Abstract
Determination of protein function requires tools that allow its detection and/or purification. As generation of specific antibodies often is laborious and insufficient, protein tagging using epitopes that are recognized by commercially available antibodies and matrices appears more promising. Also, proper spatial and temporal expression of tagged proteins is required to prevent falsification of results. We developed a new series of binary Gateway cloning vectors named pAUL1-20 for C- and N-terminal in-frame fusion of proteins to four different tags: a single (i) HA epitope and (ii) Strep-tagIII, (iii) both epitopes combined to a double tag, and (iv) a triple tag consisting of the double tag extended by a Protein A tag possessing a 3C protease cleavage site. Expression can be driven by either the 35 S CaMV promoter or, for C-terminal fusions, promoters from genes encoding the chloroplast biogenesis factors HCF107, HCF136, or HCF173. Fusions of the four promoters to the GUS gene showed that endogenous promoter sequences are functional and drive expression more moderately and consistently throughout different transgenic lines when compared to the 35 S CaMV promoter. By testing complementation of mutations affected in chloroplast biogenesis factors HCF107 and HCF208, we found that the effect of different promoters and tags on protein function strongly depends on the protein itself. Single-step and tandem affinity purification of HCF208 via different tags confirmed the integrity of the cloned tags.
Introduction
The majority of cellular processes is accomplished and regulated by proteins. To shed light on the precise function of a protein, tools for detection and/or determination of subcellular localization are required. Also, identification and characterization of interaction partners is of great importance as most proteins act in collaboration with other proteins either transiently or in stable complexes. To address all these questions diverse protein tagging strategies have been invented throughout the past years. In-frame translational fusions of the protein of interest and either a reporter protein (e.g. GFP; [1]) or an epitope tag (e.g. hemagglutinin; [2]) are created and introduced into the investigated organism. The Gateway technology (Invitrogen) based on the site-specific recombination mechanism of phage lambda [3] allows rapid cloning of DNA sequences to vectors carrying designated tag sequences. Most of the published Gateway-compatible binary vectors (reviewed by [4]) are designed for constitutive expression of transgenes therefore harboring the 35S promoter of cauliflower mosaic virus (CaMV, [5]) or the nopaline synthase (Nos) promoter of Agrobacterium tumefaciens [6]. This strategy may be disadvantageous for purposes like purification of protein complexes via epitope tagged bait proteins because overexpressed proteins might not be associated with their binding partners. Nevertheless, most purification strategies so far rely on the overexpression of the bait protein in wild-type background with the transgenic protein competing for binding partners with the endogenous protein (e.g. [7]). However, to assure proper function of tagged proteins they should be introduced into respective mutant backgrounds and reconstitute the wild-type phenotype. Furthermore, ubiquitous expression may affect complementation [8], thus demanding for proper regulation of spatial and temporal expression. In this regard, the use of endogenous promoters or promoters of genes with similar expression profiles is more promising.
We designed the binary, Gateway-compatible “pAUL“ vector series for epitope tagging of proteins that are expressed under the control of endogenous Arabidopsis thaliana promoters or the 35 S CaMV promoter [5]. The primary application is supposed to be detection and purification of nuclear encoded proteins involved in chloroplast-related processes. Thus, vectors with C-terminal tags were generated in the first instance, as N-terminal fusions would be cleaved off toward chloroplast import. The C-terminal tags are combined with A. thaliana promoter sequences of genes known to participate in those processes, namely HCF107 [9], [10], HCF136 [11], and HCF173 [12]. To make the vectors applicable for proteins involved in other biological processes vectors harboring the 35 S CaMV promoter combined with C-and N-terminal tags were also constructed.
Three epitope tags were utilized for four different C- or N-terminal fusions making possible single-, double- or triple-tagging of proteins of interest. The hemagglutinin (HA) epitope exhibits a small size (27 amino acids for 3x HA) and the availability of effective antibodies make it an ideal tool for detection. Purification can also be carried out in small scales via antibodies or anti-HA matrices and proteins can be eluted competitively by HA peptide or by low pH. The 28-amino acid Strep-tagIII [13] is an improvement of Strep-tagII and has not been described for purification of plant proteins so far. This tag has a strong binding affinity to Strep-Tactin, an engineered streptavidin derivate. Purifications can be performed under flexible binding conditions as Strep-tagIII/Strep-Tactin interactions are resistant to detergents and varying salt concentrations and do not require the availability of cofactors [13]. Also, the possibility of competitive elution via desthiobiotin makes it a suitable tool for protein and protein complex purification [13]. In the pAUL vector system, the HA epitope and Strep-tagIII can be used for single-tag-fusions of proteins of interest for detection (HA) or purification (HA and Strep-tagIII). Double tagging includes both the HA epitope and Strep-tagIII cloned in series and is supposed to serve for one-step purification via StrepTactin and subsequent detection via the HA epitope. Alternatively, two-step purification via StrepTactin and anti-HA affinity matrix may be carried out if required. Finally, we designed an alternative TAP (tandem affinity purification)-tag. The TAP tag originally developed in yeast consists of two immunglobulin-binding domains of protein A from Staphylococcus aureus (ProtA), a tobacco etch virus (TEV) cleavage site and a calmodulin binding site (CBP) [14], but has been modified in the past years (reviewed by [15]). [16] adapted this tag to plant applications and [7] further modified it. We exchanged the CBP by HA for efficient detection of the tagged protein and Strep-tagIII for purification. The ProtA tag was retained since it displays a strong binding affinity to IgG Sepharose making it well suitable for protein purification. However, the large size of the tag (116 amino acids; ∼13 kDa) may affect the function of the protein fused to it. The TEV cleavage site from the original TAP tag was replaced by the human rhinovirus (HRV) 3C protease cleavage site, which can be processed even at low temperatures according to [7].
Here, we describe cloning of the pAUL vector series. We test the 35 S CaMV promoter and promoters from HCF107, HCF136, and HCF173 for their activities by quantitative and histochemical GUS assays. Complementation with different promoter/tag combinations is tested using hcf107.2 [9] and hcf208 [17] mutants. Corresponding proteins are encoded in the nucleus and transported to chloroplast membranes where they affect thylakoid membrane biogenesis. Whereas HCF107 forms a low abundant high molecular weight complex [10] and is required for expression of the chloroplast-encoded photosystem II subunit PsbH, HCF208 is part of the system IV c-type cytochrome maturation machinery for the b 6 subunit of the cytochrome b 6 f complex [18], [19] and fulfills its function as a stable heterodimer transiently interacting with other proteins [20]. Finally, integrity of the different tags is tested by small-scale affinity purification of HCF208 from thylakoid membranes.
Materials and Methods
pAUL Vector Construction
Oligonucleotides used for pAUL vector construction are summarized in Table 1.
Table 1. Oligonucleotides used for pAUL vector construction.
| Oligonucleotide | Sequence (5′to 3′) |
| pMDC123-H | CCCTCGAGGCGCGCCAAGCTAT |
| pMDC123-R | GCGCGAGCTCTCGAACCACTTTGTACAAG |
| T NOSPstI-H | GGCTGCAGCGGAAGATCGTTCAAACATTTG |
| TNOSSacI/HindIII-R | CTATGAAGCTTGAGCTCAATTCGATCTAGTAAC |
| 3xHASacI-H | GACTGGAGCTCTACCCATATGACGTTCCAGAC |
| 3xHAstopPstI-R | GCACTGCAGTTATCAAGCGTAGTCAGGTACGTC |
| 3xHABamHI-R2 | CAGTGGATCCAGCGTAGTCAGGTACGTCG |
| HA/STREP-1 | P-GATCCTGGTCTCATCCTCAATTCGAAAAGGGTGGA |
| HA/STREP-2 | GAACCTCCACCCTTTTCGAATTGAGGATGAGACCAG |
| HA/STREP-3 | P-GGTTCTGGAGGTGGATCAGGTGGTGGATCTTGG |
| HA/STREP-4 | TGAGACCAAGATCCACCACCTGATCCACCTCCA |
| HA/STREP-5 | P-TCTCATCCTCAATTCGAAAAGTGATAAGAGCTCG |
| HA/STREP-6 | AATTCGAGCTCTTATCACTTTTCGAATTGAGGA |
| HA/STREP-7 | CGGGATCCTGGTCTCATCCTCAATTC |
| HA/STREP-9 | CGACTGCAGTTATCACTTTTCGAATTGAGGA |
| 3xHAStrepIIIXbaI-R | GCTCTAGACTTTTCGAATTGAGGATGAGAC |
| 3cIgG-BDXbaI-H | ACTCTAGACTGGAAGTTCTGTTCCAGGGGC |
| 3cIgG-BDPstI-R | CGGGCTGCAGTTATCATACCGAACTCGAATTC |
| 35SAscI-H | GTGAGCTCGGCGCGCCAAGCTTGCATGCCTGCAGGTC |
| 35SAscI-R | GCTCTAGAGGCGCGCCCCTCTCCAAATGAAATGAAC |
| HCF107-Prom-H1 | CCGGATTTGGTAGCCACATTCAATGC |
| HCF107-Prom-R1 | CCGGCTCGGGGAAGAAGAATGATGG |
| HCF107-Prom-H5-2 | GGCGCGCCGGATTTGGTAGCCACATTCAATGC |
| HCF107-Prom-R5 | GGCGCGCCGGCTCGGGGAAGAAGAATGATGG |
| HCF136-Prom-H1 | CCCTGTTCATTGGAGTCATATCAAGTC |
| HCF136-Prom-R1 | CCTCTCTTCTCTTTCTCTCTCCCGC |
| HCF173-Prom-H1 | GGCGCGCCTCTCTTACATTTTTGGGCGAACTTG |
| HCF173-Prom-R1 | GGCGCGCCAAATGCATACAATTGTTTGTTAAATGAATC |
| 3xHA-AscI/PstI-H | GCACTGCAGGGCGCGCCATGTACCCATATGACGTTCCAGAC |
| 3xHA-PstI-H | GCACTGCAGTACCCATATGACGTTCCAGAC |
| 3xHA-AscI/HindIII-R | CTATGAAGCTTGGCGCGCCAGCGTAGTCAGGTACGTCG |
| Strep-XbaI-H1 | ACTCTAGATGGTCTCATCCTCAATTCGAAAAGGGTGGAGGTTCTG |
| Strep-AscI-H2 | GACGTACCTGACTACGCTGGCGCGCCATGTGGTCT |
| Strep-SacI/AscI-H-2 | GACTGGAGCTCGGCGCGCCATGTGGTCTCATCCTC |
| Strep-PstI-R | CGGGCTGCAGCTTTTCGAATTGAGGATGAGAC |
| Strep-AscI-R | CTGGAACAGAACTTCCAGGGCGCGCCCTTTTCG |
| IgG-AscI/BstXI-H | GACTGCCACCGCGGTGGCGCGCCATGGCCACCATGGCGCAAC |
| IgG-3C-BcuI-R2 | GACACTAGTGGGCCCCTGGAACAGAACTTCCAG |
Construction of pAUL 1 to pAUL12
Cassette C1 from the pMDC123 Gateway vector [21] was modified prior to cloning of tags and promoters into the plasmid. The att cassette was amplified using primers pMDC123-H and pMDC123-R and then removed from the vector by digestion with AscI and SacI. The PCR product lacking 44 bp stop codon-containing sequence between attR2 site and SacI recognition site was digested using AscI and SacI and cloned into pMDC123 to generate pMDC123-(-)stop. The integrity of the att cassette was tested by sequencing.
The tags were first assembled in pBluescript II (pBSII) KS+ and each fused to the Nos terminator, which was obtained form the pC-TAPa plasmid [7] by PCR reaction using primers T NOSPstI-H and T NOSSacI/HindIII-R. The PstI/HindIII digested PCR product was ligated into the pBSII phagemid already containing the assembled tags, which were generated as follows.
For vectors containing only the 3x HA tag the DNA sequence from plasmid spa1g3xHA-pBS (provided by Ute Hoecker) [22] was amplified with primers 3xHASacI-H and 3xHAstopPstI-R and cloned using SacI and PstI generating pBS-3xHAPstI.
The DNA sequence of 3x HA tag cloned into the multiple tags was amplified using primers 3xHASacI-H and 3xHABamHI-R2 and cloned into pBSII after digestion with SacI and BamHI generating pBS+3xHABamHI.
Strep-tagIII [13] was obtained by annealing of the oligonucleotides HA/STREP-1 to -6 at 90°C for 15 minutes in annealing buffer (0.1 M Tris/HCl, pH 7.5; 1 M NaCl; 10 mM EDTA). The DNA sequence was adapted to plant codon usage. For the generation of 3x HA/Strep-tagIII the DNA fragment was amplified using primers HA/STREP-7 and HA/STREP-9. The BamHI/PstI digested PCR product was cloned into pBS+3xHABamHI generating pBS+3xHA/StrepIIIPstI.
The 3xHA/StrepIII/PA tag was created by amplification of the Strep-tagIII DNA fragment with primers HA/StrepIII-7 and 3xHAStrepIIIXbaI-R and amplification of the 2xProteinA tag including 3C protease cleavage site from pC-TAPa using primers 3cIgG-BDXbaI-H and 3cIgG-BDPstI-R. Both PCR products digested BamHI/XbaI and XbaI/PstI respectively were cloned into pBS+3xHABamHI generating pBS+3xHA/StrepIII/PAPstI.
The three tag constructs including nos terminator were removed from pBSII by digestion with SacI and ligated into the pMDC123-(-)stop plasmid creating plasmids pMDC123-(-)stop-3xHA, pMDC123-(-)stop-3xHA/StrepIII and pMDC123-(-)stop-3xHA/StrepIII/PA. The correct orientation of the tags was verified by restriction analysis.
The 2×35 S CaMV promoter and the three endogenous promoters from HCF107, HCF136 and HCF173 were each cloned by PCR reaction and digestion with AscI into the three pMDC123-(-)stop plasmids containing the tag constructs.
From plasmid pYL436 the 2×35 S CaMV promoter was amplified using primers 35SAscI-H and 35SAscI-R. Predicted promoter sequences of genes HCF107, HCF136 and HCF173 were amplified from genomic DNA of A. thaliana Columbia-0 ecotype. The HCF107 promoter was amplified by adapter PCR using primers HCF107-Prom-H1 and HCF107-Prom-R1 in the first step and primers HCF107-Prom-H5-2 and HCF107-Prom-R5 in the second step. Promoters of HCF136 and HCF173 were amplified with primers HCF136-Prom-H1/HCF136-Prom-R1 and HCF173-Prom-H1/HCF173-Prom-R1 respectively. AscI digested PCR products were ligated to pMDC132-(-)stop-3xHA, pMDC132-(-)stop-3xHA/StrepIII and pMDC123-(-)stop-3xHA/StrepIII/PA generating vectors pAUL1 to pAUL12. Correct orientation was checked by restriction analyses. After completion of the vectors promoters and tags were checked by sequencing.
Construction of pAUL13 to pAUL16
For the creation of pAUL13 to pAUL16 the modified att cassette from pMDC123-stop was transferred to the Gateway vector pMDC99 [21] generating pMDC99-(-)stop. The Strep-tagIII sequence was amplified with primers HA/Strep-9 and StrepIII/NotSac-H from vector pBS+3xHA/StrepIIIPstI. After digestion with NotI and PstI the fragment was ligated to a pBluescript II vector already containing the Nos terminator sequence (cloned as described above). Correct fragments were removed from pBSII via a SacI restriction site and ligated to pMDC99-(-)stop creating pMDC99-Strep. Promoters 2×35 S CaMV, HCF107, HCF136, and HCF173 were extracted from the above-described pAUL vectors via AscI and fused to pMDC99-Strep generating pAUL13 to pAUL16. Correct orientation was checked by restriction analyses. After completion of the vectors promoters and tags were checked by sequencing.
Construction of pAUL 17 to pAUL20
N-terminal versions of the pAUL vector series were produced using pMDC32 [21] containing a 2×35 S CaMV promoter and nos terminator as a backbone.
3xHA and Strep-tagIII sequences were amplified from the pAUL2 vector, pN-TAPa [7] served as template for IgG-BD+3C protease cleavage site.
In order to create vectors containing the 3x HA only, the sequence was amplified with primers 3xHA-AscI/PstI-H and 3xHA-AscI/HindIII-R. For double and triple tag primers 3xHA-PstI-H and 3xHA-AscI/HindIII-R used. Both PCR products were digested by PstI and HindIII and ligated to pBSII to create pBS-3xHA-N and pBS-PstI-3xHA-N respectively.
For the single tag Strep-tagIII was amplified via primers Strep-AscI-H2 and Strep-AscI-R, restricted with AscI and directly ligated to AscI-linearized pMDC32. The Strep-tagIII sequence needed for the 3xHA/StrepIII double tag was amplified with primers Strep-SacI/AscI-H-2 and Strep-PstI-R. The SacI/PstI digested fragment was fused to pBS-PstI-3xHA-N resulting in pBS-StrepIII/3xHA-N. Finally, primers Strep-XbaI-H1 and Strep-PstI-R were employed for amplification of Strep-tagIII needed for the triple tag. The fragment was restricted with XbaI and PstI and introduced into pBS-PstI-3xHA-N generating pBS-XbaI-StrepIII/3xHA-N.
The sequence of the 2x Protein A tag and 3C protease cleavage site was amplified with primers IgG-AscI/BstXI-H and IgG-3C-BcuI-R2 and digested with enzymes BstXI and BcuI. The fragment was ligated to pBS-XbaI-StrepIII/3xHA-N digested with XbaI and HindIII (BcuI and XbaI form compatible sticky ends) to create pBS-PA/StrepIII/3xHA-N.
All tag sequences and pMDC32 were restricted with AscI and ligated. After confirmation of the correct orientation the tags were checked by sequencing.
Cloning of Target Genes to pAUL Vectors
For characterization of the promoters the ß-glucuronidase (GUS) gene and the Nos terminator were amplified from the pBI121 vector [23] using primers GUS+Term-H and GUS+Term-R containing attB sites (Table 2).
Table 2. Oligonuleotides used for cloning of target genes.
| Oligonucleotide | Sequence (5′to 3′) |
| GUS+Term-H | GGGGACAAGTTTGTACAAAAAAGCAGGCTATGTTACGTCCTGTAGAAACC |
| GUS+Term-R | GGGGACCACTTTGTACAAGAAAGCTGGGTCGATCTAGTAACATAGATGACA |
| start107 attB1-H | GGGGACAAGTTTGTACAAAAAAGCAGGCTATGCACTTCTTCTTCGTGCCG |
| 107 attB2-R | GGGGACCACTTTGTACAAGAAAGCTGGGTCAGCACCATTTATTCTTCCTC |
| start208 attB1-H | GGGGACAAGTTTGTACAAAAAAGCAGGCTATGAGTATTCAAATTTGTAATTTC |
| 208 attB2-R | GGGGACCACTTTGTACAAGAAAGCTGGGTCACCTCTGAATTTCTCAGCCATAG |
For complementation analyses HCF107 was amplified with primers start107attB1-H and 107attB2-R (Table 2) using pPEX107PA [10] as a template. HCF208 was amplified from cDNA obtained by reverse transcription of total RNA isolated from A. thaliana wild type Columbia-0 using primers start208attB1-H and 208attB2-R (Table 2).
BP clonase reaction (Invitrogen) between the PCR products and pDONR221 were accomplished according to the Gateway manual creating pENTRY221+GUS, pENTRY+HCF107, and pENTRY+HCF208. Aliquots (5 µl) were transformed into Escherichia coli strain DH5α using heat shock. The recombinants were selected on LB agar plates containing 50 µg/ml kanamycin. After sequence analysis of the recombined DNA sequence LR clonase reactions (Invitrogen) were performed (according to the Gateway manual) to introduce the genes into the respective pAUL vectors. The ß-glucuronidase gene was introduced into pAUL1, pAUL4, pAUL7, and pAUL10 creating GUSpAUL1, GUSpAUL4, GUSpAUL7, and GUSpAUL10. HCF107 and HCF208 were recombined into pAUL1, pAUL2, pAUL3, pAUL6, and pAUL9. Resulting vectors were named HCF107pAUL1, HCF107pAUL2, HCF107pAUL3, HCF107pAUL6, HCF107pAUL9, HCF208pAUL1, HCF208pAUL2, HCF208pAUL3, HCF208pAUL6, and HCF208pAUL9.
Because pAUL vectors as well as the pENTRY221 vectors can only be selected on kanamycin containing media 5 µl of each reaction mixture were digested HpaI or Eam1105I respectively in order to linearize the pDONR221+GUS vector to avoid its transformation. The vectors were transformed into DH5α using heat shock and recombinants were selected on LB agar media supplemented with 50 µg/ml kanamycin. After restriction analyses the correct reading frame of each gene and the tags was checked by sequencing.
Plant Material, Growth Conditions, and Plant Transformation
All constructs were transformed into Agrobacterium tumefaciens strain GV3101 and introduced to A. thaliana using the floral dip method [24]. GUS constructs were transferred into wild-type Columbia-0 ecotype. pAUL vectors containing HCF107 or HCF208 cDNA were introduced into heterozygous hcf107.2 (Wassilewskija ecotype) [9] and hcf208 (Columbia-0 ecotype) [17] plants respectively.
For seed production, protein extraction, spectroscopic measurements, and measurement of GUS activity, plants were grown on soil in a growth chamber operating at a 16 h light/8 h darkness period at a photon flux density (PFD) of ∼50–70 µmol s−1 m−2 and a constant temperature of 21°C. Protein extracts used for affinity purification were isolated from plants grown under short-day conditions (8 h light/16 h darkness).
Homozygous hcf208 and hcf107.2 plants were grown on 0.5×Murashige and Skoog (MS) medium [25] containing 2% (w/v) sucrose and 0.3% (w/v) gelrite (Roth, Karlsruhe, Germany). Seedlings were exposed to a 16 h light/8 h darkness period at a PFD of ∼50–70 µmol s−1 m−2. Selection of mutant plants exhibiting high chlorophyll fluorescence phenotype was performed in the dark under UV light [11].
Transformants were selected on 0.5×MS medium as described above containing 10 µg ml−1 phosphinothricin.
Measurement of GUS Activity and Histochemical Analyses
GUS analyses were carried out with T1 plants of A. thaliana harboring GUSpAUL1, GUSpAUL4, GUSpAUL7, and GUSpAUL10, respectively. Quantitative determination of GUS activity was performed with 15- and 30-day-old plants harvested at midday after 6 hours of illumination according to [26] and [27]. The average values of the data are expressed by medians.
For histochemical analyses either intact 5-day-old seedlings or sections of 3-week-old plants cut manually with a razorblade were transferred into incubation buffer (100 mM Na2HPO4, pH 7.5; 10 mM EDTA; 50 mM K4 [Fe(CN)6]; 50 mM K3[Fe(CN)6]; 0.1% (v/v) Triton X-100; 2 mM 5-bromo-4-chloro-3-indolyl-β-D-glucuronide acid) and vacuum-infiltrated. Samples were incubated at 37°C until they stained blue and fixed in 75% ethanol and 25% acetic acid for 10 minutes. Subsequently, chlorophyll was removed by treatment with 70% ethanol.
Fluorescence Measurements
For fluorescence measurements complemented hcf107.2 or hcf208 plants carrying the respective wild-type gene in pAUL1, -2, -3, -6, or -9 vectors were employed. Chlorophyll fluorescence was imaged with a closed FluorCam FC 800-C controlled by FluorCam 6 software (Photon Systems Instruments) on 3-week-old plants. Experiments were carried out using pre-designed quenching protocol provided by the software.
Protein Extraction and Western Blot Analysis
Proteins were extracted from the same plants that were also used for fluorescence measurements described above according to [28]. 3 plants from each line were pooled, pestled in liquid nitrogen and immediately transferred to extraction buffer (10 mM Tris/HCl, pH 7.8; 4 M urea; 5% (w/v) SDS; 15% (w/v) glycerol; 10 mM β-mercaptoethanol). The samples were boiled for 4 minutes and cleared by centrifugation at 15,000 g for 5 minutes. Protein concentration was determined using RC DC Protein Assay (Bio-Rad). 50 µg total protein was separated on a 10% SDS-PAGE gel according to [29], transferred to nitrocellulose membranes, and immunoblots were decorated with anti-HCF107 and Anti-HA-Peroxidase (Roche Applied Science).
Affinity Purification of Fusion Proteins
Leaves of 4- to 6-week-old complemented hcf208 carrying either HCF208pAUL1, HCF208pAUL2 or HCF208pAUL3 constructs were homogenized in lysis buffer (10 mM Hepes/KOH, pH 7.8; 10 mM MgCl2; and 25 mM KCl). Cell debris was separated by Miracloth filtration. The suspension was centrifuged at 4°C until a speed of 5,900 g was reached and then stopped. Pelleted membranes were resuspended in Tris-buffered lysis buffer and solubilized with 1% (w/v) n-dodecyl-ß-D-maltoside (Calbiochem) for 30 minutes at 4°C and a chlorophyll concentration of 1 mg ml−1. Unsolubilized material was removed by centrifugation (20 minutes at 4°C and 15,000 g). For each purification aliquots of 200 µl supernatant were added to matrices pre-equilibrated with washing buffer (20 mM Tris/HCl, pH 7.8; 150 mM NaCl; 1 mM EDTA; 0.05% (w/v) n-dodecyl-ß-D-maltoside).
Samples from HCF208pAUL1 (containing the 3x HA tag only) plants were incubated with a bed volume of 50 µl Anti-HA affinity matrix (Roche Applied Sciences) for 1 h at 4°C on a rotator. The matrix was washed with 20 volumes of washing buffer. Proteins were eluted by incubating the affinity matrix three times with 1 volume of elution buffer (1 mg/ml HA peptide (Roche Applied Sciences)) in washing buffer for 15 minutes at 37°C.
HCF208pAUL2 (Strep-tagIII and 3x HA tag) samples were rotated with 100 µl Strep-Tactin Macroprep (IBA) at 4°C for 1 h, washed with 5 times with 1 volume of washing buffer and eluted with 3 volumes of elution buffer (2.5 mM desthiobiotin (IBA) in washing buffer). Subsequently, the eluate was purified via Anti-HA affinity matrix as described above.
For purification of HCF208pAUL3 (2x ProtA, Strep-tagIII, and 3x HA tag) samples, respective supernatants were incubated with 50 µl IgG Sepharose (GE Healthcare) for 1 h at 4°C on a rotator. After washing with 20 volumes of washing buffer the matrix was equilibrated with 5 volumes of cleavage buffer (20 mM Tris/HCl, pH 7.8; 150 mM NaCl; 1 mM EDTA; 0.05% (w/v) n-dodecyl-ß-D-maltoside; 1 mM DTT). Cleavage was performed by incubation with 20 units of PreScission Protease (GE Healthcare) in cleavage buffer for 16 h at 4°C on a rotator. The following purification with Strep-Tactin Macroprep was performed according to the procedure described above.
All final eluates were precipitated with 15% (w/v) trichloroacetic acid and separated on 12.5% SDS-PAGE gels according to [29]. Proteins were transferred to nitrocellulose membranes and immunodecorated with Anti-HA-Peroxidase (Roche Applied Sciences) and anti-ATP Synthase.
Results and Discussion
Design and Cloning of the pAUL Vector Series
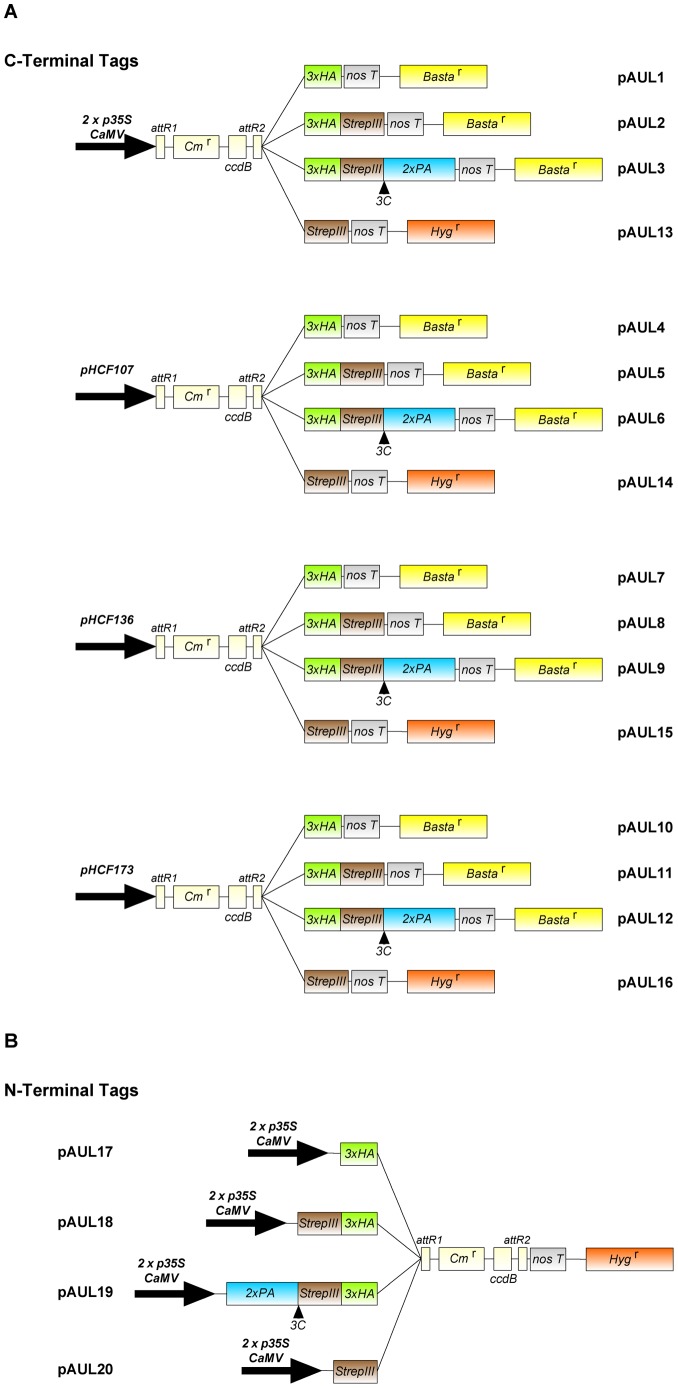
We constructed a series of 20 binary Gateway-compatible vectors containing different combinations of promoters and tags, named pAUL1-20 (Figure 1). Four different single, double or triple tags were cloned into various backbone Gateway vectors from the pMDC series [21] allowing either C- or N-terminal protein fusions to facilitate protein detection and purification. N-terminally tagged proteins can be expressed under the control of two copies of the 35 S CaMV promoter, whereas vectors for C-terminal fusions contain either the two copies of the 35 S CaMV promoter for ubiquitous and constitutive expression or one of three endogenous promoters from A. thaliana. The endogenous promoters were selected according to the function of the respective genes in chloroplast biogenesis and are described in the following section.
Figure 1. Schematic illustration of the Gateway compatible pAUL destination vector series, showing expression cassettes.
(A) C-terminal fusion vectors pAUL1-16. Expression is driven by either 2x p35 S CaMV or endogenous promoter sequences from A. thaliana (pHCF107, pHCF136, pHCF173): pAUL1-3 and pAUL13 carry p35S CaMV; pAUL4-6 and pAUL14 carry pHCF107; pAUL7-9 and pAUL15 carry pHCF136; pAUL10-12 and pAUL16 carry pHCF173. Protein tags are: 3xHA single tag (pAUL1, 4, 7, 10); Strep-tagIII single tag (pAUL13-16); 3xHA/Strep-tagIII double tag (pAUL2, 5, 8, 11); and 3xHA/Strep-tagIII/ProtA triple tag +3C protease cleavage site (pAUL3, 6, 9, 12). (B) N-terminal fusion vectors pAUL17-20. Vectors carry coding sequences for 3xHA single tag (pAUL17); 3xHA/Strep-tagIII double tag (pAUL18); 3xHA/Strep-tagIII/ProtA triple tag +3C protease cleavage site (pAUL19); and Strep-tagIII single tag (pAUL20).
Single tags are the triple HA epitope and Strep-tagIII. Both tags were combined in order to create the double tag. Addition of a 3C protease cleavage site and the ProtA tag made the triple tag. N- and C-terminal double and triple tags exhibit reverse orientations.
Sequences for C-terminal tags and a Nos terminator as well as the four promoters were cloned to the Gateway vector pMDC123 [21] resulting in vectors pAUL1 to pAUL12 (Figure 1A). Plasmid pMDC123 was used as the recipient for the tagging constructs, because this vector does not contain any preexisting promoter nor tag sequences around the Gateway att cassette but unique restriction sites (AscI upstream of the att cassette, SacI downstream of the att cassette) making it suitable for inserting promoter/tag sequences. Moreover, it harbors a bar sequence encoding for phosphinothricin (Basta) resistance driven by a 35 S CaMV pormoter.
The C-terminal Strep-tagIII and promoter sequences were introduced into pMDC99 [21] which corresponds to pMDC123 except it carries a hygromycin resistance instead the bar gene. Those vectors were named pAUL13 to pAUL16 (Figure1A).
If required, promoters from pAUL1 to pAUL16 can be exchanged easily, as they were cloned after the tag sequences by the rare cutting restriction enzyme AscI.
For vectors pAUL17 to pAUL20 N-terminal tags were inserted into pMDC32 [21], which contains a 35 S CaMV promoter, a Nos terminator and a hygromycin resistance (Figure 1B). These vectors were not tested in this study as we investigated chloroplast-localized proteins whose N-termini are cleaved off upon import into the chloroplast.
Both, N- and C-terminal tag sequences were inserted into the expression cassette in a way that allows easy cloning of sequences according to the Gateway manual (Invitrogen).
Characterization of 35S CaMV, HCF107, HCF136, and HCF173 Promoters
The three different promoter regions from genes HCF107, HCF136, and HCF173 were selected according to the respective mRNA profiles from the GENEVESTIGATOR database [30]. Experiments from [31] indicate that HCF136 and HCF173 mRNAs accumulate to similar levels but are about 4-fold higher than HCF107 mRNAs. Furthermore, HCF107 and HCF173 mRNAs are regulated diurnally, whereas the HCF136 mRNA levels are stable throughout the day. The putative promoters were defined as sequences upstream of the transcription initiation site of the respective genes ending in regions of ∼1500 bp or until a UTR of the previous gene is reached. 1525 bp of the sequence upstream of HCF107 5′UTR, 1401 bp upstream of HCF136 5′UTR, and 721 bp upstream of HCF173 5′UTR were cloned to pAUL vectors and are referred to as “HCF107 promoter” (pHCF107), “HCF136 promoter“ (pHCF136), and “HCF173 promoter“ (pHCF173) in the following.
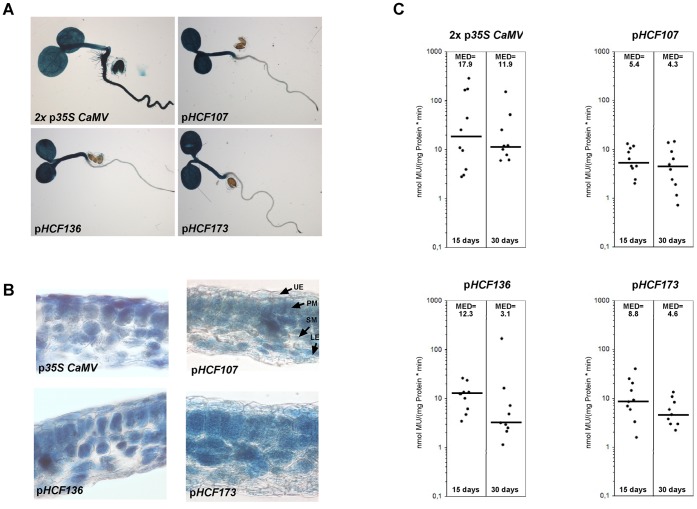
To test the ability of the selected sequences to serve as promoters and to compare them to the 35 S CaMV promoter (p35S CaMV) quantitative and histochemical GUS assays were performed. The ß-glucoronidase gene was fused to the four promoter sequences and introduced into wild-type A. thaliana plants. As presented in Figure 2 all putative promoter sequences and p35S CaMV do function as promoters.
Figure 2. Characterization of promoters 2x p35S CaMV, pHCF107, pHCF136, and pHCF173 fused to the GUS reporter gene.
(A) GUS staining of 5-day-old transgenic A. thaliana seedlings. (B) Histochemical localization of GUS activity in leaf sections of 3-week-old transgenic A. thaliana plants. UE, upper epidermis; PM, palisade mesophyll; SM, spongy mesophyll; LE, lower epidermis. (C) GUS activities in transgenic A. thaliana lines. In each case, 10 independent transgenic lines were tested 15 or 30 days after germination. Median values are shown as black bars and indicated at the top of each column. MU, 4-methylumbelliferone.
In intact A. thaliana seedlings p35S CaMV expression is detected in all plant organs, including cotyledons, hypocotyls, roots, and seed coat (Figure 2A). In contrast, pHCF107, pHCF136, and pHCF173 are only active in cotyledons and hypocotyls representing the “green” tissue of the seedling but not in roots and seed. This is consistent with the function of HCF107, HCF136, and HCF173 in chloroplast biogenesis [10], [11], [12]. To address tissue specificity of the promoters inside leaves cross sections were prepared (Figure 2B). The 35S CaMV promoter is active in all cell types, which agrees with previous studies [32]. However, the staining pattern appears spotted, suggesting that expression is not uniform throughout cell layers and types. In contrast, all endogenous promoters display even staining patterns. Expression of GUS driven by pHCF107, pHCF136, and pHCF173 is restricted to palisade and spongy mesophyll cells, which are the chloroplast possessing tissues. Together, these results show that all chosen endogenous promoter sequences are applicable for proper spatial expression of chloroplast-related proteins.
Since another aim of using endogenous promoters was to drive expression more moderately than the 35 S CaMV promoter and to ensure proper temporal expression, promoters were also analyzed quantitatively. Data was generated for two different developmental stages (15 and 30 days after germination) from plant material always harvested at the same time of day. In 15 day-old plants, pHCF107 is the weakest of the endogenous promoters, since pHCF136 and pHCF173 exhibit ∼2.3-fold and ∼1.6-fold higher GUS activity than pHCF107, respectively (Figure 2C). p35 S CaMV is the strongest promoter, presenting ∼3.3-fold higher activity than pHCF107 if median values are compared.
However, values for p35 S CaMV are strongly dispersed with their maximum and minimum at 280 and 3 mmol MU/(mg protein*min), respectively, revealing a high variation of expression in individual lines. This characteristic of p35 S CaMV has been reported previously [33]. Furthermore, the presence of multiple 35 S CaMV promoter copies is supposed to lead to silencing effects [34], which may occur in vectors that drive expression of the selectable marker by the same promoter and if plants are homozygous for the T-DNA. Expression by endogenous promoters appears more constant throughout different lines suggesting that they do not interfere with any other features of the pAUL vectors.
30 days after germination promoter activities appear to be decreased compared to values from 15 day-old plants (Figure 3C). pHCF107 activity is only slightly reduced to ∼80% and therefore is relatively stable. In contrast, pHCF173 and pHCF136 activities are drastically reduced to ∼53% and ∼25% respectively, obtaining values similar to pHCF107. However, p35 S CaMV expression is relatively stable when individual values are taken into account rather than the median value, which again is not representative due to the high spread. Additionally, p35 S CaMV driven expression is 3-fold higher than the endogenous promoters at that stage.
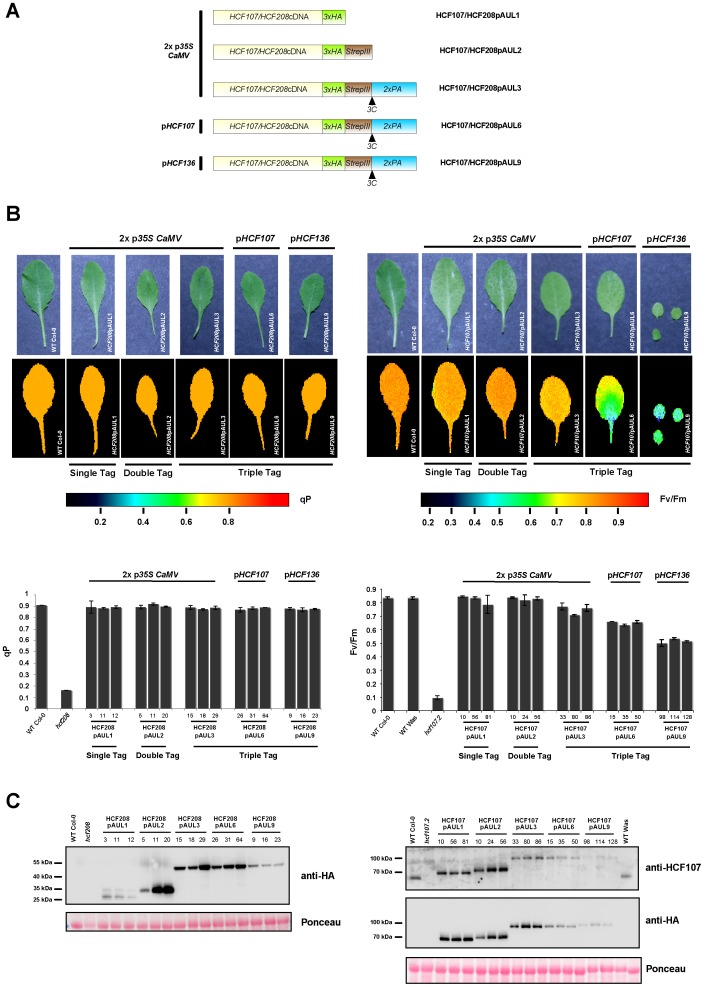
Figure 3. Complementation analysis of hcf208and hcf107.2 with a representative pAUL vector set.
(A) Schematic illustration of promoter/cDNA/tag combinations generated for transformation of hcf107.2 and hcf208. (B) Fluorometric analysis of HCF208- and HCF107 complemented plants, wild type and hcf208/hcf107.2 mutant plants. Pseudo-color images of maximum quantum efficiency of photosystem II (Fv/Fm) are displayed for HCF107 and of photochemical quenching efficiency (qP) are displayed for HCF208. 3 independent transformants were tested for each construct. Values for each line investigated are illustrated in diagrams. (C) Western blot analysis of complemented lines, wild type and mutant plants. 50 µg of crude protein extract were loaded. Membranes were decorated with Anti-HA-Peroxidase antibody for HCF208 and HCF107; HCF107 was also visualized by an HCF107-specific antibody.
Altogether, it can be stated that pHCF107 is suitable for constant and moderate expression of chloroplast-specific proteins, whereas pHCF136 and pHCF173 are optimal for proteins involved in early developmental stages (at least up to 15 days after germination) and which are not essential or would even be distracting in later stages. pHCF136 is adequate for stronger and pHCF173 for a more moderate early expression. Consequently, before starting cloning of sequences, one should check databases like GENEVESTIGATOR [30] or eFP Browser [35] to select the adequate vector.
Complementation Analyses of hcf107.2 and hcf208
Tagging of proteins, i.e. attachment of other protein sequences of up to several kDa (e.g. GFP: 27 kDa), and expression driven by foreign promoters may affect the function of fusion proteins. In order to test the influence of the presented tags on protein function and the differential expression by p35 S CaMV or endogenous promoters, we picked two proteins with different functional properties for complementation analyses. Both proteins are encoded by the nucleus and posttranslationally transported into the chloroplast where they perform different functions in chloroplast biogenesis. The HCF107 protein is part of a membrane-associated high molecular weight complex that is involved in stabilization/translation of the psbH mRNA [10]. On the other hand, HCF208 is an integral membrane protein that forms heterodimers and small complexes while assisting th c-type cytochrome maturation [20].
cDNAs of A. thaliana chloroplast biogenesis factors HCF107 and HCF208 were introduced into a subset of pAUL vectors (pAUL1, 2, 3, 6, 9), carrying either a single-, double-, or triple tag and p35 S CaMV or triple tags and pHCF107 or pHCF136 (Figure 3A). The constructs were transformed into heterozygous hcf107.2/HCF107 or hcf208/HCF208 background, as homozygous mutants cannot grow photoautotrophically. Transformants were screened for BASTA resistance and homozygous mutant backgrounds on sucrose-supplemented 0.5×MS medium and then were transferred to soil to test their capability to grow photoautotrophically.
The grade of complementation was tested by chlorophyll fluorescence measurements on 3 week-old plants (Figure 3B). Complementation of the photosystem II biogenesis factor hcf107.2 was indicated by the Fv/Fm ratio, the crucial parameter for photosystem II activity [36] indicating an estimate of the maximum portion of absorbed quanta used in photosystem II reaction centers. Since photosystem II is intact in hcf208 but the downstream cytochrome b 6 f complex is strongly reduced, qP (photochemical quenching) values were determined for indication of complementation. qP displays reduction of variable fluorescence by photosynthetic electron transport processes. From each tested line proteins were isolated and analyzed by Western blot in order to determine levels of fusion proteins.
For HCF208, all promoter/tag combinations are able to fully complement the mutant phenotype (Figure 3B). qP values of both, wild-type and complemented lines are ∼0.9 compared to the drastically lower value 0.16 of hcf208. These results indicate that none of the three tags fused to the C-terminus of HCF208 affect its functionality and that all tested promoters are able to drive transgene expression in a way that is sufficient for HCF208 complementation. However, protein levels of transgenic HCF208 vary strongly depending on the construct, as revealed by Western blot analysis using the HA antibody (Figure 3C). Among the transgenes driven by p35 S CaMV, all three independent HCF208pAUL1 lines accumulate very low protein levels unlike HCF208pAUL2- and HCF208pAUL3-constructs. One of the HCF208pAUL2 lines (line 5) also accumulates low amounts of HCF208 compared to the other two lines. There are two possible explanations for low accumulation of HCF208 in pAUL1 lines: either (i) unlike the double- and triple tag, the HA epitope destabilizes HCF208 when attached to its C-terminus or (ii) incidentally all three randomly selected HCF208pAUL1 lines and HCF208pAUL2-5 are silenced. Nevertheless, the residual amounts of HCF208 in these lines are sufficient to complement the mutant phenotype. Also, HCF208 levels in pAUL9 lines correspond to the low levels in HCF208pAUL1 and these lines, too, are fully complemented. As previously tested, the activity of pHCF136 in pAUL9 decreases during plant development, which accounts very likely for the low protein levels in three week-old HCF208pAUL9 lines. In HCF208pAUL6 lines harboring the transgene driven by pHCF107, proteins accumulate to levels similar to HCF208pAUL3. Altogether, it can be stated that protein levels in HCF208pAUL2 (except line 5), -3, and also -6 represent an overexpression of HCF208 exceeding endogenous levels. Unfortunately, no HCF208-specific antibody was available preventing comparison of protein levels in complemented lines to the wild-type situation. In conclusion, these results indicate that (i) the C-terminus of the integral membrane protein HCF208, which forms a large domain extending to the stroma [19] is not prone to attachment of large tags, although it might be influenced by the HA epitope and (ii) expression of HCF208 can be driven by all promoters to complement the mutant phenotype, but the 35 S CaMV promoter may be silenced in some lines.
The situation for hcf107.2 is different in some ways (Figure 3B). Wild-type Fv/Fm values of ∼0.83 compared to ∼0.1 in the mutant hcf107.2 are only reached by plants carrying HCF107pAUL1 and HCF107pAUL2 vectors, both driving expression by p35 S CaMV and possessing small tags. Extension of the tag sequence by ProtA (pAUL3) decreases Fv/Fm values to ∼0.74. Expression of the triple-tagged protein by pHCF107 or pHCF136 (HCF107pAUL6 and HCF107pAUL9) further decreases Fv/Fm values to ∼0.65 and ∼0.53 respectively (Figure 3B). According to these values, HCF107pAUL6 and HCF107pAUL9 plants are paler and smaller than wild type and HCF107pAUL1 to -3 plants. Even three weeks after germination, HCF107pAUL9 plants are very small and hardly produce seed, whereas the defect in HCF107pAUL6 is less severe (Figure 3B).
Western blot analysis was carried out using antibodies against the HA-epitope and HCF107, which was generated in our laboratory. As indicated in Figure 3C, HCF107pAUL1 and HCF107pAUL2 lines over-accumulate the fusion protein compared to wild-type levels, whereas levels of all triple-tagged proteins are significantly lower. Transgenic lines expressing triple-tagged HCF107 under p35 S CaMV control (pAUL3) exhibit about wild-type amounts of HCF107 but, as indicated before, the Fv/Fm ratio displaying photosystem II activity is decreased. This points to an inhibitory effect of the triple tag on protein stability and function. Expression of the triple-tagged protein by pHCF107 and pHCF136 results in protein levels below wild-type amounts. In HCF107pAUL9 plants HCF107 is hardly detectable.
Former experiments revealed that HCF107 forms a high molecular weight complex [10]. Thus, it is possible that the restriction of protein function by the triple tag may be due to inefficient complex assembly and subsequent degradation of unassembled protein. On the other hand, the protein itself may be unstable independent of its assembly state. In order to achieve nearly wild-type situation, triple tagged HCF107 needs to be overexpressed.
This detailed complementation analysis leads to the conclusion that it strongly depends on the investigated protein which promoter and tag should be chosen for experiments. For HCF208, large tags and expression by endogenous promoters were suitable for complementation, as HCF208 seems to be not required in large amounts for its function and C-terminal tags do not impair protein function, irrespective of their size. In case of HCF107 large tags impair protein function and/or result in destabilization of the protein. Thus, only smaller tags are suitable or overexpression of the incorporated gene is necessary to ensure complementation of the mutant phenotype. This also shows that protein analyses and purification should be carried out in mutant background if possible to show that the protein is not affected by its tag and ectopic expression.
Purification of HCF208 from Thylakoid Membranes
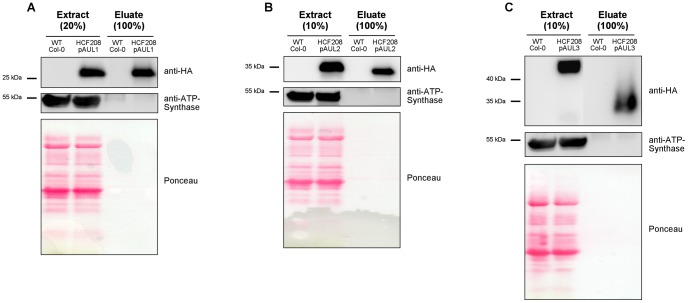
Integrity of the HA epitope, Strep-tagIII, and the ProtA tag and purification via these tags were tested on HCF208pAUL1, -pAUL2, and -pAUL3 transgenic lines. The difficulty in purification of HCF208 lies in its feature to be an integral membrane protein. First, crude membranes were treated with 1% n-dodecyl-ß-D-maltoside to solubilize proteins. Subsequently, all purification steps had to be performed in the presence of 0.05% n-dodecyl-ß-D-maltoside to keep proteins soluble.
The western blot analyses presented in the previous chapter indicate that the HA epitope is intact in all tag variants and that it is well suitable for detection using HA antibody. In contrast, detection of the Strep-tagIII in plant protein extracts at least under our conditions produced an extremely high background. Purification efficiency via anti-HA matrix was tested using solubilized protein extract from HCF208pAUL1. Elution was carried out competitively by the HA-peptide (Figure 4A). The feasibility of the double-, and triple tag for tandem purifications was tested using proteins from HCF208pAUL2 and -pAUL3 transgenic lines. Double-tagged HCF208 protein was loaded on StrepTactin matrix first, eluted competitively by desthiobiotin, and then purified via anti-HA affinity matrix (Figure 4B). Triple-tagged proteins were purified via IgG Sepharose first and eluted by 3C protease cleavage. In the second step, the eluate was incubated with StrepTactin matrix and eluted as described above (Figure 4C). From all purifications significant amounts of HCF208 could be recovered and eluates exhibited no abundant signals (ATP Synthase and Ponceau staining). Successive purification of triple tagged HCF208 from HCF208pAUL3 results in a protein of lower molecular size in the eluate compared to the input, which is consistent with the lack of the ProteinA tag cleaved off by 3C protease treatment (Figure 4C).
Figure 4. One step and tandem-purification of HCF208.
100 (A) or 200 µg (B, C) chlorophyll aliquots of solubilized membrane proteins were applied for purification. Aliquots of 20 µg chlorophyll from extracts and total amounts of eluates were separated by SDS-PAGE, transferred to a nitrocellulose membrane and immunodecorated with antibodies against the HA tag (Anti-HA-Peroxidase) and ATP-Synthase as a control. (A) One step purification of proteins from wild type and HCF208pAUL1 via the HA epitope and competitive elution. (B) Tandem purification of proteins from wild type and HCF208pAUL2 via Strep-tagIII and 3xHA (C) Tandem purification of proteins from wild type and HCF208pAUL3 via ProtA tag +3C protease cleavage and Strep-tagIII.
These experiments show that both one step and tandem affinity purifications can be carried out with our tagging system always using same buffer conditions and in the presence of detergents. Further, we introduced Strep-tagIII as a novel epitope that can be used for tagging of plant proteins and purification of proteins and protein complexes due to its property of binding at 4°C and competitive elution via desthiobiotin. Combination with the HA epitope allows easy detection of fusion proteins and, if desired, an additional low-scale purification step. Tandem affinity purification can be carried out using the triple tag via ProtA, which can be cleaved off by 3C protease, and subsequently via Strep-tagIII with all purification steps at 4°C.
In a recent study, Stoppel et al. used the pAUL11 vector (HCF173 promoter; 3xHA/Strep-tagIII) for tagging and expressing the chloroplast-localized RNase E (RNE) from Arabidopsis in rne mutant background. Using the Strep-tagIII epitope they were not only able to purify the RNE protein, but also to specifically co-precipitate the RNA-binding protein RHON1 [37]. In this way, the pAUL vector system has been proved to be effective tools for the purification of proteins as well as the identification of specific interaction partners.
Acknowledgments
We thank Stefanie Schulze for preparation and staining of leaf sections and Peter Jahns (Heinrich-Heine-Universitaet Duesseldorf) for his support with fluorescence measurements with the FlourCam system.
Funding Statement
The work described here was supported by the Deutsche Forschungsgemeinschaft (DFG) through Sonderforschungsbereich Transregio TR1. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pang SZ, DeBoer DL, Wan Y, Ye G, Layton JG, et al. (1996) An improved green fluorescent protein gene as a vital marker in plants. Plant Physiol 112: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Field J, Nikawa J, Broek D, MacDonald B, Rodgers L, et al. (1988) Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol 8: 2159–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Landy A (1989) Dynamic, structural, and regulatory aspects of lambda site-specific recombination. Annu Rev Biochem 58: 913–949. [DOI] [PubMed] [Google Scholar]
- 4. Karimi M, Depicker A, Hilson P (2007) Recombinational cloning with plant gateway vectors. Plant Physiol 145: 1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Odell JT, Nagy F, Chua NH (1985) Identification of DNA sequences required for activity of the cauliflower mosaic virus 35 S promoter. Nature 313: 810–812. [DOI] [PubMed] [Google Scholar]
- 6. Depicker A, Stachel S, Dhaese P, Zambryski P, Goodman HM (1982) Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet 1: 561–573. [PubMed] [Google Scholar]
- 7. Rubio V, Shen Y, Saijo Y, Liu Y, Gusmaroli G, et al. (2005) An alternative tandem affinity purification strategy applied to Arabidopsis protein complex isolation. Plant J 41: 767–778. [DOI] [PubMed] [Google Scholar]
- 8. Laufs P, Coen E, Kronenberger J, Traas J, Doonan J (2003) Separable roles of UFO during floral development revealed by conditional restoration of gene function. Development 130: 785–796. [DOI] [PubMed] [Google Scholar]
- 9. Felder S, Meierhoff K, Sane AP, Meurer J, Driemel C, et al. (2001) The nucleus-encoded HCF107 gene of Arabidopsis provides a link between intercistronic RNA processing and the accumulation of translation-competent psbH transcripts in chloroplasts. Plant Cell 13: 2127–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sane AP, Stein B, Westhoff P (2005) The nuclear gene HCF107 encodes a membrane-associated R-TPR (RNA tetratricopeptide repeat)-containing protein involved in expression of the plastidial psbH gene in Arabidopsis. Plant J 42: 720–730. [DOI] [PubMed] [Google Scholar]
- 11. Meurer J, Plucken H, Kowallik KV, Westhoff P (1998) A nuclear-encoded protein of prokaryotic origin is essential for the stability of photosystem II in Arabidopsis thaliana. Embo J 17: 5286–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schult K, Meierhoff K, Paradies S, Toller T, Wolff P, et al. (2007) The nuclear-encoded factor HCF173 is involved in the initiation of translation of the psbA mRNA in Arabidopsis thaliana. Plant Cell 19: 1329–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Junttila MR, Saarinen S, Schmidt T, Kast J, Westermarck J (2005) Single-step Strep-tag purification for the isolation and identification of protein complexes from mammalian cells. Proteomics 5: 1199–1203. [DOI] [PubMed] [Google Scholar]
- 14. Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, et al. (1999) A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol 17: 1030–1032. [DOI] [PubMed] [Google Scholar]
- 15. Xu X, Song Y, Li Y, Chang J, Zhang H, et al. (2010) The tandem affinity purification method: an efficient system for protein complex purification and protein interaction identification. Protein Expr Purif 72: 149–156. [DOI] [PubMed] [Google Scholar]
- 16. Rohila JS, Chen M, Cerny R, Fromm ME (2004) Improved tandem affinity purification tag and methods for isolation of protein heterocomplexes from plants. Plant J 38: 172–181. [DOI] [PubMed] [Google Scholar]
- 17. Lyska D, Paradies S, Meierhoff K, Westhoff P (2007) HCF208, a homolog of Chlamydomonas CCB2, is required for accumulation of native cytochrome b6 in Arabidopsis thaliana. Plant Cell Physiol 48: 1737–1746. [DOI] [PubMed] [Google Scholar]
- 18. Kuras R, Saint-Marcoux D, Wollman FA, de Vitry C (2007) A specific c-type cytochrome maturation system is required for oxygenic photosynthesis. Proc Natl Acad Sci U S A 104: 9906–9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lezhneva L, Kuras R, Ephritikhine G, de Vitry C (2008) A novel pathway of cytochrome c biogenesis is involved in the assembly of the cytochrome b6f complex in arabidopsis chloroplasts. J Biol Chem 283: 24608–24616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saint-Marcoux D, Wollman FA, de Vitry C (2009) Biogenesis of cytochrome b6 in photosynthetic membranes. J Cell Biol 185: 1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sato MH, Wada Y (1997) Universal template plasmid for introduction of the triple-HA epitope sequence into cloned genes. Biotechniques 23: 254–256. [DOI] [PubMed] [Google Scholar]
- 23. Jefferson RA (1987) Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol Biol Rep 5: 387–405. [Google Scholar]
- 24. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743. [DOI] [PubMed] [Google Scholar]
- 25. Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497. [Google Scholar]
- 26. Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kosugi S, Ohashi Y, Nakajima K, Arai Y (1990) An improved assay for beta-glucuronidase in transformed cells; Methanol almost completely suppresses a putative endogenous beta-glucuronidase activity. Plant Sci 70: 133–140. [Google Scholar]
- 28. Shen Y, Khanna R, Carle CM, Quail PH (2007) Phytochrome induces rapid PIF5 phosphorylation and degradation in response to red-light activation. Plant Physiol 145: 1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schagger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166: 368–379. [DOI] [PubMed] [Google Scholar]
- 30. Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 136: 2621–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith SM, Fulton DC, Chia T, Thorneycroft D, Chapple A, et al. (2004) Diurnal changes in the transcriptome encoding enzymes of starch metabolism provide evidence for both transcriptional and posttranscriptional regulation of starch metabolism in Arabidopsis leaves. Plant Physiol 136: 2687–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Battraw MJ, Hall TC (1990) Histochemical analysis of CaMV 35 S promoter-beta-glucuronidase gene expression in transgenic rice plants. Plant Mol Biol 15: 527–538. [DOI] [PubMed] [Google Scholar]
- 33. van Leeuwen W, Ruttink T, Borst-Vrenssen AW, van der Plas LH, van der Krol AR (2001) Characterization of position-induced spatial and temporal regulation of transgene promoter activity in plants. J Exp Bot 52: 949–959. [DOI] [PubMed] [Google Scholar]
- 34. Daxinger L, Hunter B, Sheikh M, Jauvion V, Gasciolli V, et al. (2008) Unexpected silencing effects from T-DNA tags in Arabidopsis. Trends Plant Sci 13: 4–6. [DOI] [PubMed] [Google Scholar]
- 35. Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, et al. (2007) An "Electronic Fluorescent Pictograph" browser for exploring and analyzing large-scale biological data sets. PLoS One 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Genty B, Briantais JM, Baker NR (1989) The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92. [Google Scholar]
- 37. Stoppel R, Manavski N, Schein A, Schuster G, Teubner M, et al. (2012) RHON1 is a novel ribonucleic acid-binding protein that supports RNase E function in the Arabidopsis chloroplast. Nucleic Acids Res 40: 8593–8606. [DOI] [PMC free article] [PubMed] [Google Scholar]