cells provides such a selective advantage.94-97 Therapy further drives tumor progression.98
Cancer Treatment with Rapamycin
Given the universal activation of the mTOR pathway in cancer, rapalogs are used for cancer treatment.99-102 Temsirolimus, a prodrug of rapamycin (Sirolimus), became the first rapalog approved for cancer treatment.103 The uses of rapalogs in the therapies of cancer and other diseases are rapidly increasing.104,105 Hundreds if not thousands of clinical trials are under the way. Yet, rapalogs (rapamycin and its analogs) are not a panacea. Although effective for approved indications (otherwise the drugs would not be approved), rapalogs still play a modest role in cancer treatment. For one, rapalogs (rapamycin and its analogs) at relevant concentrations are not toxic drugs: they do not kill cells but rather they slow their growth. While this is a disadvantage of rapalogs as anticancer drugs, this is an advantage as cancer-preventive and anti-aging agents. Rapalogs can reverse cell senescence in cancer stroma,106 potentially contributing to cancer treatment and prevention.107,108
Tumor Prevention by Rapalogs
While rapamycin and other rapalogs are modestly-potent anticancer drugs, they are effective cancer-preventive agents. Remarkably, their cancer-preventive effects were initially detected in humans. Patients, who received rapamycin due to renal transplantation, had a peculiar “side effect”: a decrease in cancer incidence.109-114 In cancer-prone mice, rapamycin dramatically delays cancer. For example, rapamycin decreased incidence and progression of tobacco carcinogen-induced lung tumors.115 Also everolimus (rapalog), delayed tumor onset and progression in a transgenic mouse model of ovarian cancer.116 Rapamycin inhibited multiple stages of tumor progression in a transgenic mouse model of HER2-positive breast cancer.117 Rapamycin also prevents skin tumors induced by phorbol esters.118 It was assumed that rapalogs delay cancer by targeting cancer cells directly. Yet, there were some indications that the effect might be indirect via targeting normal cells. Lkb1(+/−) mice were treated with rapamycin from the age of 8 weeks, well before the onset of polyposis. This decreased the number of gastric tumors. In the polyps from the treated mice, phosphorylation of S6 kinase (a marker of mTOR activity) was maintained.119 Still, in these studies, the effect of rapamycin on overall longevity and rate of aging was not determined. When the rate of aging was measured, it was revealed that rapamycin at least “formally” decreased the rate of aging and increased maximal longevity in transgenic HER-2/neu cancer-prone mice.120
Indirect “Anti-Aging” Model
It was suggested that cancer could be prevented by inhibiting aging with rapamycin or “by staying young.”121 Rapamycin decreased cancer incidence in normal (non-cancer-prone) genetically heterogeneous mice27,28 and inbred mice.29 These studies have been initiated in order to evaluate the effect of rapamycin on aging not cancer. Although retrospectively one may argue that life span was extended due to cancer prevention, this argument could (or not) be used to explain the life extending effect of calorie restriction as well. It is clear that dramatic difference in lifespan between man and C. elegans is not due cancer, but instead due to the speed of development and aging. Also in order to delay cancer and extend life span in p53+/− mice, treatment with rapamycin should be started earlier in life before tumors develop.122 Perhaps rapamycin is less effective when cancer had developed already. CR delays cancer in p53−/− mice.123-125
How to Distinguish between Two Models of Cancer Prevention
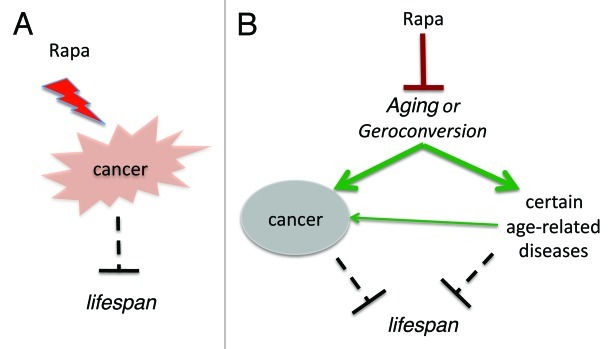
Two models of cancer prevention are very difficult to distinguish. One may believe that rapamycin prolongs life span by decreasing cancer (Fig. 1A). Yet, I believe that rapamycin prevents cancer by suppressing aging (Fig. 1B). It may be helpful to evaluate rapamycin in strains of mice with low tumor incidences. This might confirm that rapamycin increases life span independently from cancer prevention. In fact, it is already known that rapamycin delays most age-related diseases in rodents,126,32 including age-related retinopathy127 and age-related obesity.128 Second, we can test the cancer-preventive effect of rapamycin at low doses that do not inhibit cancer cell growth. Other approaches might be suggested by the readers of this paper (please address as letter to the editor).
Figure 1. Two models of cancer-prevention by rapalogs. (A) Direct anticancer effect. Rapalogs (Rapa) suppress cancer cells, prevent cancer and thus extend lifespan. (B) Indirect anticancer effect due to aging-suppression. Rapalogs (Rapa) suppress aging (gerosuppression) and thus prevent cancer and other age-related diseases, extending lifespan.
Rapalogs for Cancer Prevention
For cancer prevention via inhibiting the aging process, rapamycin (and other rapalogs) could be used at lower doses in order to target normal cells, which are very sensitive to rapamycin.129 There is no need to kill any cells. Furthermore, there is no need to inhibit mTOR completely: it could be inhibited just slightly to slow down aging. Second, administration of rapamycin can be intermittent, like intermittent fasting. In low doses or intermittent schedules, rapalogs may have no side effects. Rapamycin prevents cancer in p53-/+ mice, which could be viewed as a model of Li-Fraumeni syndrome. Currently, there is no clinically-available therapy to prevent cancer in patients with Li-Fraumeni syndrome.130-132 Definitely, there is an excellent opportunity to start cancer prevention by rapalogs.
Selective Protection of Normal Cells from Therapy Induced Cell Death and Senescence
Numerous studies have demonstrated that rapalogs potentiate chemotherapy against cancer cells. In theory, rapalogs could protect normal cells from chemotherapy and to increase therapeutic index.63 In cell culture, rapamycin in combination with the p53-inducing agent Nutlin-3 and metformin can selectively protect normal cells from chemotherapy.133-135 Remarkably, in mice rapamycin prevented epithelial stem cell senescence and protects from radiation-induced mucositis.136, 137 Like rapamycin, fasting inhibits the mTOR pathway. Fasting also increases therapeutic window and decreases side effects chemotherapy in animals and humans.138-141
Conclusions
There are several lines of reasoning suggesting that the effects of rapamycin on cancer prevention are indirect. First, while rapalogs (as monotherapy) are relatively modest anti-cancer drugs, these drugs are potent cancer-preventive and anti-aging agents. Second, typical anti-cancer drugs do not and cannot be used for cancer prevention. For example, radiation, paclitaxel and doxorubicin, just to mention a few, have no cancer-preventive effects in animals. In contrast, these treatments tend to cause secondary cancer in both animals and humans. Direct anticancer drugs are rather carcinogenic. Third, an anti-aging effect is sufficient to explain cancer-preventive effects. Yet, we might never indisputably distinguish between the anti-cancer and anti-aging effects of rapamycin because both cancer and aging share the activation of a common signaling pathway, and this pathway is targeted by rapamycin.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/22859
References
- 1.Hitt R, Young-Xu Y, Silver M, Perls T. Centenarians: the older you get, the healthier you have been. Lancet. 1999;354:652. doi: 10.1016/S0140-6736(99)01987-X. [DOI] [PubMed] [Google Scholar]
- 2.Evert J, Lawler E, Bogan H, Perls T. Morbidity profiles of centenarians: survivors, delayers, and escapers. J Gerontol A Biol Sci Med Sci. 2003;58:232–7. doi: 10.1093/gerona/58.3.M232. [DOI] [PubMed] [Google Scholar]
- 3.Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J Gerontol A Biol Sci Med Sci. 2012;67:395–405. doi: 10.1093/gerona/glr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–6. doi: 10.1093/gerona/58.4.B291. [DOI] [PubMed] [Google Scholar]
- 5.Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci. 2005;60:1369–77. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- 6.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–6. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31:89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharp ZD. Aging and TOR: interwoven in the fabric of life. Cell Mol Life Sci. 2011;68:587–97. doi: 10.1007/s00018-010-0542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R. Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci. 2010;65:695–703. doi: 10.1093/gerona/glq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blagosklonny MV. Linking calorie restriction to longevity through sirtuins and autophagy: any role for TOR. Cell Death Dis. 2010;1:e12. doi: 10.1038/cddis.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blagosklonny MV. Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans) Cell Cycle. 2010;9:683–8. doi: 10.4161/cc.9.4.10766. [DOI] [PubMed] [Google Scholar]
- 12.Blagosklonny MV. Hormesis does not make sense except in the light of TOR-driven aging. Aging (Albany NY) 2011;3:1051–62. doi: 10.18632/aging.100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye J, Keller JN. Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging (Albany NY) 2010;2:361–8. doi: 10.18632/aging.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soare A, Cangemi R, Omodei D, Holloszy JO, Fontana L. Long-term calorie restriction, but not endurance exercise, lowers core body temperature in humans. Aging (Albany NY) 2011;3:374–9. doi: 10.18632/aging.100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blagosklonny MV. NCI’s provocative questions on cancer: some answers to ignite discussion. Oncotarget. 2011;2:1352–67. doi: 10.18632/oncotarget.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoki K, Guan KL. Complexity of the TOR signaling network. Trends Cell Biol. 2006;16:206–12. doi: 10.1016/j.tcb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23:744–55. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging (Albany NY) 2009;1:357–62, 20. doi: 10.18632/aging.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle. 2010;9:3151–6. doi: 10.4161/cc.9.16.13120. [DOI] [PubMed] [Google Scholar]
- 21.Blagosklonny MV. Why men age faster but reproduce longer than women: mTOR and evolutionary perspectives. Aging (Albany NY) 2010;2:265–73. doi: 10.18632/aging.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blagosklonny MV. Why the disposable soma theory cannot explain why women live longer and why we age. Aging (Albany NY) 2010;2:884–7. doi: 10.18632/aging.100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blagosklonny MV. Rapamycin and quasi-programmed aging: four years later. Cell Cycle. 2010;9:1859–62. doi: 10.4161/cc.9.10.11872. [DOI] [PubMed] [Google Scholar]
- 24.Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–84. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskalev AA, Shaposhnikov MV. Pharmacological inhibition of phosphoinositide 3 and TOR kinases improves survival of Drosophila melanogaster. Rejuvenation Res. 2010;13:246–7. doi: 10.1089/rej.2009.0903. [DOI] [PubMed] [Google Scholar]
- 27.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–6. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- 30.Khanna A, Kapahi P. Rapamycin: killing two birds with one stone. Aging (Albany NY) 2011;3:1043–4. doi: 10.18632/aging.100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longo VD, Fontana L. Intermittent supplementation with rapamycin as a dietary restriction mimetic. Aging (Albany NY) 2011;3:1039–40. doi: 10.18632/aging.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–82. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spong A, Bartke A. Rapamycin slows aging in mice. Cell Cycle. 2012;11:845. doi: 10.4161/cc.11.5.19607. [DOI] [PubMed] [Google Scholar]
- 34.Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–6. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 35.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–90. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–4. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–74. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JH, Bodmer R, Bier E, Karin M. Sestrins at the crossroad between stress and aging. Aging (Albany NY) 2010;2:369–74. doi: 10.18632/aging.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha CW, Huh WK. The implication of Sir2 in replicative aging and senescence in Saccharomyces cerevisiae. Aging (Albany NY) 2011;3:319–24. doi: 10.18632/aging.100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pani G. P66SHC and ageing: ROS and TOR? Aging (Albany NY) 2010;2:514–8. doi: 10.18632/aging.100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stipp D. A new path to longevity. Sci Am. 2012;306:32–9. doi: 10.1038/scientificamerican0112-32. [DOI] [PubMed] [Google Scholar]
- 42.Blagosklonny MV. Cell cycle arrest is not senescence. Aging (Albany NY) 2011;3:94–101. doi: 10.18632/aging.100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle. 2008;7:3355–61. doi: 10.4161/cc.7.21.6919. [DOI] [PubMed] [Google Scholar]
- 44.Demidenko ZN, Shtutman M, Blagosklonny MV. Pharmacologic inhibition of MEK and PI-3K converges on the mTOR/S6 pathway to decelerate cellular senescence. Cell Cycle. 2009;8:1896–900. doi: 10.4161/cc.8.12.8809. [DOI] [PubMed] [Google Scholar]
- 45.Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany, NY Online) 2010;2:892–3. doi: 10.18632/aging.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wesierska-Gadek J. mTOR and its link to the picture of Dorian Gray—reactivation of mTOR promotes aging. Aging. 2010;2:924–35. doi: 10.18632/aging.100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolesnichenko M, Hong L, Liao R, Vogt PK, Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle. 2012;11:2391–401. doi: 10.4161/cc.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blagosklonny MV. Aging-suppressants: cellular senescence (hyperactivation) and its pharmacologic deceleration. Cell Cycle. 2009;8:1883–7. doi: 10.4161/cc.8.12.8815. [DOI] [PubMed] [Google Scholar]
- 49.Gems DH, Guardia YD. Alternative Perspectives on Aging in Caenorhabditis elegans: Reactive Oxygen Species or Hyperfunction? Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blagosklonny MV. TOR-driven aging: speeding car without brakes. Cell Cycle. 2009;8:4055–9. doi: 10.4161/cc.8.24.10310. [DOI] [PubMed] [Google Scholar]
- 51.Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. Am J Pathol. 2012;181:1142–6. doi: 10.1016/j.ajpath.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 52.Bjedov I, Partridge L. A longer and healthier life with TOR down-regulation: genetics and drugs. Biochem Soc Trans. 2011;39:460–5. doi: 10.1042/BST0390460. [DOI] [PubMed] [Google Scholar]
- 53.Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22:R741–52. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 54.Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, et al. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and enhancing NMDA signaling. Aging Cell. 2012;11:326–35. doi: 10.1111/j.1474-9726.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tacutu R, Budovsky A, Yanai H, Fraifeld VE. Molecular links between cellular senescence, longevity and age-related diseases - a systems biology perspective. Aging (Albany NY) 2011;3:1178–91. doi: 10.18632/aging.100413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 57.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 58.Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276–88. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 60.Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–9. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 61.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blagosklonny MV, Darzynkiewicz Z. Four birds with one stone: RAPA as potential anticancer therapy. Cancer Biol Ther. 2002;1:359–61. doi: 10.4161/cbt.1.4.6. [DOI] [PubMed] [Google Scholar]
- 64.Mita MM, Mita A, Rowinsky EK. The molecular target of rapamycin (mTOR) as a therapeutic target against cancer. Cancer Biol Ther. 2003;2(Suppl 1):S169–77. [PubMed] [Google Scholar]
- 65.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–92. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 66.Dbouk HA, Backer JM. A beta version of life: p110β takes center stage. Oncotarget. 2010;1:729–33. doi: 10.18632/oncotarget.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hart JR, Vogt PK. Phosphorylation of AKT: a mutational analysis. Oncotarget. 2011;2:467–76. doi: 10.18632/oncotarget.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garrett JT, Chakrabarty A, Arteaga CL. Will PI3K pathway inhibitors be effective as single agents in patients with cancer? Oncotarget. 2011;2:1314–21. doi: 10.18632/oncotarget.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhattacharya B, Akram M, Balasubramanian I, Tam KK, Koh KX, Yee MQ, et al. Pharmacologic synergy between dual phosphoinositide-3-kinase and mammalian target of rapamycin inhibition and 5-fluorouracil in PIK3CA mutant gastric cancer cells. Cancer Biol Ther. 2012;13:34–42. doi: 10.4161/cbt.13.1.18437. [DOI] [PubMed] [Google Scholar]
- 70.Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway--beyond rapalogs. Oncotarget. 2010;1:530–43. doi: 10.18632/oncotarget.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Major P. Potential of mTOR inhibitors for the treatment of subependymal giant cell astrocytomas in tuberous sclerosis complex. Aging (Albany NY) 2011;3:189–91. doi: 10.18632/aging.100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martelli AM, Evangelisti C, Chiarini F, McCubrey JA. The phosphatidylinositol 3-kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients. Oncotarget. 2010;1:89–103. doi: 10.18632/oncotarget.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li H, Gao Q, Guo L, Lu SH. The PTEN/PI3K/Akt pathway regulates stem-like cells in primary esophageal carcinoma cells. Cancer Biol Ther. 2011;11:950–8. doi: 10.4161/cbt.11.11.15531. [DOI] [PubMed] [Google Scholar]
- 74.Barger JF, Gallo CA, Torni KA, Merk L, Seibel WL, Nelson S, et al. Identification of Akt-selective cytotoxic compounds that enhance cytotoxic responses to rapamycin. Cancer Biol Ther. 2011;10:1256–61. doi: 10.4161/cbt.10.12.13442. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt-Kittler O, Zhu J, Yang J, Liu G, Hendricks W, Lengauer C, et al. PI3Kα inhibitors that inhibit metastasis. Oncotarget. 2010;1:339–48. doi: 10.18632/oncotarget.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sokolosky ML, Stadelman KM, Chappell WH, Abrams SL, Martelli AM, Stivala F, et al. Involvement of Akt-1 and mTOR in sensitivity of breast cancer to targeted therapy. Oncotarget. 2011;2:538–50. doi: 10.18632/oncotarget.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dancey JE. Therapeutic targets: MTOR and related pathways. Cancer Biol Ther. 2006;5:1065–73. doi: 10.4161/cbt.5.9.3175. [DOI] [PubMed] [Google Scholar]
- 78.Weber GL, Parat MO, Binder ZA, Gallia GL, Riggins GJ. Abrogation of PIK3CA or PIK3R1 reduces proliferation, migration, and invasion in glioblastoma multiforme cells. Oncotarget. 2011;2:833–49. doi: 10.18632/oncotarget.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A. 2005;102:8204–9. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–75. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 81.Matthew EM, Hart LS, Astrinidis A, Navaraj A, Dolloff NG, Dicker DT, et al. The p53 target Plk2 interacts with TSC proteins impacting mTOR signaling, tumor growth and chemosensitivity under hypoxic conditions. Cell Cycle. 2009;8:4168–75. doi: 10.4161/cc.8.24.10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci U S A. 2010;107:9660–4. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leontieva OV, Gudkov AV, Blagosklonny MV. Weak p53 permits senescence during cell cycle arrest. Cell Cycle. 2010;9:4323–7. doi: 10.4161/cc.9.21.13584. [DOI] [PubMed] [Google Scholar]
- 84.Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging (Albany NY). 2010;2:344-352. doi: 10.18632/aging.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blagosklonny MV. The power of chemotherapeutic engineering: arresting cell cycle and suppressing senescence to protect from mitotic inhibitors. Cell Cycle. 2011;10:2295–8. doi: 10.4161/cc.10.14.16819. [DOI] [PubMed] [Google Scholar]
- 86.Maki CG. Decision-making by p53 and mTOR. Aging (Albany NY) 2010;2:324–6. doi: 10.18632/aging.100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Serrano M. Shifting senescence into quiescence by turning up p53. Cell Cycle. 2010;9:4256–7. doi: 10.4161/cc.9.21.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Darzynkiewicz Z. Another “Janus paradox” of p53: induction of cell senescence versus quiescence. Aging (Albany NY) 2010;2:329–30. doi: 10.18632/aging.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Galluzzi L, Kepp O, Kroemer G. TP53 and MTOR crosstalk to regulate cellular senescence. Aging (Albany NY) 2010;2:535–7. doi: 10.18632/aging.100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chao SK, Horwitz SB, McDaid HM. Insights into 4E-BP1 and p53 mediated regulation of accelerated cell senescence. Oncotarget. 2011;2:89–98. doi: 10.18632/oncotarget.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lane DP, Verma C, Fang CC. The p53 inducing drug dosage may determine quiescence or senescence. Aging (Albany NY) 2010;2:748. doi: 10.18632/aging.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Poyurovsky MV, Prives C. P53 and aging: A fresh look at an old paradigm. Aging (Albany NY) 2010;2:380–2. doi: 10.18632/aging.100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vigneron A, Vousden KH. p53, ROS and senescence in the control of aging. Aging (Albany NY) 2010;2:471–4. doi: 10.18632/aging.100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blagosklonny MV. Molecular damage in cancer: an argument for mTOR-driven aging. Aging (Albany NY) 2011;3:1130–41. doi: 10.18632/aging.100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blagosklonny MV. Oncogenic resistance to growth-limiting conditions. Nat Rev Cancer. 2002;2:221–5. doi: 10.1038/nrc743. [DOI] [PubMed] [Google Scholar]
- 96.Greaves M. Leukemogenesis and ageing: 'fit for transformation'? Aging (Albany, NY Online) 2011;3:79–80. doi: 10.18632/aging.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Henry CJ, Marusyk A, DeGregori J. Aging-associated changes in hematopoiesis and leukemogenesis: what’s the connection? Aging (Albany NY) 2011;3:643–56. doi: 10.18632/aging.100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blagosklonny MV. Antiangiogenic therapy and tumor progression. Cancer Cell. 2004;5:13–7. doi: 10.1016/S1535-6108(03)00336-2. [DOI] [PubMed] [Google Scholar]
- 99.Garber K. Rapamycin’s resurrection: a new way to target the cancer cell cycle. J Natl Cancer Inst. 2001;93:1517–9. doi: 10.1093/jnci/93.20.1517. [DOI] [PubMed] [Google Scholar]
- 100.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–48. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 101.Choo AY, Blenis J. TORgeting oncogene addiction for cancer therapy. Cancer Cell. 2006;9:77–9. doi: 10.1016/j.ccr.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 102.Kwitkowski VE, Prowell TM, Ibrahim A, Farrell AT, Justice R, Mitchell SS, et al. FDA approval summary: temsirolimus as treatment for advanced renal cell carcinoma. Oncologist. 2010;15:428–35. doi: 10.1634/theoncologist.2009-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Global ARCC Trial Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 104.Altman JK, Sassano A, Platanias LC. Targeting mTOR for the treatment of AML. New agents and new directions. Oncotarget. 2011;2:510–7. doi: 10.18632/oncotarget.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sacco A, Roccaro A, Ghobrial IM. Role of dual PI3/Akt and mTOR inhibition in Waldenstrom’s Macroglobulinemia. Oncotarget. 2010;1:578–82. doi: 10.18632/oncotarget.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mercier I, Camacho J, Titchen K, Gonzales DM, Quann K, Bryant KG, et al. Caveolin-1 and accelerated host aging in the breast tumor microenvironment: chemoprevention with rapamycin, an mTOR inhibitor and anti-aging drug. Am J Pathol. 2012;181:278–93. doi: 10.1016/j.ajpath.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lewis DA, Travers JB, Machado C, Somani AK, Spandau DF. Reversing the aging stromal phenotype prevents carcinoma initiation. Aging (Albany NY) 2011;3:407–16. doi: 10.18632/aging.100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smit MA, Peeper DS. Epithelial-mesenchymal transition and senescence: two cancer-related processes are crossing paths. Aging (Albany NY) 2010;2:735–41. doi: 10.18632/aging.100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446–9. doi: 10.1111/j.1399-0012.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 110.Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, et al. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317–23. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- 111.Campistol JM, Eris J, Oberbauer R, Friend P, Hutchison B, Morales JM, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol. 2006;17:581–9. doi: 10.1681/ASN.2005090993. [DOI] [PubMed] [Google Scholar]
- 112.Cullis B, D’Souza R, McCullagh P, Harries S, Nicholls A, Lee R, et al. Sirolimus-induced remission of posttransplantation lymphoproliferative disorder. Am J Kidney Dis. 2006;47:e67–72. doi: 10.1053/j.ajkd.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 113.Yakupoglu YK, Buell JF, Woodle S, Kahan BD. Individualization of immunosuppressive therapy. III. Sirolimus associated with a reduced incidence of malignancy. Transplant Proc. 2006;38:358–61. doi: 10.1016/j.transproceed.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 114.Zmonarski SC, Boratyńska M, Rabczyński J, Kazimierczak K, Klinger M. Regression of Kaposi’s sarcoma in renal graft recipients after conversion to sirolimus treatment. Transplant Proc. 2005;37:964–6. doi: 10.1016/j.transproceed.2004.12.172. [DOI] [PubMed] [Google Scholar]
- 115.Granville CA, Warfel N, Tsurutani J, Hollander MC, Robertson M, Fox SD, et al. Identification of a highly effective rapamycin schedule that markedly reduces the size, multiplicity, and phenotypic progression of tobacco carcinogen-induced murine lung tumors. Clin Cancer Res. 2007;13:2281–9. doi: 10.1158/1078-0432.CCR-06-2570. [DOI] [PubMed] [Google Scholar]
- 116.Mabuchi S, Altomare DA, Connolly DC, Klein-Szanto A, Litwin S, Hoelzle MK, et al. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res. 2007;67:2408–13. doi: 10.1158/0008-5472.CAN-06-4490. [DOI] [PubMed] [Google Scholar]
- 117.Mosley JD, Poirier JT, Seachrist DD, Landis MD, Keri RA. Rapamycin inhibits multiple stages of c-Neu/ErbB2 induced tumor progression in a transgenic mouse model of HER2-positive breast cancer. Mol Cancer Ther. 2007;6:2188–97. doi: 10.1158/1535-7163.MCT-07-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Checkley LA, Rho O, Moore T, Hursting S, DiGiovanni J. Rapamycin is a potent inhibitor of skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Prev Res (Phila) 2011;4:1011–20. doi: 10.1158/1940-6207.CAPR-10-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robinson J, Lai C, Martin A, Nye E, Tomlinson I, Silver A. Oral rapamycin reduces tumour burden and vascularization in Lkb1(+/-) mice. J Pathol. 2009;219:35–40. doi: 10.1002/path.2562. [DOI] [PubMed] [Google Scholar]
- 120.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176:2092–7. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blagosklonny MV. Prevention of cancer by inhibiting aging. Cancer Biol Ther. 2008;7:1520–4. doi: 10.4161/cbt.7.10.6663. [DOI] [PubMed] [Google Scholar]
- 122.Komarova EA, Antoch MP, Novototskaya LR, Chernova OB, Paszkiewicz G, Leontieva OV, et al. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/− mice. Aging (Albany, NY Online) 2012:4. doi: 10.18632/aging.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23:817–22. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- 124.Hursting SD, Perkins SN, Phang JM. Calorie restriction delays spontaneous tumorigenesis in p53-knockout transgenic mice. Proc Natl Acad Sci U S A. 1994;91:7036–40. doi: 10.1073/pnas.91.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hursting SD, Perkins SN, Brown CC, Haines DC, Phang JM. Calorie restriction induces a p53-independent delay of spontaneous carcinogenesis in p53-deficient and wild-type mice. Cancer Res. 1997;57:2843–6. [PubMed] [Google Scholar]
- 126.Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle. 2006;5:2087–102. doi: 10.4161/cc.5.18.3288. [DOI] [PubMed] [Google Scholar]
- 127.Kolosova NG, Muraleva NA, Zhdankina AA, Stefanova NA, Fursova AZ, Blagosklonny MV. Prevention of age-related macular degeneration-like retinopathy by rapamycin in rats. Am J Pathol. 2012;181:472–7. doi: 10.1016/j.ajpath.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 128.Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron. 2012;75:425–36. doi: 10.1016/j.neuron.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Blagosklonny MV. Increasing healthy lifespan by suppressing aging in our lifetime: preliminary proposal. Cell Cycle. 2010;9:4788–94. doi: 10.4161/cc.9.24.14360. [DOI] [PubMed] [Google Scholar]
- 130.Sherif ZA, Sultan AS. Divergent control of Cav-1 expression in non-cancerous Li-Fraumeni syndrome and human cancer cell lines. Cancer Biol Ther. 2012;14:11. doi: 10.4161/cbt.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Herbert BS, Chanoux RA, Liu Y, Baenziger PH, Goswami CP, McClintick JN, et al. A molecular signature of normal breast epithelial and stromal cells from Li-Fraumeni syndrome mutation carriers. Oncotarget. 2010;1:405–22. doi: 10.18632/oncotarget.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Glazer RI, et al. A new therapeutic basis for treating Li-Fraumeni Syndrome breast tumors expressing mutated TP53. Oncotarget. 2010;1:470–1. doi: 10.18632/oncotarget.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Darzynkiewicz Z, Leontieva OV, Demidenko ZN, Li F, Blagosklonny MV. Novel strategies of protecting non-cancer cells during chemotherapy: are they ready for clinical testing? Oncotarget. 2011;2:107–8. doi: 10.18632/oncotarget.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van Leeuwen IM, Laín S. Pharmacological manipulation of the cell cycle and metabolism to protect normal tissues against conventional anticancer drugs. Oncotarget. 2011;2:274–6. doi: 10.18632/oncotarget.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo AA, Mitchell JB, et al. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell. 2012;11:401–14. doi: 10.1016/j.stem.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Iglesias-Bartolome R, Gutkind JS. Exploiting the mTOR paradox for disease prevention. Oncotarget. 2012;3:1061–3. doi: 10.18632/oncotarget.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, et al. Fasting and cancer treatment in humans: A case series report. Aging (Albany NY) 2009;1:988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, et al. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–72. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Raffaghello L, Safdie F, Bianchi G, Dorff T, Fontana L, Longo VD. Fasting and differential chemotherapy protection in patients. Cell Cycle. 2010;9:4474–6. doi: 10.4161/cc.9.22.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4:24ra27. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Blagosklonny MV. Carcinogenesis, cancer therapy and chemoprevention. Cell Death Differ. 2005;12:592–602. doi: 10.1038/sj.cdd.4401610. [DOI] [PubMed] [Google Scholar]