Abstract
Advanced non-small lung cancer (NSCLC) remains almost uniformly lethal with marginal long-term survival despite efforts to target specific oncogenic addiction pathways that may drive these tumors with small molecularly targeted agents and biologics. The EML4-ALK fusion gene encodes a chimeric tyrosine kinase that activates the Ras signaling pathway, and this fusion protein is found in approximately 5% of NSCLC. Targeting EML4-ALK with Crizotinib in this subset of NSCLC has documented therapeutic efficacy, but the vast majority of patients eventually develop recurrent disease that is often refractory to further treatments. We present the clinicopathologic features of three patients with metastatic NSCLC harboring the EML4-ALK translocation that developed isolated central nervous system (CNS) metastases in the presence of good disease control elsewhere in the body. These cases suggest a differential response of NSCLC to Crizotinib in the brain in comparison to other sites of disease, and are consistent with a previous report of poor CNS penetration of Crizotinib. Results of ongoing clinical trials will clarify whether the CNS is a major sanctuary site for EML4-ALK positive NSCLC being treated with Crizotinib. While understanding molecular mechanisms of resistance is critical to overcome therapeutic resistance, understanding physiologic mechanisms of resistance through analyzing anatomic patterns of failure may be equally crucial to improve long-term survival for patients with EML4-ALK translocation positive NSCLC.
Keywords: Non-small cell lung cancer, anaplastic lymphoma kinase (ALK), brain metastasis, stereotactic radiosurgery, Crizotinib
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer mortality in the United States with over 225,000 new cases and 160,000 deaths expected in 2012.1 While greater than 85% of NSCLC is causally attributed to smoking or tobacco exposure, a minority of NSCLC occurs in non-smokers, and they are often associated with specific oncogenic mutations.2 Such mutations are found in genes such as the Rat sarcoma kinase (Ras), epidermal growth factor receptor (EGFR), and the anaplastic lymphoma kinase (ALK).3 The identification of novel molecular targets associated with NSCLC development has brought about the simultaneous development of rational target-based molecularly targeted agents and biologics for the purpose of overcoming therapeutic resistance in this subset of NSCLC. Currently, the National Comprehensive Cancer Network (NCCN) guidelines recommend molecular profiling of NSCLC with adenocarcinoma histology to detect genetic alterations potentially susceptible to targeted therapy regardless of history of smoking.4 With an increasing number of patients treated with molecularly targeted inhibitors and biologics, it is critical to understand the role of these agents in altering the natural history of NSCLC by analyzing the clinicopathologic features of patients in order to tailor therapy, advance care, and improve survival.
It is estimated that less than 5% of NSCLC are associated with the gene fusion product of the echinoderm microtubule-associated protein-like 4 (EML4) and ALK.5,6 The EML4-ALK fusion protein auto-phosphorylates and constitutively activates the Ras signaling cascade.7 Constitutive Ras activation promotes NSCLC initiation, growth, and metastasis by allowing aberrant cell cycle progression and maintenance of putative cancer stem cell homeostasis,8 conferring a poor clinical prognosis.9,10 Due to activation of Ras pathway mediators, even early tumors harboring the EML4-ALK translocation tends to be aggressive with a higher chance of early relapse.7 The clinical efficacy of targeted ALK inhibition in advanced NSCLC was shown in the PF-02341066 phase I clinical trial in which the oral ALK, c-ros proto-oncogene 1 receptor tyrosine kinase (ROS1), and c-Met inhibitor Crizotinib had a response rate of 57% in patients harboring EML4-ALK translocation with only mild toxicity.11 These results compared favorably to the 20–30% response rates for conventional platinum-based chemotherapy,12,13 leading to the Food and Drug Administration (FDA) approval of Crizotinib for patients with metastatic NSCLC harboring EML4-ALK translocation.
With increasing use of Crizotinib for NSCLC, it is important to understand patterns of failure and resistance to therapy, with the goal of further improving therapeutic efficacy. While pre-clinical pharmacologic studies suggest good plasma distribution of Crizotinib,14-16 an isolated central nervous system failure associated with low cerebrospinal concentrations of Crizotinib has previously been reported in a patient with EML4-ALK positive NSCLC with brain metastases.17 In this case series, we report three patients with EML4-ALK positive NSCLC treated with Crizotinib who developed isolated brain metastases despite otherwise well controlled systemic disease. The clinical response of these patients suggests poor control of CNS disease by Crizotinib, and underscores the necessity to improve CNS bio-availability, as well as using combined-modality approaches to overcome the apparent potential Achilles heel of this treatment approach.
Clinical Case Series
The clinical, histopathologic, and molecular characteristics of these patients are summarized in Table 1. All three of the patients are non-smoking Caucasian females with metastatic moderately-differentiated NSCLC, harboring the EML4-ALK gene re-arrangement as shown by fluorescent in situ hybridization (FISH). The status of EGFR, K-RAS, and B-RAF were all wild-type. Throughout Crizotinib treatment, all patients tolerated Crizotinib well with minimal side effects.
Table 1. The clinicopathologic features of three patients with EML4-ALK translocation positive NSCLC are summarized that developed progressive brain metastases in spite of good systemic control elsewhere.
| Patient | Age at diagnosis | Histopathology | Primary tumor | Sites of Disease at diagnosis | Sites of disease progression | No. of CNS lesions | Smoking status | Brain RT |
|---|---|---|---|---|---|---|---|---|
| 1 |
41 y |
Moderately-differentiated adenocarcinoma |
Left lower lobe |
Skeleton |
Brain |
12 |
Never smoker |
SRS 20 Gy |
| Bilateral lungs | ||||||||
| Mediastinum | ||||||||
| 2 |
51 y |
Moderately-differentiated adenocarcinoma |
Right middle lobe |
Brain |
Brain |
36 |
Never smoker |
SRS 15 Gy |
| Skeleton |
|
|||||||
| Mediastinum |
WBRT 37.5 Gy |
|||||||
| 3 | 59 y | Moderately-differentiated adenocarcinoma | Right hilum | Brain |
Brain | 22 | Never smoker | WBRT 35 Gy |
| Supraclavicular | ||||||||
| Bilateral Pleural Efusion | ||||||||
| Mediastinum |
CNS, central nervous system; SRS, stereotactic radiosurgery; RT, radiation therapy; No., number; WBRT, whole brain radiotherapy; Gy, Grey (joule per kilogram).
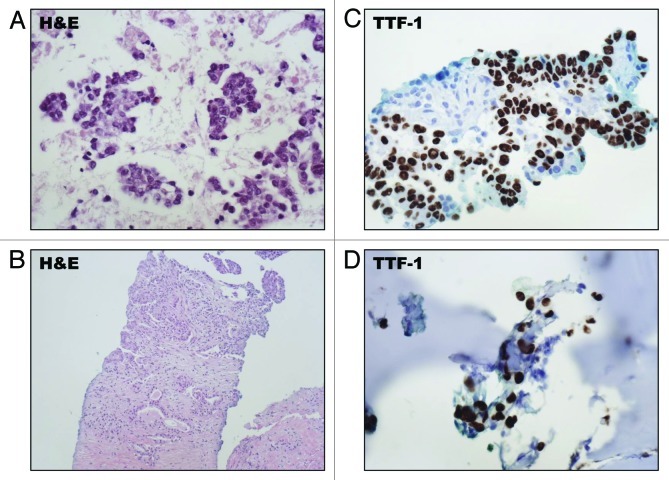
The first patient was diagnosed at age 41 with metastatic NSCLC after a chest X-ray, obtained for cough and night sweats, showed a large left lower lobe mass. A CT (CT)-scan of the chest confirmed the presence of a left lower lobe mass (Fig. 1A), and a positron emission tomography (PET) CT-scan showed diffuse skeletal changes suggestive of metastatic disease (Fig. 1B). An endobronchial fine needle aspiration (FNA) of the primary tumor showed malignant cells, and a core needle biopsy of her left lung tumor showed moderately-differentiated adenocarcinoma (Fig. 2A and B) which expressed the lung-specific thyroid transcription factor 1 (TTF-1) by immunohistochemistry (Fig. 2C), and due to facial pain, a nasal bone biopsy was done which confirmed metastatic disease that similarly expressed TTF-1 (Fig. 2D). Molecular characterization of the tumor using FISH showed that it harbored the EML4-ALK 2p23 gene re-arrangement. Further metastatic work-up by a PET CT-scan showed bilateral lung disease and extensive skeletal metastases, and an initial MRI of the brain showed no evidence of CNS metastases. The patient was then treated with single-agent Crizotinib with partial response and good control of her skeletal and pulmonary disease at 6 mo (Fig. 1C). However, a magnetic resonance image (MRI) of the brain at 6 mo showed the development of 12 new metastatic lesions not present on initial brain imaging (Fig. 1D). Each lesion was treated with stereotactic radiosurgery to a dose of 20 Gy in a single fraction. Prior to radiosurgery, Crizotinib was stopped for one week to avoid potentially adverse interactions with concurrent radiation, and re-started one week after radiation treatment.
Figure 1. Radiographic findings in patient 1. (A) An initial CT-scan of the chest showed a large tumor arising from the left lower lobe of the lung. (B) An initial staging PET CT-scan showed diffuse FDG avidity suggestive of widespread metastases involving the skeleton. (C) After 6 mo of Crizotinib monotherapy, the patient had a partial response with remarkable improvement in Fluorodeoxyglucose (FDG) avidity seen in a PET CT-scan. (D) An MRI of the brain at 6 mo showed development of 12 brain metastases, two of which are visible in this axial T1-post contrast image.
Figure 2. Histopathologic features of the EML4-ALK translocation positive tumor from patient 1. (A) An FNA of the patients left lung tumor showed malignant cells with hematoxylin and eosin (H&E) staining. (B) A core needle biopsy of the primary left lower lobe lung tumor showed moderately-differentiated adenocarcinoma. (C) The tumor cells exhibited nuclear expression of TTF-1 by IHC. (D) A biopsy of a nasal bone lesion confirmed metastatic disease which similarly expressed TTF-1 by IHC.
The second patient was diagnosed with metastatic NSCLC with the EML4-ALK gene rearrangement at age 51 following a motor vehicle accident associated with blurred vision. A CT-scan and MRI of the brain showed 3 large intracranial lesions suggestive of metastatic disease (Fig. 3A), and a CT-scan of the chest showed a primary tumor likely arising from the right middle lobe (Fig. 3B), with widespread involvement of the mediastinal lymph nodes, and skeletal metastases. A CT-guided biopsy of the presumed primary tumor in the right middle lobe showed moderately-differentiated adenocarcinoma, and FISH showed the EML4-ALK fusion product. The patient then underwent whole brain radiation therapy to a dose of 37.5 Gy in 15 fractions, and was subsequently treated with single-agent Crizotinib with good systemic response. At 8 mo, re-staging with a PET CT-scan showed durable partial response and control of previously identified systemic disease (Fig. 3C), but an MRI of the brain showed the development of multiple brain metastases, all of which were new (Fig. 3D). These lesions were treated with stereotactic radiosurgery to a dose of 15 Gy in a single fraction. Again, Crizotinib was held prior to radiosurgery to avoid potential complications, and re-started one week following radiation treatment.
Figure 3. Radiographic findings in patient 2. (A) An initial CT-scan of the brain showed 3 large lesions, one of which is seen in this axial image abutting the posterior right lateral ventricle. (B) The primary tumor likely arised from the right middle lobe adjacent to the hilum. (C) After 6 mo of Crizotinib monotherapy, the tumor in the right lower lobe exhibited a significant partial response. (D) An MRI of the brain after undergoing 8 mo of Crizotinib showed the interval development of multiple new metastases.
The third patient was diagnosed with metastastic NSCLC after the development of progressive dyspnea at age 59. Her initial chest CT-scan showed a large right hilar mass, bilateral pulmonary nodules, extensive mediastinal and supraclavicular adenopathy, and bilateral pleural effusions (Fig. 4A), and no initial brain metastases on MRI of the brain. An endoscopic bronchial biopsy of the right hilar mass showed moderately-differentiated adenocarcinoma (Fig. 4B), that expressed TTF-1 by immunohistochemistry. The patient was then treated with multiple chemotherapeutic regimens including Erlotonib monotherapy, Carboplatin and Paclitaxel, and Pemetrexed, with disease progression on each regimen. Due to symptomatic airway compression, the patient underwent right bronchial stenting. After one year of disease progression on multiple chemotherapeutic regimens, further molecular analysis of her tumor was done which revealed the presence of the EML4-ALK fusion gene by FISH. The patient was then enrolled on clinical trial where she received single-agent Crizotinib. After 3 mo of Crizotinib, she had clinical improvement in her dyspnea, with good partial response of the intrathoracic disease. However, at this time, she developed new-onset dizziness and dysmetria, and an MRI of the brain showed numerous new cystic metastases (Fig. 4C and D). The patient then underwent whole brain irradiation to a dose of 35 Gy in 14 fractions without concurrent Crizotinib. In spite of whole brain irradiation, the patient developed worsening neurologic symptoms, became obtunded, and died less than 2 mo later from CNS progression in spite of Crizotinib and maximal symptomatic support with high-dose steroids.
Figure 4. Radiographic findings in patient 3. (A) an initial CT-scan of the chest showed a large right hilar tumor with bilateral pleural effusions. (B) A biopsy of her right hilar tumor showed moderately-differentiaed adenocarcinoma. (C) Sn axial image and (D), a frontal/coronal image from a brain MRI showing development of multiple large metastases.
Molecular Features: Targeting the EML4-ALK Chimeric Kinase in NSCLC
The EML4-ALK gene translocation was initially identified in NSCLC in 2007 through analysis of cDNA from tumor tissue derived from a human pulmonary adenocarcinoma.5 Prior to its identification in NSCLC, translocations involving ALK had been identified in anaplastic lymphomas, inflammatory myofiroblastic tumors, and neuroblastomas.7 The typical activating ALK fusion gene identified in NSCLC involves an intra-chromosomal inversion of the short arm of chromosome 2 joined with exons 1 and 13 of EML4. The chimeric protein product of this chromosomal rearrangement causes auto-phosphorylation and constitutive activation of the ALK tyrosine kinase domain as well as the cytoplasmic localization of the kinase.7
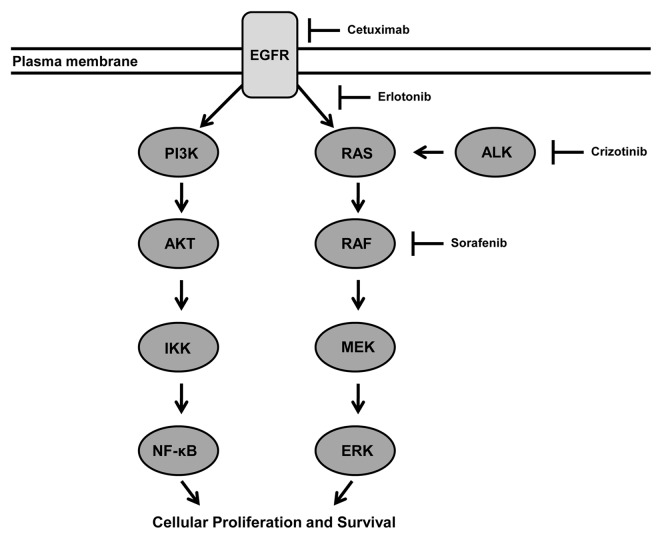
There is substantial pre-clinical evidence showing that constitutive kinase activity of the EML4-ALK fusion protein acts as a potent oncogenic driver of NSCLC progression. Phosphorylation targets of ALK signaling include the well characterized Ras signaling pathway. Activation of Ras signaling pathway independent of putative ALK-activating ligands promotes unregulated cell proliferation and survival through the activation of MAP and PI3 kinases (Fig. 5).3,18 Similar to NSCLC that expresses oncogenic mutations in Ras signaling, the phenotypes of NSCLC tumors with EML4-ALK mutations tend to be aggressive and resistant to conventional antineoplastic agents, portending a poor clinical prognosis.9,10 Moreover, transgenic mouse models expressing the EML4-ALK protein have a propensity to develop lung adenocarcinomas,19 similar to the human disease. In translational NSCLC models that overexpress ALK-signaling, Crizotinib has consistently been shown to have a potent antineoplastic effect,19,20 providing the impetus for human trials.20,21
Figure 5. Working model for the role of EML4-ALK chimeric kinase in driving the progression of NSCLC through activation of the EGFR pathway. The EML4-ALK protein phosphorylates and activates Ras signaling leading to a cascade of events driving cellular proliferation and growth. There are multiple redundancies in this pathway susceptible to targeted small molecule therapy. We propose that using agents such as Sorafenib with good CNS penetration distal to ALK-signaling represents an opportunity to overcome therapeutic resistance and reduce CNS failure.41 EGFR, epidermal growth factor receptor dimer; AKT, a serine/threonine-specific protein kinase; ERK, extracellular-signal related kinases; IKK, IκB kinase; NFκB, nuclear transcription factor kappa B; MEK, mitogen-activated protein kinase; RAF, protein serine/threonine protein kinase. →, activates; —|, inhibits.
While constitutive ALK activity has a potent oncogenic effect on tumorigenesis and cancer progression, the role of ALK in normal cellular and tissue homeostasis remains unclear.22 With multiple redundancies in the EGFR signaling pathway, it is not surprising that there are no gross phenotypic effects in ALK-knockout mice.23 Potential ALK activating ligands in normal cellular homeostasis include heparin-binding growth factors such as pleotrophin and midkine.7,24 It is possible that ALK plays a role in development and maintenance of CNS physiology in mammals where it is almost exclusively expressed in mouse CNS, particularly in the hippocampus.25 We speculate that this pattern of ALK expression in the CNS may account for the visual disturbances noted in 41% of patients in phase I clinical trial with Crizotinib.11
Since initial application of ALK inhibitors in phase I and II human clinical trials for advanced EML4-ALK translocation positive NSCLC, multiple phase III clinical trials have opened to evaluate its efficacy as a single agent and in combination with other standard NSCLC chemotherapeutics.26,27 The initial phase I clinical trial assessing toxicity of Crizotinib in NSCLC prospectively selected patients whose advanced NSCLC harbored ALK re-arrangement by FISH and absence of Met amplification.11 The majority of patients treated with Crizotinib had good response, with an overall response rate of 57%, and 6-mo progression free survival was 72%, comparing very favorably with historic experiences with conventional chemotherapy in advanced NSCLC. This effect was especially encouraging given that 94% of patients had previously received cytotoxic chemotherapy which likely selected for more aggressive tumors potentially resistant to further therapy.9,11
Discussion
Advanced metastatic NSCLC remains almost uniformly fatal with dismal long-term prognosis, even in cases where molecularly targeted agents and biologic inhibitors are used to target specific oncogenic mutations that promote cellular proliferation and survival. Even in patients with EML4-ALK positive NSCLC treated with Crizotinib, durability of disease control is transient with mean response duration of 6.2 mo,11 and the vast majority of patients die from progressive metastatic disease refractory to all known oncologic therapy. Overcoming therapeutic resistance through understanding molecular and cellular mechanisms of resistance is critical to improve outcomes in these patients. However, equally important is the understanding of physiologic and anatomic mechanisms of resistance to facilitate effective combined modality care. Analysis of anatomic patterns of failure will facilitate development of effective novel treatment strategies to address these physiologic and anatomic compartments providing a sanctuary and enabling resistance to targeted therapy. We have presented three cases of isolated CNS progression of EML4-ALK translocation-positive NSCLC while being treated with Crizotinib. These cases taken together suggest that the CNS provides an important sanctuary site for NSCLC with EM4-ALK translocation treated with Crizotinib.
This clinical case series intimates that EML4-ALK translocation positive NSCLC has a propensity to metastasize to the brain, and that Crizotinib has impaired control of brain metastases in comparison to other sites of systemic disease. These cases are consistent with the one previously reported case of diffuse CNS failure of NSCLC associated with low cerebrospinal concentration of Crizotinib of 0.617 ng/mL, in comparison to serum concentration of 237 ng/mL.17 In two of our cases, high dose stereotactic radiosurgery to numerous lesions was used to control their brain metastases given the excellent response to Crizotinib extracranially and previous failure of prior whole brain radiotherapy or patient refusal of whole brain radiotherapy.28,29 Although there is conflicting evidence regarding brain metastasis predisposition by EML4-ALK translocation in NSCLC with some retrospective analyses suggesting an increased predisposition for brain metastases while others show no significant increase in brain metastases,10,30 early data presented in abstract form at the American Society of Clinical Oncology Annual Meeting in 2012 by Weickhardt et al., suggest that the CNS is the primary site of initial treatment failure in 46% of EML4-ALK positive NSCLC patients treated with Crizotinib. The observation of high rates of CNS failure in EML4-ALK positive NSCLC is consistent with an abundance of evidence suggesting that activation of the EGFR pathway and Ras signaling promotes metastasis in NSCLC.31,32 Conceivably, EML4-ALK signaling confers a more aggressive clinical phenotype by maintaining putative cancer stem cell homeostasis thereby promoting metastatic potential to the CNS. The final results from current prospective trials of EML4-ALK positive NSCLC treated with Crizotinib will clarify whether Crizotinib changes the natural history in such a way that the brain becomes a major site of treatment failure and disease progression.
There are several plausible explanations for the differential treatment effect of Crizotinib in the brain in comparison to other systemic sites of disease. Possibilities include poor CNS penetration of Crizotinib,17 altered tumor biology within the brain parenchyma, and development of secondary mutations in the kinase domain of ALK conferring resistance to Crizotinib.33 While impaired drug delivery to the CNS is implicated as a potential mechanism of resistance by Costa et al.,17 a recent report of dramatic clinical response of glioblastoma with c-met amplification to Crizotinib suggests that even low concentrations of Crizotinib may be sufficient to inhibit its molecular targets such as ALK and c-met.34 It is even possible that Crizotinib itself may induce CNS metastases by activating signal transduction pathways that cause preferential metastasis to the brain, or that tumor cells capable of metastasis to the brain have an inherently more resistant and aggressive biology. A number of cancer pathways predisposing brain metastases have been identified,35 and determining the effect of Crizotinib on the expression pattern of such genes will help to clarify this hypothesis.
We propose a number of potential therapeutic strategies to overcome resistance to Crizotinib in the CNS. Given the poor CSF penetration of Crizotinib as previously reported,17 we speculate that further modification of Crizotinib to improve its bioavailability and possibly the development of an intrathecal formulation could be helpful in preventing and treating CNS disease. Additionally, should CNS progression be confirmed as a major site of disease failure in analyses of current Crizotinib trials, prophylactic cranial irradiation may be another feasible option to improve CNS control and survival. While prophylactic cranial irradiation has not shown a survival benefit in the heterogeneous overall NSCLC population,36 EML4-ALK positive NSCLC may represent a subset that has a propensity for early brain dissemination, similar to small-cell lung cancer, where combined modality intervention could improve survival.37,38 It is noteworthy that whole brain radiation therapy provided an 8 mo of CNS disease control in patient 2, supporting future studies examining prophylactic cranial irradiation for EML4-ALK positive NSCLC. Moreover, the typical rate of brain metastases in NSCLC is 30%,39,40 which argues for better CNS penetration of systemic agents in general. With respect to other targeted agents, Sorafenib and other Ras pathway inhibitors downstream of ALK with good CNS penetration may also be helpful in overcoming therapeutic resistance in the brain (Fig. 5).41 It is likely that a combination of strategies including novel targeted therapies, modification of current therapeutics to improve bio-availability, and aggressive combined modality approaches will be necessary to meaningfully improve long-term survival for these patients.
Presently, the interaction of targeted ALK inhibition with concurrent radiotherapy is poorly understood. In our case series, stereotactic radiosurgery and whole brain radiation therapy were used to improve CNS control without concurrent Crizotinib. The feasibility and toxicity of treating patients with whole brain radiation therapy or stereotactic radiosurgery concurrently with Crizotinib will require further study. There is currently insufficient evidence to support a therapeutic advantage for concurrent therapy and we propose that caution should be applied for concurrent use at this time. As nearly half of patients treated with Crizotinib have visual disturbances,11 it is possible that concurrent radiotherapy could exacerbate ocular toxicity. Moreover, the toxicity and efficacy of using Crizotinib in combination with concurrent thoracic radiotherapy as a definitive treatment for non-metastatic disease remains an unanswered question. A number of well-designed clinical trials will be necessary to answer these important clinical questions.
While the cases reported are suggestive of the CNS as a sanctuary site in EML4-ALK positive NSCLC treated with Crizotinib, we recognize the limitations of this small case series. It is entirely possible that these cases represent outliers in the spectrum of such patients treated with Crizotinib. Moreover, there is inherent selection bias in our reported patients, all of whom are being treated at a major academic center. Nevertheless, the pattern of CNS failure observed in our patients may still prove to be a relevant clinical phenomenon that will be clarified by the results of ongoing clinical trials assessing Crizotinib in advanced NSCLC.
Initial results of a phase II clinical trial (PROFILE 1005) were presented in abstract form at the American Society for Clinical Oncology in 2011, where an 83% tumor response rate was reported. The results of the phase III PROFILE 1007 trial comparing Crizotinib vs. second line chemotherapy in EML4-ALK positive NSCLC consisting of Taxotere or Premetrexed, and PROFILE 1014 comparing Crizotininb vs. platinum agent and Premetrexed are highly anticipated.26 The long-term results of PROFILE 1002 and 1006 phase I/II clinical trials will also help clarify the feasibility and toxicity profiles of Crizotinib combined with anti-EGFR therapies in EML4-ALK positive NSCLC which could potentially mitigate therapeutic resistance.
In summary, we have presented a case series of isolated CNS progression in patients with EML4-ALK translocation positive metastatic NSCLC being treated with single-agent Crizotinib. By understanding molecular, cellular, and anatomic mechanisms of treatment failure, we may be able to devise the optimal treatment strategy that will lead to improved disease control and ultimately survival. The results of multiple ongoing prospective clinical trials of Crizotinib in NSCLC will direct future efforts to advance care for this unique subset of patients.
Disclosure of Potential Conflicts of Interest
The authors have no financial interests to disclose.
Acknowledgments
The authors express gratitude to Dr. Jonathan Baker for his assistance in generating histologic images in this manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/22255
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25:561–70. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 3.Janku F, Stewart DJ, Kurzrock R. Targeted therapy in non-small-cell lung cancer--is it becoming a reality? Nat Rev Clin Oncol. 2010;7:401–14. doi: 10.1038/nrclinonc.2010.64. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Solomon B, Kenudson MM. Crizotinib and testing for ALK. J Natl Compr Canc Netw. 2011;9:1335–41. doi: 10.6004/jnccn.2011.0115. [DOI] [PubMed] [Google Scholar]
- 5.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 6.Martelli MP, Sozzi G, Hernandez L, Pettirossi V, Navarro A, Conte D, et al. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol. 2009;174:661–70. doi: 10.2353/ajpath.2009.080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw AT, Solomon B. Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res. 2011;17:2081–6. doi: 10.1158/1078-0432.CCR-10-1591. [DOI] [PubMed] [Google Scholar]
- 8.Mossé YP, Wood A, Maris JM. Inhibition of ALK signaling for cancer therapy. Clin Cancer Res. 2009;15:5609–14. doi: 10.1158/1078-0432.CCR-08-2762. [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–12. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang T, Liu H, Chen J. [EML4-ALK fusion gene in lung cancer and its biological function] Zhongguo Fei Ai Za Zhi. 2012;15:112–6. doi: 10.3779/j.issn.1009-3419.2012.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YH, Mishima M. Maintenance chemotherapy for non-small-cell lung cancer. Cancer Treat Rev. 2011;37:505–10. doi: 10.1016/j.ctrv.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Eastern Cooperative Oncology Group Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 14.Cui JJ, Tran-Dubé M, Shen H, Nambu M, Kung PP, Pairish M, et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK) J Med Chem. 2011;54:6342–63. doi: 10.1021/jm2007613. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki S, Vicini P, Shen Z, Zou HY, Lee J, Li Q, et al. Pharmacokinetic/pharmacodynamic modeling of crizotinib for anaplastic lymphoma kinase inhibition and antitumor efficacy in human tumor xenograft mouse models. J Pharmacol Exp Ther. 2012;340:549–57. doi: 10.1124/jpet.111.188870. [DOI] [PubMed] [Google Scholar]
- 16.Joshi M, Jiang Y, Belani CP. Maintenance therapy for advanced non-small-cell lung cancer: switch versus continuation. Expert Opin Pharmacother. 2012;13:685–97. doi: 10.1517/14656566.2012.668530. [DOI] [PubMed] [Google Scholar]
- 17.Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443–5. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 18.Okayama H, Kohno T, Ishii Y, Shimada Y, Shiraishi K, Iwakawa R, et al. Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res. 2012;72:100–11. doi: 10.1158/0008-5472.CAN-11-1403. [DOI] [PubMed] [Google Scholar]
- 19.Soda M, Takada S, Takeuchi K, Choi YL, Enomoto M, Ueno T, et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci U S A. 2008;105:19893–7. doi: 10.1073/pnas.0805381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Ye X, Liu J, Zha J, Pei L. Evaluation of EML4-ALK fusion proteins in non-small cell lung cancer using small molecule inhibitors. Neoplasia. 2011;13:1–11. doi: 10.1593/neo.101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–83. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009;420:345–61. doi: 10.1042/BJ20090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulford K, Morris SW, Turturro F. Anaplastic lymphoma kinase proteins in growth control and cancer. J Cell Physiol. 2004;199:330–58. doi: 10.1002/jcp.10472. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Pinera P, Chang Y, Deuel TF. Pleiotrophin, a multifunctional tumor promoter through induction of tumor angiogenesis, remodeling of the tumor microenvironment, and activation of stromal fibroblasts. Cell Cycle. 2007;6:2877–83. doi: 10.4161/cc.6.23.5090. [DOI] [PubMed] [Google Scholar]
- 25.Bilsland JG, Wheeldon A, Mead A, Znamenskiy P, Almond S, Waters KA, et al. Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology. 2008;33:685–700. doi: 10.1038/sj.npp.1301446. [DOI] [PubMed] [Google Scholar]
- 26.Galetta D, Rossi A, Pisconti S, Colucci G. The emerging role of ALK inhibitors in the treatment of advanced non-small cell lung cancer. Expert Opin Ther Targets. 2012;16(Suppl 2):S45–54. doi: 10.1517/14728222.2011.642372. [DOI] [PubMed] [Google Scholar]
- 27.Gandhi L, Jänne PA. Crizotinib for ALK-rearranged non-small cell lung cancer: a new targeted therapy for a new target. Clin Cancer Res. 2012;18:3737–42. doi: 10.1158/1078-0432.CCR-11-2393. [DOI] [PubMed] [Google Scholar]
- 28.Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427–34. doi: 10.1016/S0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 29.Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–41. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer. 2012:26. doi: 10.1002/cncr.27409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han C, Ma J, Zhao J, Zhou Y, Jing W, Zou H. EGFR mutations, gene amplification, and protein expression and KRAS mutations in primary and metastatic tumors of nonsmall cell lung cancers and their clinical implications: a meta-analysis. Cancer Invest. 2011;29:626–34. doi: 10.3109/07357907.2011.621914. [DOI] [PubMed] [Google Scholar]
- 32.Kim HR, Shim HS, Chung JH, Lee YJ, Hong YK, Rha SY, et al. Distinct clinical features and outcomes in never-smokers with nonsmall cell lung cancer who harbor EGFR or KRAS mutations or ALK rearrangement. Cancer. 2012;118:729–39. doi: 10.1002/cncr.26311. [DOI] [PubMed] [Google Scholar]
- 33.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. ALK Lung Cancer Study Group EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–9. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 34.Chi AS, Batchelor TT, Kwak EL, Clark JW, Wang DL, Wilner KD, et al. Rapid radiographic and clinical improvement after treatment of a MET-amplified recurrent glioblastoma with a mesenchymal-epithelial transition inhibitor. J Clin Oncol. 2012;30:e30–3. doi: 10.1200/JCO.2011.38.4586. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Wang Y, Chen Y, Sun S, Li N, Lv D, et al. Identification and validation of S100A7 associated with lung squamous cell carcinoma metastasis to brain. Lung Cancer. 2007;57:37–45. doi: 10.1016/j.lungcan.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 36.Gore E. RTOG 0214: a phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small cell lung cancer. Clin Adv Hematol Oncol. 2005;3:625–6. [PubMed] [Google Scholar]
- 37.Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. EORTC Radiation Oncology Group and Lung Cancer Group Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–72. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 38.Aupérin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ, et al. Prophylactic Cranial Irradiation Overview Collaborative Group Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999;341:476–84. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 39.Ma S, Xu Y, Deng Q, Yu X. Treatment of brain metastasis from non-small cell lung cancer with whole brain radiotherapy and Gefitinib in a Chinese population. Lung Cancer. 2009;65:198–203. doi: 10.1016/j.lungcan.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 40.Kawabe T, Phi JH, Yamamoto M, Kim DG, Barfod BE, Urakawa Y. Treatment of brain metastasis from lung cancer. Prog Neurol Surg. 2012;25:148–55. doi: 10.1159/000331188. [DOI] [PubMed] [Google Scholar]
- 41.Kim A, McCully C, Cruz R, Cole DE, Fox E, Balis FM, et al. The plasma and cerebrospinal fluid pharmacokinetics of sorafenib after intravenous administration in non-human primates. Invest New Drugs. 2012;30:524–8. doi: 10.1007/s10637-010-9585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]