Abstract
Purpose: Human Immunodefiency Virus (HIV) protease inhibitors (PI) remain a crucial component of highly active therapy (HAART) and recently have been demonstrated to have potent antitumor effect on a wide variety of tumor cell lines. However, discontinuation of therapy is an important issue, which may be related to various side-effects, especially diarrhea. The aim of this study was to evaluate the effects of nelfinavir (NFV), an HIV PI, and of alanyl-glutamine (AQ) supplementation, on intestinal cell migration, proliferation, apoptosis and necrosis, using IEC-6 cells and on intestinal crypt depth, villus length, villus area, mitotic index and apoptosis in Swiss mice. Methods: Migration was evaluated at 12 and 24 h after injury using a wound healing assay. Cellular proliferation was measured indirectly at 24 and 48 h using tetrazolium salt WST-1. Apoptosis and necrosis were measured by flow cytometry using the Annexin V assay. Intestinal morphometry and mitotic index in vivo were assessed following a seven-day treatment with 100 mg/kg of NFV, given orally. In vivo proliferation and apoptosis were evaluated by intestinal crypt mitotic index and immunohistochemistry, respectively. Results: In vitro, AQ supplementation enhanced IEC-6 cell migration and proliferation, following challenge with NFV. In vivo, AQ increased intestinal villus length, villus area, crypt depth and cell proliferation and cell migration, following treatment with NFV. AQ did not decrease cell death induced by NFV both in vivo and in vitro. Conclusions: AQ supplementation is potentially beneficial in preventing the effects of PIs, such as NFV, in the intestinal tract.
Keywords: Nelfinavir, chemotherapy, alanyl-glutamine, intestine, proliferation, migration, apoptosis, necrosis
Introduction
Protease inhibitors (PI) remain important components of highly active antiretroviral therapy (HAART) in the treatment of Human Immunodeficiency Virus (HIV) infection. However, approximately 60% of patients receiving HAART suffer from diarrhea and other gastrointestinal side effects that can compromise adherence to treatment and lead to treatment discontinuation.1,2 HAART associated diarrhea also reduces the absorption of oral antiretrovirals exposing patients to subtherapeutic serum levels of these medications and increasing the risk of drug resistance. Protease inhibitors alter cell survival by inducing apoptosis, necrosis3-5 and blocking DNA synthesis6 which may explain some of the mechanisms involved in intestinal damage. Indeed, we have demonstrated that the PIs induce weight loss, decrease intestinal villi height, and increase intestinal epithelial cell apoptosis in mice and reduce cell proliferation and increase apoptosis in rat intestinal epithelial cells in vitro.7
Recently, several studies have suggested that HIV PI’s could be repositioned as cancer therapeutics. Nelfinavir may have the most potent and broad antitumor activity of the HIV PI’s8-10 and has been suggested to have antitumor activity in many human cancer cell lines by inducing ER stress, autophagy, unfolded protein response and both caspase-dependent and independent cell death.10 There are currently, 20 clinical trials, listed by the National Institutes of Health, using nelfinavir as a single-agent or in combination with chemotherapy and radiation for cancer, most of which were not associated with HIV infection (reference http://clinicaltrials.gov/ct2/results?term=nelfinavir+cancer). Better understanding of the mechanisms underlying the intestinal epithelial damage and diarrhea induced by nelfinavir can potentially lead to novel treatment or preventive strategies with important clinical impact.
Glutamine, like epidermal growth factor (EGF) and insulin-like growth factor (IGF)-1, stimulates crypt cell proliferation and has an additional mitogenic effect on cultured intestinal IEC-6 rat crypt cells.11 Glutamine has been reported to have the ability to ‘spark’ proliferation, as a result of activation of extracellular signal-related kinases (ERK) 1 and 2 and phosphorylation of nuclear transcription factors, such as Elk-1 and c-Jun. Thus, glutamine may be a unique nutrient for enterocytes, capable of dual signaling and augmenting the effects of growth factors that regulate cellular proliferation and repair.12-14 The importance of glutamine as an essential precursor for nucleotide biosynthesis explains its critical requirement by proliferating cells such as those in the intestinal epithelium.15 This may be especially important during diarrheal diseases and malnutrition when the mucosal barrier function is often disrupted and under stressful conditions, when glutamine uptake by the body is enhanced.16 However, glutamine has limited solubility and a tendency to hydrolyze to potentially toxic glutamate. Alanyl-glutamine (AQ) is more stable, soluble and well tolerated and has been shown to be at least as effective as glutamine in intestinal injury repair in vitro, in vivo and in patients.17-24 Our group has demonstrated that AQ or glutamine supplementation given to patients on HAART, decreased diarrhea rates and increased antiretroviral serum concentration, indirectly indicating improvement of the intestinal epithelial function.21 Similarly, a clinical trial demonstrated that AQ supplementation ameliorated clinical symptoms of gastrointestinal toxicity in patients on chemotherapy.25
Although the protective effects of alanyl-glutamine in HIV positive patients taking anti-retroviral agents was previously reported,21 the mechanisms of how nelfinavir induces diarrhea and how alanyl-glutamine protects against this nelfinavir effect remains largely unknown. The aim of this study was to evaluate the potential benefits of AQ supplementation on NFV-induced intestinal epithelial damage, by examining its effects on cell migration, proliferation, necrosis, apoptosis and gut absorptive area, using both in vitro and in vivo models.
Results
Individual effects of NFV and AQ on IEC-6 cell migration
Pretreatment with NFV for 1 h caused reduction of cell migration at 12 and 24h in a dose-dependent manner. The most significant reduction of cell migration was seen with NFV at 100 μg/mL at 12 h (46.7% reduction vs control; p < 0.05) and with 70 μg/mL at 24h (63.3% reduction vs control; p < 0.05). NFV at 70 μg/mL was the lowest dose that caused significant reduction (p < 0.05) at both 12 and 24h (40.7% and 63.3%, respectively, vs control), therefore, this dose was chosen to evaluate the effects of AQ supplementation. Of note, NFV at 70 μg/mL is 10 times higher than the drug’s serum concentration but lower than the estimated intestinal lumen concentration (750 μg/mL) in vivo, taking into account the recommended oral dose and an intestinal volume of 1L.28,29 The tissue concentration is unknown but may be significantly higher in the intestinal epithelium, since after an oral uptake of the drug a concentration gradient is likely formed.3
The effect of AQ on cell migration was also examined. AQ supplementation caused a significant increase in cell migration in a dose-dependent manner. The most significant increase on cell migration was observed with AQ 10mM at both 12 and 24 h (increases of 24.2% and 30.2%, respectively, compared with control; p < 0.05), therefore, this dose was chosen to carry out the supplementation experiment following 1 h of NFV exposure. Supplementation with 10 mM of AQ, after pretreatment for 1 h with NFV, significantly improved cell migration by 49.9% and 56.6%, respectively, at 12 and 24 h (p < 0.001, by student’s unpaired t-test) (Fig. 1).
Figure 1. (A) Protective effect of 10 mM alanyl-glutamine (NFV+AQ) supplementation on IEC-6 cell migration following 1 h incubation with 70 μg/mL of nelfinavir (NFV) at 12 and 24 h. After reaching confluency, IEC-6 monolayers were scratched, wells were incubated with 70 μg/mL of NFV for 1h and washed with media without glutamine, followed by incubation with 10mM of AQ. The bars represent means ± SE for the number of migrating cells per square millimeter of scraped area. *p < 0.05, compared with control group with media without glutamine, by student’s unpaired t test. #p < 0.05, compared with group with NFV, by student’s unpaired t test. (B) Representative images of migration of IEC-6 cells at 24 h from the control group, NFV group, and AQ supplemented group, following NFV exposure for 1 h. Diagram shows scraping area with grid (each square = 0.1 mm2), overlapping the column of the farthest migration. The IEC-6 cells were tracked by traced dots for counting. The dots were counted digitally by Image Pro Plus software.
Effect of NFV and AQ on IEC-6 cell proliferation
NFV pretreatment for 1 h caused a significant reduction on cell proliferation at 24 and 48 h compared with the control group as seen in Figure 2A (reduction of 7% and 19.3%, respectively, compared with control, p < 0.05, by student’s unpaired t test). Supplementation with 10 mM of AQ significantly improved cell proliferation in NFV-pretreated wells at 24 and 48 h (increase of 27.1% and 63% vs control, p < 0.05, by student’s unpaired t-test). The effect of AQ alone on cell proliferation was also investigated with 1, 5, 10 and 50 mM of AQ. Cell proliferation increased in a dose dependent fashion following supplementation with AQ (Fig. 2B). The strongest proliferation increase was observed with 10 mM of AQ at both 24 h and 48 h (increase of 61% and 88.8% compared with control group; p < 0.05).
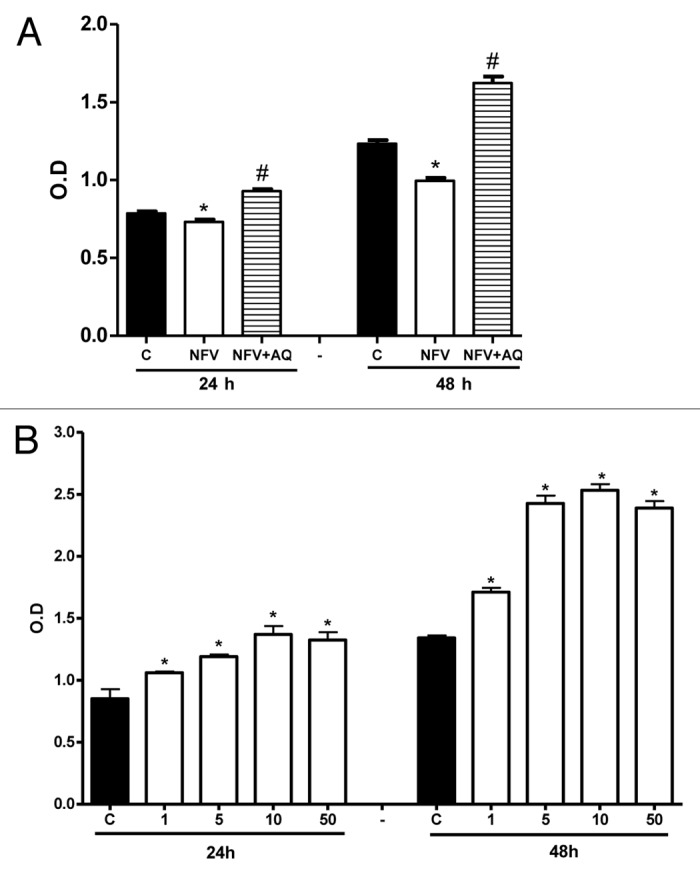
Figure 2. (A) Protective effect of supplementation with 10mM of alanyl-glutamine (AQ) on IEC-6 cell proliferation following 1h of exposure to nelfinavir (NFV) at 70μg/mL . (B) Effect of 1, 5, 10 and 50mM of AQ supplementation on IEC-6 cell proliferation, evaluated with a colorimetric assay by detecting absorbance using an ELISA microplate-reader at 450 nm. After 24 and 48 h, wells were incubated for 4 h with 10 μL of tetrazolium salt and the absorbance was measured. After 24 and 48 h, wells were incubated for 4 h with 10 μL of tetrazolium salt and the absorbance was measured. Values are expressed as mean ± standard error. *p < 0.05, compared with control group with media without glutamine, by student’s unpaired t test. #p < 0.05, compared with group with NFV, by student’s unpaired t-test.
Effect of NFV and AQ on cell apoptosis and necrosis
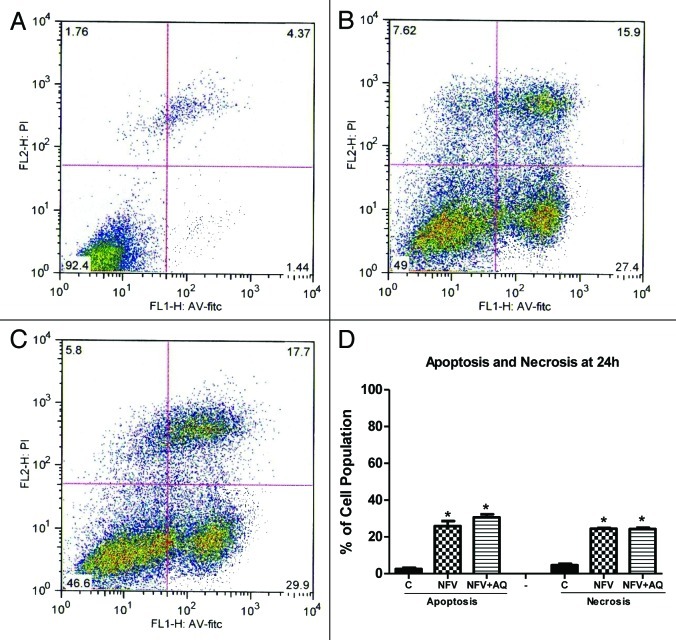
NFV pretreatment for 1 h at a dose of 70 μg/mL did not cause a significant change on rates of cell apoptosis after 24 h (Control: 1.23% vs NFV: 1.14%) or on cell necrosis (Control: 5.42% vs NFV: 5.94%). However, NFV pretreatment for 24h at a dose of 70 μg/mL caused a significant increase in the rates of apoptosis (Control: 2.51% vs NFV: 25.9%; p = 0.001) and necrosis (Control: 4.61% vs NFV: 24.42%; p < 0.0001) (Fig. 3). Supplementation with AQ at 10 mM, during the incubation period with NFV, did not cause a significant change at 24h on the rates of apoptosis (NFV: 25.9% vs NFV plus AQ:30.63% p = 0.21) and necrosis (NFV: 24.42% vs NFV + AQ: 24.26%; p = 0.86).
Figure 3. Flow cytometry analysis for apoptosis and necrosis rates on intestinal epithelial IEC-6 cell apoptosis and necrosis following 24 h of NFV treatment at 70 μg/mL, with or without supplementation with 10 mM of alanylglutamine (AQ). Control group (A), NFV (B), NFV+AQ (C). Lower-right quadrant represent apoptotic cells (high annexin V-FITC and low propidium iodide staining), lower-left quadrant indicate viable cells (low annexin V-FITC and propidium iodide staining), and upper-right quadrant show necrotic cells (high propidium iodide and annexin V-FITC staining). Graph (D) indicates the effect of both 1 h and 24 h exposure to NFV at 70 μg/mL and supplementation with AQ at 10 mM.
Effect of NFV and AQ on mouse body weight curves
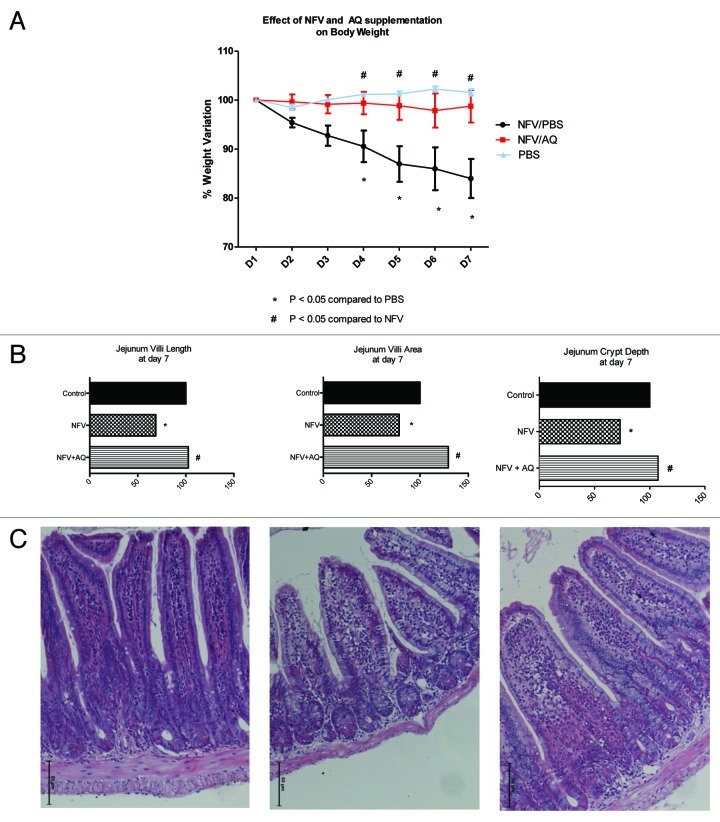
As shown in Figure 4A, NFV induced a significant reduction of body weight at days 4, 5, 6 and 7 (p < 0.001). Supplementation with 100mM of AQ prevented weight loss throughout the experiment (p < 0.001).
Figure 4. (A) Effect of nelfinavir at 100 mg/kg (NFV+PBS) and supplementation with 10 mM of alanyl-glutamine (NFV+AQ) compared with control (PBS), on body weight variation during 7-d treatment. For each animal (n = 6), daily body weight was measured. (B) Effect of NFV and AQ supplementation (NFV+AQ) on jejunum villi area, villli height, crypt depth and (C) morphometry after 7 d of treatment. For each animal (n = 6), 10 measurements of each small intestine segment were taken. Values are expressed as percentage of control. *p < 0.05, compared with PBS control group, by student’s unpaired t-test. #p < 0.05, compared with group with NFV, by one-way ANOVA, with Bonferroni’s post test.
Effect of NFV and AQ on intestinal morphometry and histopathology
As shown in Figure 4B and C, NFV significantly reduced jejunum villus height, surface area and crypt depth by 31.3% (p < 0.05), 21.4% (p < 0.05) and 26.8% (p < 0.05), respectively. AQ supplementation increased jejunal villus height, villus area and crypt depth by 33.9%, 50.8% and 34.4%, respectively.
Effect of NFV and AQ on intestinal tissue mitotic index
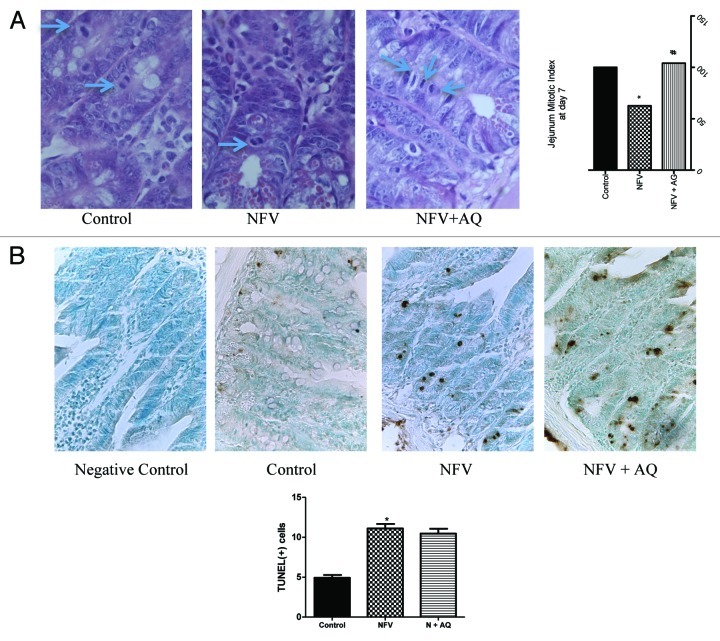
As shown in Figure 5A, seven-day course treatment with 100 mg/kg of NFV significantly reduced jejunum mitotic index by 37.4% when compared with control (p < 0.05). AQ supplementation increased mitotic index by 41.4% (p < 0.05).
Figure 5. (A) Effect of nelfinavir at 100 mg/kg (NFV) and supplementation with 10 mM of alanyl-glutamine (NFV+AQ) on jejunum intestinal morphometry and mitotic index compared with control group (CONTROL). Values are expressed as percentage of control. (B) Effects of NFV and AQ supplementation (NFV+AQ) on cell death in the jejunum after 7 d of treatment compared with the PBS control (C). For each animal (n = 6), intestinal samples were collected and stained with TUNEL for immunohistochemistry. All slices were counterstained with methyl green, designed for nuclear counterstaining (stained light green, similar to blue color), which provide excellent contrast to brown. A strong methyl green stain, observed in the negative control slice, means that no immunostaining was detected, as expected. Negative control represents a sample of the jejunum where the antibody was replaced by 5% PBS/BSA. *p < 0.05, compared with PBS control group, by student’s unpaired t test. #p < 0.05, compared with group with NFV, by one-way ANOVA, with Bonferroni’s post-test.
Effect of NFV and AQ on intestinal cell death
As seen in Figure 5B, seven-day course treatment with NFV at 100 mg/kg significantly increased the number of TUNEL-positive cells in the jejunum segments by 125% when compared with control (p < 0.05). Supplementation with 100 mM of AQ did not cause a significant reduction in the number of TUNEL-positive cells.
Discussion
This study demonstrates the beneficial effects of AQ supplementation on NFV-induced impairment of intestinal cell migration, proliferation and intestinal barrier but no effect on cell death both in vitro and in vivo. AQ prevented weight loss associated with NFV treatment in mice, indicating that the beneficial effects on cell proliferation and migration, outweighed its inability to prevent apoptosis and necrosis.
In a recent study evaluating the effects of PIs and reverse transcriptase inhibitors in the intestinal epithelium, we demonstrated that NFV and indinavir decreased cell proliferation.7 Additionally, both PIs and reverse transcriptase inhibitors altered intestinal tissue morphology and induced sodium secretion. Interestingly, only NFV increased intestinal permeability as measured by mannitol and lactulose excretion in mice. Furthermore, NFV caused significant weight loss as early as day five of treatment. In this current study, we confirmed that NFV causes reduction of body weight associated with notable histopathologic changes. We postulate that weight loss may be explained by decreased in either nutrient absorption or food ingestion. Indeed, although we did not observe diarrhea in the mice, jejunal villus lengths and area were decreased with NFV-treatment. Interestingly, another study evaluating the effect of NFV treatment in rabbits, found that the drug at 1000 mg/kg/day caused significant weight loss and decreased food consumption during the first 3 d of study with no report of diarrhea.30
The intestinal epithelium has a very dynamic cell population with a high turnover rate of approximately 3 days. Enterocytes proliferate in the crypt and migrate toward the villi where they may undergo apoptosis. The balance between proliferation, migration and apoptosis is crucial for the intestinal homeostasis.31 Danaher RJ et al. demonstrated that HIV-PI caused a significant reduction in cell viability in normal human keratinocytes NHOK and immortalized keratinocyte cell lines through blockage of DNA synthesis and not through cell death, as had been demonstrated in other tumor cell-lines.10,32 In this study we have found that NFV decreased cell proliferation in IEC-6 cells, an undifferentiated intestinal non-tumor cell line derived from rats, corroborating the hypothesis that PIs are able to decrease cell proliferation in non-tumor cell lines as well. In vivo, we demonstrated that NFV reduced jejunal villus height, villus area, crypt depth and crypt mitotic index. These alterations in villus and crypt morphometry in the presence of NFV may be explained by the drug-induced inhibition of cell proliferation (corroborated by decreased mitotic index in jejunal crypts) and migration observed in vitro.
HIV protease inhibitors have been suggested to have antitumor effects. In HT-29/B6 intestinal cells, PIs induced massive cell apoptosis, with no significant changes in necrosis rates or tight junction expression.3 Our group has reported that PIs can induce both apoptosis and necrosis in IEC-6 cells.8 Similarly, a recent study noted that ritonavir and lopinavir, but not amprenavir, induced apoptosis and necrosis by activating endoplasmic reticulum (ER) stress.4 Among the different PIs tested, NFV was found to be a potent inducer of ER stress, autophagy and apoptosis in various cell lines.10 In our study, we observed that prolonged exposure to NFV caused apoptosis and necrosis in IEC-6 cells as well as apoptosis in the intestinal epithelium in mice.
AQ enhanced cell proliferation and migration in IEC-6 cells even after exposure to NFV. These findings are consistent with our previous studies which have shown that glutamine and AQ were able to ameliorate intestinal epithelial damage induced by a variety of agents such as Clostridium difficile toxin A and 5-fluorouracil, a chemotherapy drug.27,33 As others have previously reported, glutamine is able to enhance crypt turnover activity through distinct growth factors, such as EGF and IGF-1, and is involved in mitotic signaling pathways including ERK 1 and 2, resulting in a shift of the intestinal balance toward more proliferative effects, rather than programmed cell death.34,35 Interestingly, we found that both glutamine and AQ were able to improve cell migration and apoptosis induced by toxin A, but not apoptosis induced by 5-flourouracil suggesting that glutamine may act through specific pathways on preventing cell death.27,33 Glutamine and AQ inhibited caspase 8 activation induced by toxin A and did not interfer with caspase 6 and 9 activation.36 We postulate that AQ might regulate cell apoptosis through a mechanism different from the one involved in 5FU- or NFV-induced apoptosis, resulting in its inability to revert or prevent cell death in this condition. That AQ was able to improve intestinal restitution in untransformed cells and mice, without affecting the pro-apoptotic effect of NFV, indicates AQ’s potential benefit in decreasing intestinal side-effects during chemotherapy with PIs. However, further studies evaluating the anti-tumor effects of NFV in the presence of alanyl-glutamine (which our study did not address) are warranted. Whether enhancement of intestinal absorption and ability to use increased doses of the drug may improve the effectiveness of NFV treatment against cancer in vivo are yet to be proven.
In summary, we provide evidence that AQ supplementation enhances intestinal epithelial repair in vitro and in vivo, after exposure to NFV, while not altering cell death induced by the PI. These results support exogenous AQ supplementation following treatment with PIs to ameliorate gastrointestinal side-effects and possibly allow increases in the maximum tolerated dose and enhance intestinal drug absorption, which may positively impact treatment efficacy and adherence. Further studies are warranted to elucidate the mechanisms involved in intestinal epithelial damage caused by PIs and repair by AQ.
Materials and Methods
Reagents and drugs
Glutamine (Gln) and AQ were obtained from Sigma. Tetrazolium salt WST-1 reagent was obtained from Roche. Mitomycin C was obtained from Roche. Nelfinavir (NFV) was obtained through the NIH-AIDS Reagent Program.
Cell culture
Rat intestinal jejunal crypt cells (IEC-6, passages 10–25) were purchased from American Type Culture Collection and were cultured at 37°C in a 5% CO2 incubator. The maintenance cell media was Dulbecco’s Modified Eagle Media (DMEM; Gibco BRL) supplemented with 5% fetal calf serum (FCS), 5mg bovine insulin, 50μg/ml of penicillin/streptomycin (DMEM; Gibco BRL) and a final concentration of 1mM of sodium pyruvate. The media was changed thrice a week, according to standard culture protocols. Dulbecco’s Modified Eagle Medium without glutamine (DMEM; Gibco BRL) was used whenever the supplementation effect of Gln or AQ was evaluated. The cultured cells were trypsinized with 0.25% EDTA trypsin when 90–95% confluence was achieved.
Wound healing assay of IEC-6 cell monolayers (cell migration)
IEC-6 cells were seeded in 12-well plates in a concentration of 6.25 × 104 cells/well and cultivated in DMEM media with 5% FCS (Gibco BRL). IEC-6 cells were confluent after 2 d following seeding. In order to rule out the effect of cell proliferation on cell migration, wells were incubated for 20 min with 5 μg/mL of mitomycin C, which inhibits DNA synthesis and cell mitosis. Wells were then scratched along their diameter and extended 30mm in length to the right center corner, using a sterile razor blade. Prior to scratching, 50% of the media volume was removed from each study well. The media was changed and the cells were then incubated in DMEM media without glutamine (Gln) with 0, 7, 10, 70 and 100 μg/mL of NFV, diluted in 0.5% DMSO. After one hour of incubation, wells were washed using DMEM media without Gln and incubated with DMEM media without Gln for 12 or 24 h. To evaluate the effect of AQ on cell migration, after incubating the wells with mitomycin C for 20min and scratching, the wells were incubated with either DMEM media without Gln or DMEM media without Gln supplemented with 1, 5, 10 or 50 mM of AQ. To evaluate the effect of AQ supplementation after NFV exposure, wells were incubated for 1 h with either DMEM media without Gln or 70 μg/mL of NFV. Afterwards, wells were washed with DMEM media without Gln and incubated for 12 or 24 h with either DMEM media without Gln or 10 mM of AQ. Wells were digitally photographed after 12 and 24 h.26,27 The digital pictures were analyzed using Image Pro Plus software version 5.0 (Media Cybernetics). A grid of 0.1 mm2 for each square was drawn, overlaying the digital pictures of the wells. The column of farthest migration was chosen. Cells within the column were then tracked using red dots, and digitally counted using the Image Pro Plus software.
Cell proliferation assay
Cell proliferation was measured indirectly using the tetrazolium salt WST-1 (4-[3-(4-iodophenyl)-2H-5-tetrazolio]-1-3-benzene disulfonate), according to the manufacturer recommendations. A 96-well plate was seeded with IEC-6 cells in a total concentration of 4 × 103 cells/well in 100 μL of DMEM media. Cells were allowed to attach for 48 h when and washed with 100 μL of DMEM media without Gln. The wells were incubated for 1 h with 70 μg/mL of NFV (dose chosen based on the migration results) diluted in 0.5% DMSO. The control groups were washed with DMEM media without glutamine and incubated for 1 h with 0.5% of DMSO. The cells were washed again with media without Gln and incubated for 24 and 48 h with either DMEM media without Gln or DMEM media supplemented with 10mM of AQ. To evaluate the effects of AQ on cell proliferation wells were incubated for 24 and 48h with either media without Gln or supplemented with 1, 5, 10 or 50 mM of AQ. After 24 and 48 h, wells were incubated for 2 h with 10 μL of the tetrazolium salt and the absorbance was measured using an ELISA microplate reader at 450 nm (reference range 420–480 nm). Tetrazolium salts are cleaved to formazan by mitochondrial enzymes in viable cells. Enhancement of the number of viable cells will result in an increase of the amount of the formazan dye, which is detectable by the ELISA reader.
Flow cytometry for apoptosis and necrosis
Apoptosis and necrosis were measured by flow cytometry analyses using the ApoAlert annexin V kit. Annexin V is a molecule that binds to phosphatidylserine (PS) and when conjugated to a fluorochrome detects apoptotic cells expressing PS on the reversed membrane surface. For this protocol, propidium iodide (PI) was used to detect necrotic and late apoptotic cells, which express PI inside the membrane. The cells were seeded on 12-well plates in a concentration of 5 × 105cells/well. These cells were allowed to attach on the plate surface for 24 h. Afterwards, cells were washed with DMEM media and incubated with NFV at 70 μg/mL for 1h or 24h. In the first group, after 1 h of exposure, media was replaced with Gln-free DMEM and incubated with or without 10 mM of AQ for 24 h. In the second group, cells were exposed to 70 μg/mL of NFV for 24h in DMEM media without Gln and supplemented or not with 10 mM of AQ during the 24h period. Cells were trypsinized, centrifuged, and washed with serum-containing media, before incubation with annexin V. Cells were counted and diluted to 105–106 cells and rinsed with 1 × Binding Buffer, and re-suspended in 200 μL of Binding Buffer. Five μL of annexin V and 10 μL of PI were added and cells were incubated for 5–15 min in the dark. The samples were then processed at the University of Virginia’s Flow Cytometry Core, using a FACS Calibur dual laser (Becton Dickinson).
Experimental animals
Male Swiss mice, 25–35 g body weight, were supplied from Center Vivarium at the Federal University of Ceará. The animals were kept in cages at room temperature, day-night cycle of 12 h, water and commercial balanced food were given ad libitum. All experiments were conducted in accordance with National Institute of Health guidelines on the welfare of experimental animals and with the approval of the Ethical Committee for animal research from the Federal University of Ceará. The animals were divided into four groups with six animals per group. NFV at a dose of 100 mg/kg/day, based on previous studies evaluating toxicity,7 or AQ (AQ: 100 mM, based on previous studies17 was given orally, via gavage, during seven days mixed with phosphate buffer saline (PBS; 0.25 ml). PBS was used as a control for either NFV or AQ. The following groups were utilized: (a) Control PBS; (b) NFV; and (c) NFV + AQ. Daily body weight was recorded during the 7-d treatment period. Diarrhea rates were assessed by observing loose stools and “wet tail.”
Histology and intestinal morphometry
Mice were sacrificed by a lethal injection of a euthanasia solution (chloral hydrate, 250 mg/kg, i.p), under anesthesia, on day 8. Immediately after euthanasia, 0.5 cm-samples were harvested from the duodenum, jejunum, and ileum, based on anatomical hallmarks. Tissue specimens were fixed in 10% neutral buffered formalin, and dehydrated for 12 h. On the following day, specimens were cut with a razor blade and then stored in 70% ethanol for paraffin embedding. 5 μm-thick cross-sections were prepared for hematoxylin-eosin staining (H&E). Crypt depth and villus height were measured from H&E stained slides on a light microscope equipped with a digital camera, and a computer-aid image capture system. Villus height was measured from the tip to the villus-crypt junction. The crypt depth was measured from the villus-crypt junction to the crypt bottom. All morphometric analyses were conducted blindly regarding experimental groups and diarrheal outcomes. The mounted intestinal segments were photographed at low power magnification (10x). Image J computer software version 1.33μ (NIH, Bethesda, MD) was used to measure crypt depth and villus height on ten randomly selected points for each experimental group.
Mitotic index
The effect of NFV and AQ supplementation on intestinal cell proliferation was evaluated following 7 d of treatment. The mitotic figures were blindly counted in 5 longitudinal crypt sections per animal (n = 4/group). Light microscopy at 100× magnification was used for measurements. The mitotic index for each group was obtained by averaging the absolute values.
In vivo analysis for cell death
After seven days of treatment with NFV with or without supplementation with AQ, intestinal tissue samples were collected as described above.
Intestinal tissue sections embedded in paraffin were hydrated and incubated with 20 μg/ml of proteinase K (Sigma) for 15 min at room temperature (RT) for analysis of apoptosis and necrosis using the ApopTag Plus Peroxidase In Situ Detection Kit (Serologicals Corp.) for TUNEL (terminal deoxynucleotidyltransferase [TdT]-mediated dUTP-biotin nick end labeling). Endogenous peroxidase was blocked by treatment with 3% (wt/vol) hydrogen peroxide in PBS for 5 min at RT. Afterwards, slides were washed with PBS and incubated in a humidified chamber at 37°C for 1 h with TdT buffer containing TdT enzyme and reaction buffer. Samples were then incubated for 10 min at RT with a stop/wash buffer and placed in a humidified chamber for 30 min with anti-digoxigenin-peroxidase conjugate at RT. Sections were then washed 3 times in PBS, the slides covered with peroxidase substrate to develop color, washed in three changes of distilled H2O and counterstained in 0.5% (vol/vol) methyl green for 10 min at RT. At least 10 randomly selected sections from each sample were counted and cell death measured by counting, under a light microscope, the number TUNEL positive cells.
Statistical analyses
Results are expressed as mean ± standard error (SEM), as generated by GraphPad Prism version 4.0 (GraphPad software). The differences between the experimental groups were compared by using either one-way ANOVA, with Bonferroni’s post-test, or unpaired Student’s t-test. Statistical significance was accepted at the level of p < 0.05.
Disclosure of Potential Conflicts of Interest
RLG co-founded AlGlutamine, LLC.
Acknowledgments
We would like to thank Dr. Benedito Carneiro for reviewing our manuscript, Joanne Lennigan for technical assistance with flow cytometry.
Financial support. This study was supported by the National Research Council (CNPq), Brazil and by the National Institutes of Health GIDRT Grant # 5 D43 TW006578.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/22251
References
- 1.Guarino A, Bruzzese E, De Marco G, Buccigrossi V. Management of gastrointestinal disorders in children with HIV infection. Paediatr Drugs. 2004;6:347–62. doi: 10.2165/00148581-200406060-00003. [DOI] [PubMed] [Google Scholar]
- 2.Tramarin A, Parise N, Campostrini S, Yin DD, Postma MJ, Lyu R, et al. Palladio Study Group Association between diarrhea and quality of life in HIV-infected patients receiving highly active antiretroviral therapy. Qual Life Res. 2004;13:243–50. doi: 10.1023/B:QURE.0000015282.24774.36. [DOI] [PubMed] [Google Scholar]
- 3.Bode H, Lenzner L, Kraemer OH, Kroesen AJ, Bendfeldt K, Schulzke JD, et al. The HIV protease inhibitors saquinavir, ritonavir, and nelfinavir induce apoptosis and decrease barrier function in human intestinal epithelial cells. Antivir Ther. 2005;10:645–55. [PubMed] [Google Scholar]
- 4.Wu X, Sun L, Zha W, Studer E, Gurley E, Chen L, et al. HIV protease inhibitors induce endoplasmic reticulum stress and disrupt barrier integrity in intestinal epithelial cells. Gastroenterology. 2010;138:197–209. doi: 10.1053/j.gastro.2009.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badley AD. In vitro and in vivo effects of HIV protease inhibitors on apoptosis. Cell Death Differ. 2005;12(Suppl 1):924–31. doi: 10.1038/sj.cdd.4401580. [DOI] [PubMed] [Google Scholar]
- 6.Danaher RJ, Wang C, Roland AT, Kaetzel CS, Greenberg RN, Miller CS. HIV protease inhibitors block oral epithelial cell DNA synthesis. Arch Oral Biol. 2010;55:95–100. doi: 10.1016/j.archoralbio.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braga Neto MB, Aguiar CV, Maciel JG, Oliveira BM, Sevilleja JE, Oriá RB, et al. Evaluation of HIV protease and nucleoside reverse transcriptase inhibitors on proliferation, necrosis, apoptosis in intestinal epithelial cells and electrolyte and water transport and epithelial barrier function in mice. BMC Gastroenterol. 2010;10:90. doi: 10.1186/1471-230X-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow WA, Jiang C, Guan M. Anti-HIV drugs for cancer therapeutics: back to the future? Lancet Oncol. 2009;10:61–71. doi: 10.1016/S1470-2045(08)70334-6. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Ikezoe T, Takeuchi T, Adachi Y, Ohtsuki Y, Takeuchi S, et al. HIV-1 protease inhibitor induces growth arrest and apoptosis of human prostate cancer LNCaP cells in vitro and in vivo in conjunction with blockade of androgen receptor STAT3 and AKT signaling. Cancer Sci. 2005;96:425–33. doi: 10.1111/j.1349-7006.2005.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJ, Abu-Asab MS, et al. Nelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res. 2007;13:5183–94. doi: 10.1158/1078-0432.CCR-07-0161. [DOI] [PubMed] [Google Scholar]
- 11.Rhoads JM, Argenzio RA, Chen W, Rippe RA, Westwick JK, Cox AD, et al. L-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol. 1997;272:G943–53. doi: 10.1152/ajpgi.1997.272.5.G943. [DOI] [PubMed] [Google Scholar]
- 12.Rhoads JM, Argenzio RA, Chen W, Graves LM, Licato LL, Blikslager AT, et al. Glutamine metabolism stimulates intestinal cell MAPKs by a cAMP-inhibitable, Raf-independent mechanism. Gastroenterology. 2000;118:90–100. doi: 10.1016/S0016-5085(00)70417-3. [DOI] [PubMed] [Google Scholar]
- 13.Marc Rhoads J, Wu G. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids. 2009;37:111–22. doi: 10.1007/s00726-008-0225-4. [DOI] [PubMed] [Google Scholar]
- 14.Duggan C. Glutamine-based oral rehydration solutions: the magic bullet revisited? J Pediatr Gastroenterol Nutr. 1998;26:533–5. doi: 10.1097/00005176-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Neu J, DeMarco V, Li N. Glutamine: clinical applications and mechanisms of action. Curr Opin Clin Nutr Metab Care. 2002;5:69–75. doi: 10.1097/00075197-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Lima NL, Soares AM, Mota RM, Monteiro HS, Guerrant RL, Lima AA. Wasting and intestinal barrier function in children taking alanyl-glutamine-supplemented enteral formula. J Pediatr Gastroenterol Nutr. 2007;44:365–74. doi: 10.1097/MPG.0b013e31802eecdd. [DOI] [PubMed] [Google Scholar]
- 17.Carneiro-Filho BA, Oriá RB, Wood Rea K, Brito GA, Fujii J, Obrig T, et al. Alanyl-glutamine hastens morphologic recovery from 5-fluorouracil-induced mucositis in mice. Nutrition. 2004;20:934–41. doi: 10.1016/j.nut.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Blikslager A, Hunt E, Guerrant R, Rhoads M, Argenzio R. Glutamine transporter in crypts compensates for loss of villus absorption in bovine cryptosporidiosis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G645–53. doi: 10.1152/ajpgi.2001.281.3.G645. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro Júnior H, Ribeiro T, Mattos A, Palmeira C, Fernandez D, Sant’Ana I, et al. Treatment of acute diarrhea with oral rehydration solutions containing glutamine. J Am Coll Nutr. 1994;13:251–5. doi: 10.1080/07315724.1994.10718405. [DOI] [PubMed] [Google Scholar]
- 20.Yalçin SS, Yurdakök K, Tezcan I, Oner L. Effect of glutamine supplementation on diarrhea, interleukin-8 and secretory immunoglobulin A in children with acute diarrhea. J Pediatr Gastroenterol Nutr. 2004;38:494–501. doi: 10.1097/00005176-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Bushen OY, Davenport JA, Lima AB, Piscitelli SC, Uzgiris AJ, Silva TM, et al. Diarrhea and reduced levels of antiretroviral drugs: improvement with glutamine or alanyl-glutamine in a randomized controlled trial in northeast Brazil. Clin Infect Dis. 2004;38:1764–70. doi: 10.1086/421394. [DOI] [PubMed] [Google Scholar]
- 22.Silva AC, Santos-Neto MS, Soares AM, Fonteles MC, Guerrant RL, Lima AAM. Efficacy of a glutamine-based oral rehydration solution on the electrolyte and water absorption in a rabbit model of secretory diarrhea induced by cholera toxin. J Pediatr Gastroenterol Nutr. 1998;26:513–9. doi: 10.1097/00005176-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Haque SM, Chen K, Usui N, Iiboshi Y, Okuyama H, Masunari A, et al. Alanyl-glutamine dipeptide-supplemented parenteral nutrition improves intestinal metabolism and prevents increased permeability in rats. Ann Surg. 1996;223:334–41. doi: 10.1097/00000658-199603000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satoh J, Tsujikawa T, Fujiyama Y, Bamba T. Nutritional benefits of enteral alanyl-glutamine supplementation on rat small intestinal damage induced by cyclophosphamide. J Gastroenterol Hepatol. 2003;18:719–25. doi: 10.1046/j.1440-1746.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Ping X, Yu B, Liu F, Ni X, Li J. Clinical trial: prophylactic intravenous alanyl-glutamine reduces the severity of gastrointestinal toxicity induced by chemotherapy--a randomized crossover study. Aliment Pharmacol Ther. 2009;30:452–8. doi: 10.1111/j.1365-2036.2009.04068.x. [DOI] [PubMed] [Google Scholar]
- 26.McCormack SA, Viar MJ, Johnson LR. Migration of IEC-6 cells: a model for mucosal healing. Am J Physiol. 1992;263:G426–35. doi: 10.1152/ajpgi.1992.263.3.G426. [DOI] [PubMed] [Google Scholar]
- 27.Brito GA, Carneiro-Filho B, Oriá RB, Destura RV, Lima AA, Guerrant RL. Clostridium difficile toxin A induces intestinal epithelial cell apoptosis and damage: role of Gln and Ala-Gln in toxin A effects. Dig Dis Sci. 2005;50:1271–8. doi: 10.1007/s10620-005-2771-x. [DOI] [PubMed] [Google Scholar]
- 28.Viracept. Prescribing Information. 25 January2001 APILJ, CA 92037, USA Item #634200MV.
- 29.Wu EY, Wilkinson JM, 2nd, Naret DG, Daniels VL, Williams LJ, Khalil DA, et al. High-performance liquid chromatographic method for the determination of nelfinavir, a novel HIV-1 protease inhibitor, in human plasma. J Chromatogr B Biomed Sci Appl. 1997;695:373–80. doi: 10.1016/S0378-4347(97)00193-X. [DOI] [PubMed] [Google Scholar]
- 30.Burns-Naas LA, Webber S, Stump DG, Holson JF, Masarjian L, Zorbas M. Absence of embryo-fetal toxicity in rats or rabbits following oral dosing with nelfinavir. Regul Toxicol Pharmacol. 2003;38:291–303. doi: 10.1016/S0273-2300(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 31.Bjerknes M, Cheng H. Gastrointestinal stem cells. II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G381–7. doi: 10.1152/ajpgi.00160.2005. [DOI] [PubMed] [Google Scholar]
- 32.Jiang W, Mikochik PJ, Ra JH, Lei H, Flaherty KT, Winkler JD, et al. HIV protease inhibitor nelfinavir inhibits growth of human melanoma cells by induction of cell cycle arrest. Cancer Res. 2007;67:1221–7. doi: 10.1158/0008-5472.CAN-06-3377. [DOI] [PubMed] [Google Scholar]
- 33.Braga-Neto MB, Warren CA, Oriá RB, Monteiro MS, Maciel AA, Brito GA, et al. Alanyl-glutamine and glutamine supplementation improves 5-fluorouracil-induced intestinal epithelium damage in vitro. Dig Dis Sci. 2008;53:2687–96. doi: 10.1007/s10620-008-0215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu Y, Wu ZH. The anabolic effects of recombinant human growth hormone and glutamine on parenterally fed, short bowel rats. World J Gastroenterol. 2002;8:752–7. doi: 10.3748/wjg.v8.i4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziegler TR, Mantell MP, Chow JC, Rombeau JL, Smith RJ. Gut adaptation and the insulin-like growth factor system: regulation by glutamine and IGF-I administration. Am J Physiol. 1996;271:G866–75. doi: 10.1152/ajpgi.1996.271.5.G866. [DOI] [PubMed] [Google Scholar]
- 36.Carneiro BA, Fujii J, Brito GA, Alcantara C, Oriá RB, Lima AA, et al. Caspase and bid involvement in Clostridium difficile toxin A-induced apoptosis and modulation of toxin A effects by glutamine and alanyl-glutamine in vivo and in vitro. Infect Immun. 2006;74:81–7. doi: 10.1128/IAI.74.1.81-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]