Abstract
Rhodiola imbricata Edgew. (Rose root or Arctic root or Golden root or Shrolo), belonging to the family Crassulaceae, is an important food crop and medicinal plant in the Indian trans-Himalayan cold desert. Chemometric profile of the n-hexane, chloroform, dichloroethane, ethyl acetate, methanol, and 60% ethanol root extracts of R. imbricata were performed by hyphenated gas chromatography mass spectrometry (GC/MS) technique. GC/MS analysis was carried out using Thermo Finnigan PolarisQ Ion Trap GC/MS MS system comprising of an AS2000 liquid autosampler. Interpretation on mass spectrum of GC/MS was done using the NIST/EPA/NIH Mass Spectral Database, with NIST MS search program v.2.0g. Chemometric profile of root extracts revealed the presence of 63 phyto-chemotypes, among them, 1-pentacosanol; stigmast-5-en-3-ol, (3β,24S); 1-teracosanol; 1-henteracontanol; 17-pentatriacontene; 13-tetradecen-1-ol acetate; methyl tri-butyl ammonium chloride; bis(2-ethylhexyl) phthalate; 7,8-dimethylbenzocyclooctene; ethyl linoleate; 3-methoxy-5-methylphenol; hexadecanoic acid; camphor; 1,3-dimethoxybenzene; thujone; 1,3-benzenediol, 5-pentadecyl; benzenemethanol, 3-hydroxy, 5-methoxy; cholest-4-ene-3,6-dione; dodecanoic acid, 3-hydroxy; octadecane, 1-chloro; ethanone, 1-(4-hydroxyphenyl); α-tocopherol; ascaridole; campesterol; 1-dotriacontane; heptadecane, 9-hexyl were found to be present in major amount. Eventually, in the present study we have found phytosterols, terpenoids, fatty acids, fatty acid esters, alkyl halides, phenols, alcohols, ethers, alkanes, and alkenes as the major group of phyto-chemotypes in the different root extracts of R. imbricata. All these compounds identified by GC/MS analysis were further investigated for their biological activities and it was found that they possess a diverse range of positive pharmacological actions. In future, isolation of individual phyto-chemotypes and subjecting them to biological activity will definitely prove fruitful results in designing a novel drug.
Introduction
To identify and evaluate the therapeutic potential of medicinal herbs, isolation of active components and structural elucidation of these compounds is very essential in medicinal chemistry and natural product research. In recent years a lot of attention has been given towards the study of organic compounds from medicinal herbs and to elucidate their pharmacological activities. Numerous extraction techniques and analytical systems like spectrophotometry, capillary electrophoresis, high performance liquid chromatography (HPLC), high performance thin layer chromatography (HPTLC), gas chromatography (GC) with flame ionization detection (FID), gas chromatography/mass spectrometry (GC/MS) have been developed for the analysis and characterization of active compounds from medicinal plants. GC/MS has become an ideal technique for qualitative and quantitative analysis of volatile and semi-volatile compounds of plant origin. It has the unique combination of a perfect separation system (GC) with the excellent identification and confirmation technique (MS) which has made it the best suited analytical system for plant compound characterization. Additionally, for rapid extraction and precise analysis of these active phyto-compounds, the experimental design should also be optimized to obtain enhanced recoveries, low solvent consumption, and reduced extraction time [1]–[5].
Rhodiola imbricata Edgew. (Rose root/Arctic root/Golden root/Shrolo), belonging to the family Crassulaceae, is an important food crop and medicinal plant in the high altitude region of Indian trans-Himalayan cold desert. It is a popular medicinal plant in Pakistan, Nepal, India, Tibet, China, and many other countries and is widely used as food and traditional medicine around the world. A number of metabolites like phenylpropanoids, phenylethanol derivatives, flavanoids, terpenoids, and phenolic acids have been found in good quantity from these Rhodiola species and extracts of these plant species, particularly those from roots, have been shown to possess pharmacological activities. A survey of the literature showed that Rhodiola species influence a number of physiological functions including neurotransmitter levels, central nervous system activity, and cardiovascular function. It is being used to stimulate the nervous system, decrease depression, enhance work performance, eliminate fatigue, and prevents high-altitude sickness. Most of these effects have been ascribed to constituents such as salidrosides (rhodiolosides), rosavins, and p-tyrosol. Many pharmacological studies on R. imbricata have demonstrated that this plant exhibits cardioprotective, anti-inflammatory, antistress, dermal wound healing, and adaptogenic activities. It has also been found to possess antioxidant, antiaging, immuno-stimulant, radioprotective, and anticarcinogenic properties [6]–[24]. All these reports validate its use in traditional system of medicine.
However, the phytochemistry of the most important plant part having the medicinal and therapeutic potential, the root of R. imbricata has not been studied in considerable details. Hence, aim of the present investigation was to identify and quantify the chemotypes extracted successively in different solvents such as n-hexane, chloroform, dichloroethane, ethyl acetate, methanol, and 60% ethanol, from roots of R. imbricata from trans-Himalayan cold desert of Ladakh, India, by hyphenated GC/MS technique.
Materials and Methods
Chemicals
n-Hexane, chloroform, dichloroethane, ethyl acetate, methanol, ethanol, and water CHROMASOLV HPLC grade and all other chemicals used were of analytical grade and purchased from Sigma-Aldrich (St. Louis, MO, USA).
Ethics statement
All necessary permits were obtained for the described field studies. The permit was issued by Dr. B. Balaji (IFS), Divisional Forest Officer, Leh Forest Division, Jammu & Kashmir, India.
Plant materials and extraction
R. imbricata roots were collected from the trans-Himalayan region (Chang-La Top, altitude = 5330 m above mean sea level, Indus valley, Ladakh) of India in the month of October, 2011 after the period of senescence, with the prior permission from the local authorities. The plant roots were washed thoroughly and cut into small pieces and shade dried at room temperature for 15 days. Then they were finely powdered and used for extraction. The root powder (20 gm) was taken for the sequential extraction in six solvent systems with increasing polarity viz. n-hexane, chloroform, dichloroethane, ethyl acetate, methanol, and 60% ethanol by Soxhlet apparatus (Borosil GlassWorks Limited, Worli, Mumbai, India) at 40°C. The extracted fractions were concentrated under vacuum and reduced pressure (BUCHI Rotavapor R-205, BUCHI Labortechnik AG CH-9230, Flawil, Switzerland) at 40°C by circulation of cold water using thermostat maintained at 4°C in order to minimize the degradation of thermolabile compounds. The dry extracts were then stored in a −80°C freezer till further analysis.
Preparation of sample for GC/MS analysis
The 25 mg of concentrated n-hexane, chloroform, dichloroethane, ethyl acetate, methanol, and 60% ethanol root extracts were redissolved in the respective solvents, vortexed properly and filtered through 0.22 µm syringe filter (Millipore Corp., Bedford, MA, USA). One microlitre aliquot of the sample solution was injected into the GC/MS MS system for the requisite analysis.
Instrumentation and chromatographic conditions
GC/MS analysis was carried out on a Thermo Finnigan PolarisQ Ion Trap GC/MS MS system comprising of an AS2000 liquid autosampler (Thermo Finnigan, Thermo Electron Corporation, Austin, TX, USA). The gas chromatograph was interfaced to a mass spectrometer instrument employing the following conditions viz. Durabond DB-5 ms column (30 m×0.25 mm×0.25 µm), operating in electron impact [electron ionisation positive (EI+)] mode at 70 eV, helium (99.999%) was used as carrier gas at a constant flow of 1 ml/min, an injection volume of 0.5 EI was employed (split ratio of 10∶1), injector temperature 280°C, and transfer line temperature 300°C. The oven temperature was programmed from 50°C (isothermal for 2 min), with gradual increase in steps of 10°C/min, to 300°C. Mass spectra were taken at 70 eV, a scan interval of 0.5 s, and full mass scan range from 25 m/z to 1000 m/z. The data acquisition was performed on Finnigan Xcalibur data acquisition and processing software version 2.0 (ThermoQuest, LC and LC/MS Division, San Jose, California, USA).
Identification of components
Interpretation of mass spectrum of GC/MS was done using the NIST/EPA/NIH Mass Spectral Database (NIST11), with NIST MS search program v.2.0g [National Institute Standard and Technology (NIST), Scientific Instrument services, Inc., NJ, USA]. The mass spectrum of the unknown component was compared with the spectrum of the known components stored in the NIST library. The name, molecular weight, and structure of the components of the test materials were ascertained.
Results
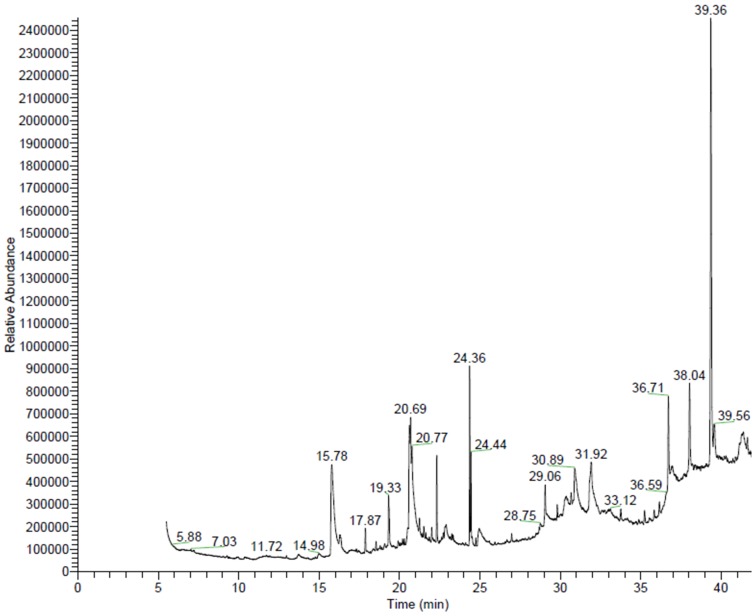
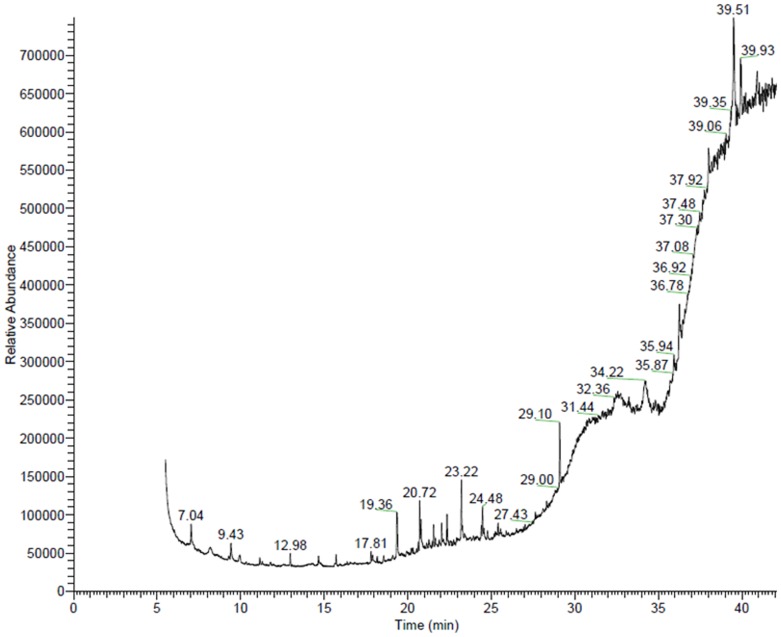
GC/MS chromatograms of n-hexane (Fig. 1), chloroform (Fig. 2), dichloroethane (Fig. 3), ethyl acetate (Fig. 4), methanol (Fig. 5), and 60% ethanol (Fig. 6) root extracts of R. imbricate as per the experimental procedure discussed above, showed various peaks indicating the presence of different chemotypes in the respective extracts.
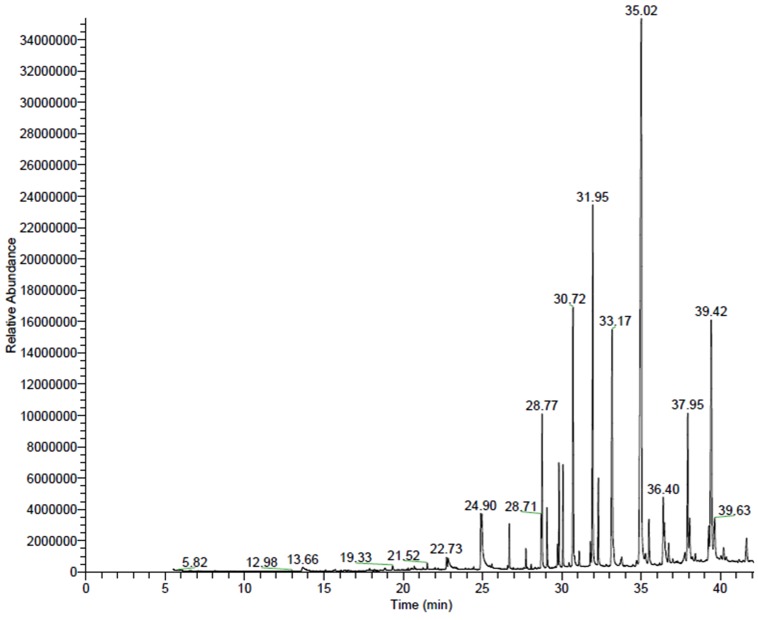
Figure 1. GC/MS chromatogram of n-hexane root extract of R. imbricata.
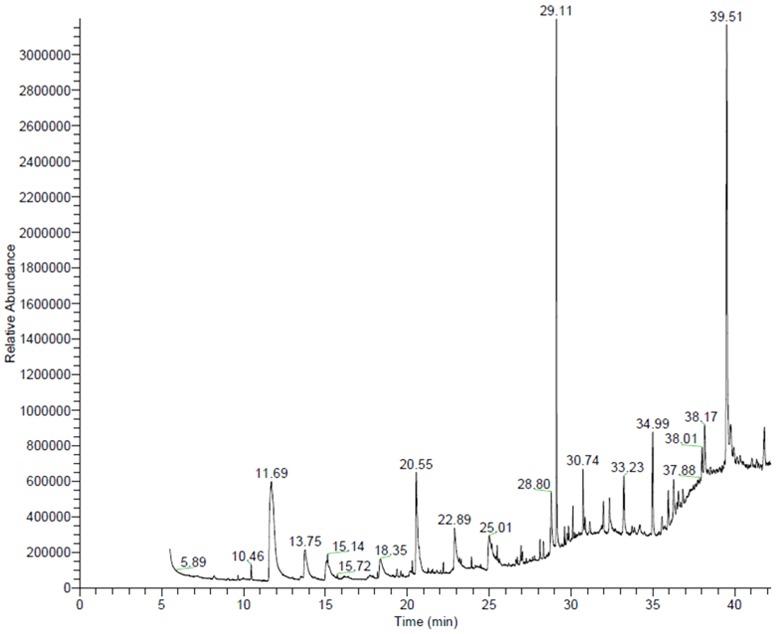
Figure 2. GC/MS chromatogram of chloroform root extract of R. imbricata.
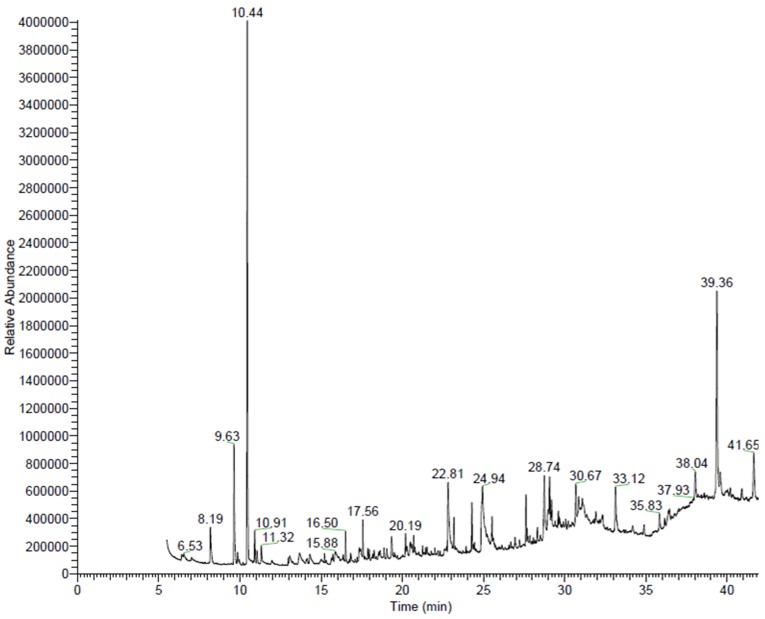
Figure 3. GC/MS chromatogram of dichloroethane root extract of R. imbricata.
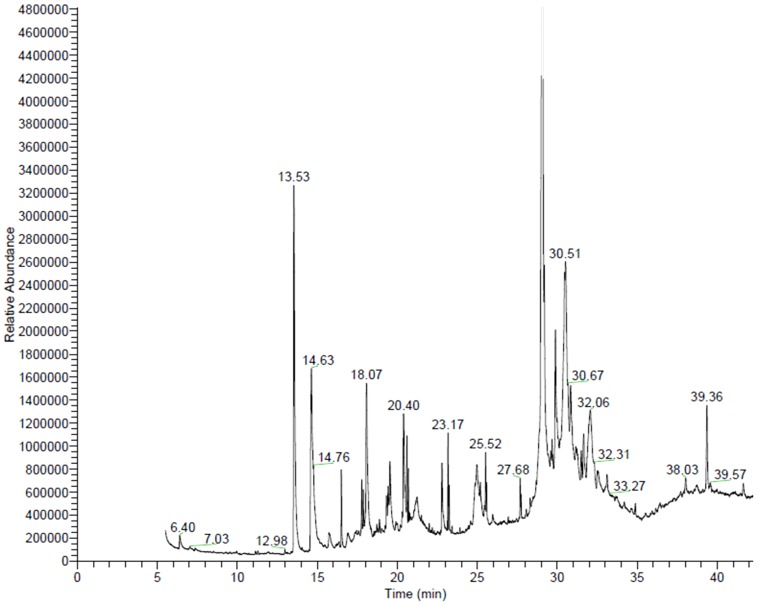
Figure 4. GC/MS chromatogram of ethyl acetate root extract of R. imbricata.
Figure 5. GC/MS chromatogram of methanol root extract of R. imbricata.
Figure 6. GC/MS chromatogram of 60% ethanol root extract of R. imbricata.
GC/MS chemometric profile
n-Hexane root extract
The n-hexane root extract revealed the presence of 22 different chemotypes which were characterized and identified (Table 1, Fig. 1) by comparison of their mass fragmentation patterns with the similar in NIST database library. Of these 22 chemotypes, 1-pentacosanol (28.21%), stigmast-5-en-3-ol, (3β,24S) (13.40%), 1-teracosanol (9.23%), 1-henteracontanol (8.53%), 17-pentatriacontene (7.01%), and 13-tetradecen-1-ol acetate (6.40%) were found to be major constituents whereas 1-hentriacontane (3.66%), 1-heptacosane (3.47%), 1-tericosanol (2.51%), 13-docosan-1-ol, (Z) (2.12%), eicosen-1-ol, cis-9 (1.99%) 1,30-triacontanediol (1.49%), stigmast-4-en-3-one (1.28%), bis(2-ethylhexyl) phthalate (1.20%), hexadecanoic acid (1.16%), 1-tetrateracontane (0.90%), campesterol (0.90%), α-Tocopherol-β-D-mannoside (0.73%), stigmastanol (0.71%), 1-pentatriacontane (0.48%), and 3-methoxy-5-methylphenol (0.46%) were found to be present in trace amount.
Table 1. Phyto-chemotypes identified in the n-hexane root extract of R. imbricata by GC/MS.
| S. No. | Peak RT (min) | Peak area | Peak area (%) | Compound detected | Hit | SI | RSI | Prob | CAS No | Mol. Formula | Mol. Wt. |
| 1 | 13.66 | 3489208 | 0.46 | 3-Methoxy-5-methylphenol | 1 | 838 | 874 | 72.98 | 3209-13-0 | C8H10O2 | 138 |
| 2 | 22.84 | 9266063 | 1.16 | Hexadecanoic acid | 1 | 801 | 842 | 73.13 | 57-10-3 | C16H32O2 | 256 |
| 3 | 24.91 | 34516141 | 4.16 | Ethyl linoleate | 1 | 839 | 876 | 17.81 | 544-35-4 | C20H36O2 | 308 |
| 4 | 26.69 | 6791237 | 0.9 | 1-Tetratetracontane | 1 | 827 | 829 | 10.27 | 7098-22-8 | C44H90 | 618 |
| 5 | 27.74 | 3769798 | 0.48 | 1-Pentatriacontane | 1 | 823 | 838 | 11.01 | 630-07-9 | C35H72 | 492 |
| 6 | 28.77 | 30375043 | 3.66 | 1-Hentriacontane | 1 | 848 | 855 | 14.33 | 630-04-6 | C31H64 | 436 |
| 7 | 29.07 | 9600422 | 1.2 | Bis(2-ethylhexyl) phthalate | 3 | 855 | 866 | 18.65 | 117-81-7 | C24H38O4 | 390 |
| 8 | 29.82 | 20564432 | 2.51 | 1-Tricosanol | 4 | 807 | 823 | 6.18 | 05-01-3133 | C23H48O | 340 |
| 9 | 30.08 | 16467246 | 1.99 | Eicosen-1-ol, cis-9 | 1 | 810 | 847 | 10.25 | 112248-30-3 | C20H40O | 296 |
| 10 | 30.7 | 56408242 | 7.01 | 17-Pentatriacontene | 1 | 799 | 800 | 7.41 | 6971-40-0 | C35H70 | 490 |
| 11 | 31.95 | 74961756 | 9.23 | 1-Tetracosanol | 2 | 804 | 821 | 12.41 | 506-51-4 | C24H50O | 354 |
| 12 | 33.31 | 16764245 | 2.12 | 13-Docosen-1-ol, (Z) | 1 | 798 | 820 | 9.17 | 629-98-1 | C22H44O | 324 |
| 13 | 33.17 | 68435403 | 8.53 | 1-Hentetracontanol | 1 | 848 | 870 | 37.4 | 40710-42-7 | C41H84O | 592 |
| 14 | 35.01 | 229932016 | 28.21 | 1-Pentacosanol | 1 | 812 | 823 | 31.85 | 26040-98-2 | C25H52O | 368 |
| 15 | 35.5 | 12157246 | 1.49 | 1,30-Triacontanediol | 1 | 769 | 786 | 7.65 | 36645-68-8 | C30H62O2 | 454 |
| 16 | 36.4 | 29211487 | 3.47 | 1-Heptacosane | 3 | 781 | 825 | 10.3 | 593-49-7 | C27H56 | 380 |
| 17 | 36.74 | 5914760 | 0.73 | α-Tocopherol-β-D-mannoside | 1 | 814 | 865 | 57.26 | CID 597057 | C35H60O7 | 592 |
| 18 | 37.95 | 54630744 | 6.4 | 13-Tetradecen-1-ol acetate | 5 | 743 | 801 | 4.89 | 56221-91-1 | C16H30O2 | 254 |
| 19 | 38.08 | 6914671 | 0.9 | Campesterol | 1 | 771 | 794 | 53.27 | 474-62-4 | C28H48O | 400 |
| 20 | 39.39 | 107745880 | 13.4 | Stigmast-5-en-3-ol, (3β,24S) | 1 | 848 | 855 | 45.49 | 83-47-6 | C29H50O | 414 |
| 21 | 39.62 | 5803953 | 0.71 | Stigmastanol | 1 | 720 | 732 | 58.69 | 19466-47-8 | C29H52O | 416 |
| 22 | 41.64 | 10789041 | 1.28 | Stigmast-4-en-3-one | 1 | 699 | 874 | 18.43 | 1058-61-3 | C29H48O | 412 |
Chloroform root extract
GC/MS chemometric profile of chloroform root extract showed the presence of 18 different chemotypes (Table 2, Fig. 2). Amongst these, stigmast-5-en-3-ol, (3β,24S) (24.30%), methyl tri-butyl ammonium chloride (14.64%), bis(2-ethylhexyl) phthalate (11.50%), 7,8-dimethylbenzocyclooctene (7.97%), ethyl linoleate (4.75%), 3-methoxy-5-methylphenol (4.16%), and hexadecanoic acid (4.13%) were found to constitute major amount while, campesterol (3.94%), 1-pentacosanol (3.82%), 17-pentariacontene (3.38%), benzene sulfonic acid, 4-amino-3-nitro (3.21%), orcinol (2.93%), benzenemethanol, 3-hydroxy, 5-methoxy (2.62%), 1-hentetracontanol (2.54%), 1-tetracosanol (1.86%), stigmast-4-en-3-one (1.82%); and α-tocopherol (1.31%), and eicosen-1-ol, cis-9 (1.13%) were found to be present in trace quantity.
Table 2. Phyto-chemotypes identified in the chloroform root extract of R. imbricata by GC/MS.
| S. No. | Peak RT (min) | Peak area | Peak area % | Compound detected | Hit | SI | RSI | Prob | CAS No | Mol. Formula | Mol. Wt. |
| 1 | 11.69 | 9872792 | 14.64 | Methyl tri-butyl ammonium chloride | 1 | 792 | 797 | 56.37 | 56375-79-2 | C13H30ClN | 235 |
| 2 | 13.75 | 2364627 | 4.16 | 3-Methoxy-5-methylphenol | 1 | 808 | 858 | 67.78 | 3209-13-0 | C8H10O2 | 138 |
| 3 | 15.14 | 1927265 | 2.93 | 1,3-Benzenediol, 5-methyl | 1 | 708 | 865 | 22.77 | 504-15-4 | C7H8O2 | 124 |
| 4 | 18.35 | 1520584 | 2.62 | Benzenemethanol, 3-hydroxy-5-methoxy | 1 | 811 | 860 | 85.91 | 30891-29-3 | C8H10O3 | 154 |
| 5 | 20.55 | 5041042 | 7.97 | 7,8-Dimethylbenzocyclooctene | 1 | 770 | 849 | 30.04 | 99027-76-6 | C14H14 | 182 |
| 6 | 22.89 | 2762363 | 4.13 | Hexadecanoic acid | 1 | 749 | 813 | 60.75 | 57-10-3 | C16H32O2 | 256 |
| 7 | 25.01 | 3042142 | 4.75 | Ethyl linoleate | 4 | 736 | 854 | 17.03 | 544-35-4 | C20H36O2 | 308 |
| 8 | 28.8 | 2066650 | 3.21 | Benzene sulfonic acid, 4-amino-3-nitro | 4 | 582 | 652 | 2.29 | 616-84-2 | C6H6N2O5S | 218 |
| 9 | 29.11 | 7589941 | 11.5 | Bis(2-ethylhexyl) phthalate | 4 | 793 | 806 | 9.51 | 117-81-7 | C24H38O4 | 390 |
| 10 | 30.12 | 670458 | 1.13 | Eicosen-1-ol, cis-9 | 1 | 810 | 847 | 10.25 | 112248-30-3 | C20H40O | 296 |
| 11 | 30.74 | 2150315 | 3.38 | 17-Pentariacontene | 1 | 799 | 800 | 7.41 | 6971-40-0 | C35H70 | 490 |
| 12 | 31.98 | 1129772 | 1.86 | 1-Tetracosanol | 2 | 804 | 821 | 12.41 | 506-51-4 | C24H50O | 354 |
| 13 | 33.23 | 1665168 | 2.54 | 1-Hentetracontanol | 1 | 848 | 870 | 37.4 | 40710-42-7 | C41H84O | 592 |
| 14 | 34.99 | 2475797 | 3.82 | 1-Pentacosanol | 8 | 616 | 763 | 0.8 | 26040-98-2 | C25H52O | 368 |
| 15 | 36.83 | 898525 | 1.31 | α-Tocopherol | 3 | 571 | 702 | 11.35 | 59-02-9 | C29H50O2 | 430 |
| 16 | 38.17 | 2267712 | 3.94 | Campesterol | 1 | 682 | 779 | 20.92 | 474-62-4 | C28H48O | 400 |
| 17 | 39.51 | 16343303 | 24.29 | Stigmast-5-en-3-ol, (3β,24S) | 1 | 798 | 823 | 43.31 | 83-47-6 | C29H50O | 414 |
| 18 | 41.82 | 1236875 | 1.82 | Stigmast-4-en-3-one | 1 | 540 | 625 | 8.51 | 1058-61-3 | C29H48O | 412 |
Dichloroethane root extract
GC/MS chemometric profile of dichloroethane root extract illustrated the presence of 25 different chemotypes (Table 3, Fig. 3). Among these, camphor (17.78%), stigmast-5-en-3-ol, (3β,24S) (15.42%), ethyl linoleate (9.95%), 1,3-dimethoxybenzene (8.15%), hexadecanoic acid (6.55%), and thujone (4.73%) were present in major amount, whereas, benzene sulfonic acid, 4-amino-3-nitro (3.96%), campesterol (3.88%), methanol, (4-carboxymethoxy) benzoyl (3.27%), stigmast-4-en-3-one (2.84%), 1-hentetracontanol (2.74%), oleic acid (2.18%), bis(2-ethylhexyl) adipate (2.16%), bacteriochlorophyll-c-stearyl (2.11%), eucalyptol (1.95%), ethanone, 1-(2,6-dihydroxy-4-methoxyphenyl) (1.66%), 1-dotriacontane (1.58%), linalyl isovalerate (1.49%), 3-methoxy-5-methylphenol (1.47%), 1-chloro-2,4-dimethoxybenzene (1.44%), borneol (1.22%), 4-chlorothiophenol (1.02%), phenol, 2,4-bis(1,1-dimethylethyl) (0.94%), fenchyl alcohol (0.91%), and stigmast-3,5-dien-7-one (0.60%) were found to be present in trace.
Table 3. Phyto-chemotypes identified in the dichloroethane root extract of R. imbricata by GC/MS.
| S. No. | Peak RT (min) | Peak area | Peak area % | Compound detected | Hit | SI | RSI | Prob | CAS No | Mol. Formula | Mol. Wt. |
| 1 | 8.19 | 1187393 | 1.95 | Eucalyptol | 2 | 800 | 829 | 51.1 | 470-82-6 | C10H18O | 154 |
| 2 | 9.64 | 2910856 | 4.73 | Thujone | 1 | 835 | 845 | 23.35 | 546-80-5 | C10H16O | 152 |
| 3 | 10.44 | 11188301 | 0.85 | Camphor | 1 | 837 | 854 | 25.13 | 76-22-2 | C10H16O | 152 |
| 4 | 10.91 | 742204 | 1.22 | Borneol | 1 | 866 | 878 | 28.4 | 464-45-9 | C10H18O | 154 |
| 5 | 11.32 | 552901 | 0.91 | β-fenchyl alcohol | 1 | 765 | 836 | 10.05 | 470-08-6 | C10H18O | 154 |
| 6 | 13.06 | 631861 | 1.02 | Benzenethiol, 4-chloro | 1 | 599 | 670 | 34.81 | 106-54-7 | C6H5ClS | 144 |
| 7 | 13.66 | 898266 | 1.47 | 3-Methoxy-5-methylphenol | 1 | 759 | 851 | 50.29 | 3209-13-0 | C8H10O2 | 138 |
| 8 | 15.88 | 2126709 | 3.27 | Methanol, (4-carboxymethoxy) benzoyl | 1 | 694 | 746 | 16.43 | 80099-44-1 | C10H10O5 | 210 |
| 9 | 16.5 | 571000 | 0.94 | Phenol, 2,4-bis(1,1-dimethylethyl) | 2 | 842 | 865 | 22.66 | 96-76-4 | C14H22O | 206 |
| 10 | 17.36 | 885243 | 1.44 | 1-Chloro-2,4-dimethoxybenzene | 1 | 635 | 756 | 24.81 | 7051-13-0 | C8H9ClO2 | 172 |
| 11 | 17.56 | 926151 | 1.49 | Linalyl isovalerate | 1 | 751 | 812 | 14.87 | 50649-12-2 | C15H26O2 | 238 |
| 12 | 19.33 | 953645 | 1.58 | 1-Dotriacontane | 1 | 787 | 803 | 45.91 | 544-85-4 | C32H66 | 450 |
| 13 | 20.49 | 975679 | 1.66 | Ethanone, 1-(2,6-dihydroxy-4-methoxyphenyl) | 1 | 661 | 835 | 45.49 | 7507-89-3 | C9H10O4 | 182 |
| 14 | 22.81 | 4154976 | 6.55 | Hexadecanoic acid | 1 | 801 | 855 | 70.5 | 57-10-3 | C16H32O2 | 256 |
| 15 | 23.18 | 1089256 | 2.18 | Oleic acid | 1 | 772 | 798 | 49.32 | 112-80-1 | C18H34O2 | 282 |
| 16 | 24.28 | 1364685 | 2.11 | Bacteriochlorophyll-c-stearyl | 1 | 755 | 767 | 13.97 | CID5367801 | C52H72MgN4O4 | 840 |
| 17 | 24.94 | 6245279 | 9.95 | Ethyl linoleate | 1 | 783 | 883 | 10.73 | 544-35-4 | C20H36O2 | 308 |
| 18 | 27.61 | 1330635 | 2.16 | Hexanedioic acid, bis(2-ethylhexyl) ester | 1 | 695 | 773 | 29.62 | 103-23-1 | C22H42O4 | 370 |
| 19 | 28.74 | 2480706 | 3.96 | Benzene sulfonic acid, 4-amino-3-nitro | 6 | 590 | 655 | 1.81 | 616-84-2 | C6H6N2O5S | 218 |
| 20 | 29.1 | 5070293 | 8.15 | 1,3-Dimethoxybenzene | |||||||
| 21 | 33.12 | 1747914 | 2.74 | 1-Hentetracontanol | 4 | 675 | 828 | 4.63 | 40710-42-7 | C41H84O | 592 |
| 22 | 38.04 | 2440601 | 3.88 | Campesterol | 1 | 652 | 759 | 13.55 | 474-62-4 | C28H48O | 400 |
| 23 | 39.36 | 9725435 | 15.42 | Stigmast-5-en-3-ol, (3β,24S) | 1 | 814 | 853 | 63.99 | 83-47-6 | C29H50O | 414 |
| 24 | 40.9 | 366122 | 0.6 | Stigmast-3,5-dien-7-one | 1 | 512 | 760 | 71 | 2034-72-2 | C29H46O | 410 |
| 25 | 41.65 | 1869647 | 2.84 | Stigmast-4-en-3-one | 1 | 628 | 856 | 22.86 | 1058-61-3 | C29H48O | 412 |
Ethyl acetate root extract
Nineteen different chemotypes were identified in ethyl acetate extract (Table 4, Fig. 4). Amongst these 19 chemotypes, 1,3-dimethoxybenzene (27.61%), 1,3-benzenediol, 5-pentadecyl (16.90%), 3-methoxy-5-methylphenol (10.11%), 1,3-benzenediol, 5-methyl (8.40%), benzenemethanol, 3-hydroxy, 5-methoxy (5.75%), cholest-4-ene-3,6-dione (5.75%), and dodecanoic acid, 3-hydroxy (4.46%) were found to constitute major amount, whereas, 7,8-dimethylbenzocyclooctene (3.57%) 3,5-dimethoxyphenyl acetate (3.44%), α-D-glucopyranoside, O-α-D-glucopyranosyl-(1.fwdarw.3)-β-D-fructofuranosyl (2.95%), stigmast-5-en-3-ol, (3β,24S) (2.12%), eicosen-1-ol, cis-9 (2.12%), hexadecanoic acid (1.84%), oleic acid (1.34%), bacteriochlorophyll-c-stearyl (1.14%), phenol, 2,4-bis(1,1-dimethylethyl) (0.85%), 1-pentatricontene (0.72%), 1-dodecanol, 3,7,11-trimethyl (0.61%), and stigmast-4-en-3-one (0.32%) were found to be present in trace.
Table 4. Phyto-chemotypes identified in the ethyl acetate root extract of R. imbricata by GC/MS.
| S. No. | Peak RT (min) | Peak area | Peak area % | Compound detected | Hit | SI | RSI | Prob | CAS No | Mol. Formula | Mol. Wt. |
| 1 | 10.11 | 18961417 | 4.16 | 3-Methoxy-5-methylphenol | 1 | 880 | 881 | 78.08 | 3209-13-0 | C8H10O2 | 138 |
| 2 | 14.63 | 17158116 | 8.4 | 1,3-Benzenediol, 5-methyl | 1 | 909 | 929 | 70.69 | 504-15-4 | C7H8O2 | 124 |
| 3 | 16.5 | 1721777 | 0.85 | Phenol, 2,4-bis(1,1-dimethylethyl) | 1 | 860 | 887 | 30.17 | 96-76-4 | C14H22O | 206 |
| 4 | 17.78 | 1213849 | 0.61 | 1-Dodecanol, 3,7,11-trimethyl | 1 | 696 | 721 | 3.99 | 6750-34-1 | C15H32O | 228 |
| 5 | 18.07 | 11340896 | 5.75 | Benzenemethanol, 3-hydroxy-5-methoxy | 1 | 866 | 868 | 75.89 | 30891-29-3 | C8H10O3 | 154 |
| 6 | 19.54 | 7034263 | 3.44 | Phenol, 3,5-dimethoxy acetate | 7 | 636 | 829 | 4.41 | 23133-74-6 | C10H12O4 | 196 |
| 7 | 20.4 | 7323033 | 3.57 | 7,8-Dimethylbenzocyclooctene | 1 | 803 | 870 | 51.6 | 99027-76-6 | C14H14 | 182 |
| 8 | 20.6 | 4129209 | 2.12 | Eicosen-1-ol, cis-9 | 1 | 750 | 771 | 5.19 | 629-96-9 | C20H40O | 296 |
| 9 | 21.22 | 6328149 | 2.95 | α-D-glucopyranoside, O-α-D-glucopyranosyl-(1.fwdarw.3)-β-D-fructofuranosyl | 1 | 720 | 759 | 37.89 | 597-12-6 | C18H32O16 | 504 |
| 10 | 22.8 | 3545343 | 1.84 | Hexadecanoic Acid | 1 | 795 | 837 | 71.82 | 57-10-3 | C16H32O2 | 256 |
| 11 | 23.17 | 2659100 | 1.34 | Oleic acid | 1 | 779 | 794 | 11.53 | 112-80-1 | C18H34O2 | 282 |
| 12 | 24.97 | 9179686 | 4.46 | Dodecanoic acid, 3-hydroxy | 1 | 674 | 706 | 35.46 | 1883-13-2 | C12H24O3 | 216 |
| 13 | 25.52 | 2261098 | 1.14 | Bacteriochlorophyll-c-stearyl | 1 | 722 | 739 | 6.88 | CID5367801 | C52H72MgN4O4 | 840 |
| 14 | 27.68 | 1352685 | 0.72 | 17-Pentatriacontene | 1 | 720 | 737 | 46.41 | 6971-40-0 | C35H70 | 490 |
| 15 | 29.08 | 76996880 | 27.61 | 1,3-Dimethoxybenzene | 2 | 712 | 767 | 11.52 | 151-10-0 | C8H10O2 | 138 |
| 16 | 30.51 | 29595091 | 16.9 | 1,3-Benzenediol, 5-pentadecyl | 1 | 664 | 797 | 23.45 | 3158-56-3 | C21H36O2 | 320 |
| 17 | 32.06 | 11993388 | 5.75 | Cholest-4-ene-3,6-dione | 1 | 710 | 773 | 32.12 | 984-84-9 | C27H42O2 | 398 |
| 18 | 39.36 | 4575754 | 2.12 | Stigmast-5-en-3-ol, (3β,24S) | 1 | 757 | 821 | 54.07 | 83-47-6 | C29H50O | 414 |
| 19 | 41.64 | 684162 | 0.32 | Stigmast-4-en-3-one | 1 | 508 | 755 | 16.81 | 1058-61-3 | C29H48O | 412 |
Methanol root extract
The methanol root extract revealed the presence of 18 different chemotypes (Table 5, Fig. 5). Among the identified chemotypes, stigmast-5-en-3-ol, (3β,24S) (21.91%), octadecane, 1-chloro (17.01%), ethanone, 1-(4-hydroxyphenyl) (11.07%), α-tocopherol (8.42%), ascaridole (5.92%), and campesterol (4.98%) were found to present in major amount, while, linolein, 2-mono (3.99%), hexadecanoic acid (3.67%), 1,3-dimethoxybenzene (3.57%), ethyl linoleate (3.35%), 1-dotriacontane (2.21%), linolein, 1-mono (1.74%), methyl palmitate (1.73%), stigmast-4-en-3-one (1.55%), 1-dodecane (0.66%), δ-tocopherol (0.56%), and 3-methoxy-5-methylphenol (0.43%) were found to be present in trace.
Table 5. Phyto-chemotypes identified in the methanol root extract of R. imbricata by GC/MS.
| S. No. | Peak RT (min) | Peak area | Peak area % | Compound detected | Hit | SI | RSI | Prob | CAS No | Mol. Formula | Mol. Wt. |
| 1 | 13.73 | 224745 | 0.43 | 3-Methoxy-5-methylphenol | 1 | 730 | 810 | 72.09 | 3209-13-0 | C8H10O2 | 138 |
| 2 | 15.78 | 5548712 | 11.07 | Ethanone, 1-(4-hydroxyphenyl) | 1 | 884 | 910 | 60.39 | 99-93-4 | C8H8O2 | 136 |
| 3 | 17.87 | 345721 | 0.66 | 1-Dodecane | 1 | 699 | 751 | 16.38 | 112-40-3 | C12H26 | 170 |
| 4 | 19.33 | 1157289 | 2.21 | 1-Dotriacontane | 1 | 777 | 785 | 14.73 | 544-85-4 | C32H66 | 450 |
| 5 | 20.69 | 8405167 | 17.01 | Octadecane, 1-chloro | 1 | 734 | 738 | 22.3 | 386-33-2 | C18H37Cl | 288 |
| 6 | 22.32 | 905705 | 1.73 | Hexadecanoic acid, methyl ester | 1 | 793 | 866 | 61.21 | 112-39-0 | C17H34O2 | 270 |
| 7 | 22.89 | 1901742 | 3.67 | Hexadecanoic acid | 4 | 660 | 762 | 11.87 | 57-10-3 | C16H32O2 | 256 |
| 8 | 24.36 | 1592833 | 3.99 | 9,12-Octadecadienoic acid (Z,Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 5 | 807 | 833 | 6.93 | 3443-82-1 | C21H38O4 | 354 |
| 9 | 24.44 | 821776 | 1.74 | 9,12,15-Octadecatrienoic acid, 2,3-dihydroxypropyl ester, (Z,Z,Z) | 2 | 804 | 825 | 32.86 | 18465-99-1 | C21H36O4 | 352 |
| 10 | 24.96 | 1495805 | 3.35 | Ethyl linoleate | 10 | 681 | 835 | 2.99 | 544-35-4 | C20H36O2 | 308 |
| 11 | 29.06 | 1614826 | 3.57 | 1,3-Dimethoxybenzene | 34 | 471 | 675 | 0.33 | 151-10-0 | C8H10O2 | 138 |
| 12 | 30.91 | 3109377 | 5.92 | Ascaridole | 1 | 619 | 700 | 57.05 | 512-85-6 | C10H16O2 | 168 |
| 13 | 31.92 | 3533697 | 7.23 | Unknown | - | - | - | - | - | - | - |
| 14 | 33.77 | 174824 | 0.56 | δ-Tocopherol | 1 | 667 | 763 | 89.78 | 119-13-1 | C27H46O2 | 402 |
| 15 | 36.71 | 4419092 | 8.42 | α-Tocopherol | 1 | 731 | 853 | 46.36 | 59-02-9 | C29H50O2 | 430 |
| 16 | 38.04 | 2615057 | 4.98 | Campesterol | 1 | 726 | 810 | 24.4 | 474-62-4 | C28H48O | 400 |
| 17 | 39.36 | 11245680 | 21.91 | Stigmast-5-en-3-ol, (3β,24S) | 1 | 813 | 850 | 67.6 | 83-47-6 | C29H50O | 414 |
| 18 | 41.38 | 902689 | 1.55 | Stigmast-4-en-3-one | 45 | 395 | 709 | 0.74 | 1058-61-3 | C29H48O | 412 |
60% Ethanol root extract
GC/MS chemometric profile of 60% ethanol root extracts illustrated the presence of 12 different chemotypes (Table 6, Fig. 6). Amongst the identified chemotypes, dotriacontane (5.69%), and heptadecane, 9-hexyl (5.44%) were found to be present in major amount, whereas, bis(2-ethylhexyl) phthalate (3.58%), hexadecanoic acid, methyl ester (2.27%), and dibutyl phthalate (1.23%) were found to be present in trace.
Table 6. Phyto-chemotypes identified in the 60% ethanol root extract of R. imbricata by GC/MS.
| S. No. | Peak RT (min) | Peak area | Peak area % | Compound detected | Hit | SI | RSI | Prob | CAS No | MF | MW |
| 1 | 19.37 | 258681 | 5.69 | 1-Dotriacontane | 1 | 769 | 805 | 39.82 | 544-85-4 | C32H66 | 450 |
| 2 | 20.72 | 276232 | 5.44 | Heptadecane, 9-hexyl | 1 | 696 | 719 | 24.93 | 55124-79-3 | C23H48 | 324 |
| 3 | 21.56 | 62567 | 1.23 | Dibutyl phthalate | 5 | 799 | 875 | 5.32 | 84-74-2 | C16H22O4 | 278 |
| 4 | 22.36 | 115053 | 2.27 | Hexadecanoic acid, methyl ester | 1 | 624 | 700 | 20.11 | 112-39-0 | C17H34O2 | 270 |
| 5 | 23.22 | 296422 | 5.84 | Unknown | - | - | - | - | - | - | - |
| 6 | 24.48 | 194215 | 3.83 | Unknown | - | - | - | - | - | - | - |
| 7 | 25.41 | 56168 | 1.11 | Unknown | - | - | - | - | - | - | - |
| 8 | 29.1 | 181582 | 3.58 | Bis(2-ethylhexyl) phthalate | 2 | 697 | 786 | 11.81 | 117-81-7 | C24H38O4 | 390 |
| 9 | 34.21 | 644518 | 12.7 | Unknown | - | - | - | - | - | - | - |
| 10 | 39.51 | 1832781 | 35.53 | Unknown | - | - | - | - | - | - | - |
| 11 | 39.95 | 550771 | 10.86 | Unknown | - | - | - | - | - | - | - |
| 12 | 40.92 | 604576 | 11.92 | Unknown | - | - | - | - | - | - | - |
Discussion
We have conducted the present investigation to identify the major volatile and semivolatile components in the root of R. imbricata. The presence of various bioactive compounds justifies the use of the plant by traditional practitioners of ‘Amchi’ system of medicine in trans-Himalayan Ladakh region. Also, extensive pharmacological studies were conducted by different researchers with the plant root extracts [6]–[23] and the results were very promising to justify the use of this plant as therapeutic agent.
However, the phytochemical profiling of the plant root still remains to be unexplored and to the best of our knowledge, this is the first ever study of its kind on the GC/MS chemometric profiling of the root extracts. In medicinal chemistry, it is very essential to ascertain the chemotyping of medicinal plant parts that are responsible for its numerous pharmacological properties and by this technique we may be able to scientifically determine and validate the traditional uses, pharmacological activities, and therapeutic potential of these plant parts. Profiling of metabolites in plant extracts permits the complete phenotyping of genetically or environmentally adapted plant systems and such investigations draw on simple extraction procedures that have been shown to be very robust and have permitted broad range of high-throughput applications in plant metabolomics. [25]–[27].
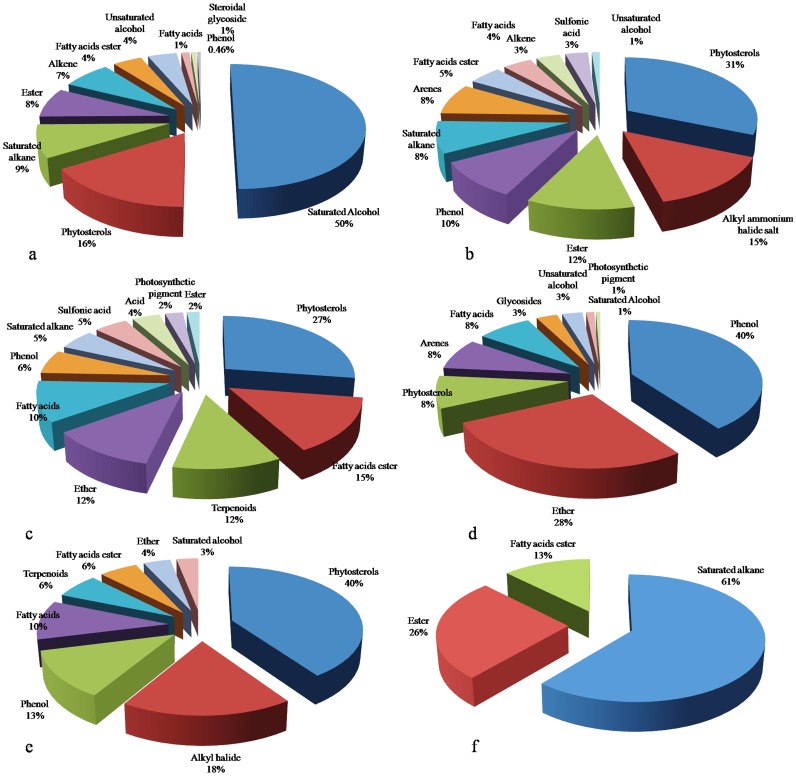
The major phytochemical groups in n-hexane, ethyl acetate, and 60% ethanol extracts were saturated alcohol (50%), phenols (40%), and alkanes (61%) respectively. On the other hand, phytosterols were the major group in chloroform (31%), dichloroethane (27%), and methanol (40%) extracts. The total of various volatile and semi volatile groups present in different root extracts of R. imbricata had the following distribution order: phytosterols (122%), alkanes (83%), phenols (69.46%), esters (48%), ethers (44%), fatty acid esters (43%), fatty acids (33%), terpenoids (18%), arenes (16%), alkyl ammonium halide salt (15%), alkenes (10%), sulfonic acid (8%), unsaturated alcohols (8%), organic acids (4%), saturated alcohols (4%), glycosides (3%), photosynthetic pigments (3%), steroidal glycoside (1%). The order of extraction capacities of different polarity solvents for phytosterols, phenols, fatty acids, alkanes, esters, fatty acid esters, ethers, unsaturated alcohols, arenes, terpenoids, alkenes, sulfonic acid, photosynthetic pigment, and saturated alcohols was as follows:
Phytosterols: methanol (40%), chloroform (31%), dichloroethane (27%), n-hexane (16%), ethyl acetate (8%)
Phenols: ethyl acetate (40%), methanol (13%), chloroform (10%), dichloroethane (6%), n-hexane (0.46%)
Fatty acids: dichloroethane (10%) = methanol (10%), ethyl acetate (8%), chloroform (4%), n-hexane (1%)
Alkane: 60% ethanol (61%), n-hexane (9%), chloroform (8%), dichloroethane (5%)
Esters: 60% ethanol (26%), chloroform (12%), n-hexane (8%), dichloroethane (2%)
Fatty acid esters: dichloroethane (15%), 60% ethanol (13%), methanol (6%), chloroform (5%), n-hexane (4%)
Ethers: ethyl acetate (28%), dichloroethane (12%), methanol (4%)
Unsaturated alcohols: n-hexane (4%), ethyl acetate (3%), chloroform (1%)
Arenes: chloroform (8%) = ethyl acetate (8%)
Terpenoids: dichloroethane (12%), methanol (6%)
Alkenes: n-hexane (7%), chloroform (3%)
Sulfonic acid: dichloroethane (5%), chloroform (3%)
Photosynthetic pigment: dichloroethane (2%), ethyl acetate (1%)
Saturated alcohols: methanol (3%), ethyl acetate (1%)
The steroidal glycoside, alkyl ammonium halide salt, organic acids, and glycoside were found only in n-hexane (1%), chloroform (15%), dichloroethane (4%), and ethyl acetate (3%), respectively. Eventually, in the present study we have found phytosterols, terpenoids, fatty acids, fatty acid esters, alkyl halides, phenols, alcohols, ethers, alkanes, and alkenes as the major group of phyto-chemotypes in the different root extracts of R. imbricate (Fig. 7, Table 7). All these compounds identified by GC/MS analysis (Fig. 8) were further investigated for their biological activities [28] and most of them were found to possess a diverse range of positive pharmacological actions (Table 8).
Figure 7. Estimation of major phytochemical groups in different root extracts of R. imbricata, a) n-hexane extract, b) chloroform extract, c) dichloroethane extract, d) ethyl acetate extract, e) methanol extract, f) 60% ethanol extract.
Table 7. Distribution of phyto-chemotypes in different root extracts of R. imbricatea.
| Phyto-chemotypes | Root extracts | |||||
| n-Hexane | Chloroform | Dichloroethane | Ethyl acetate | Methanol | 60% Ethanol | |
| 1,30-Triacontanediol | √ | – | – | – | – | – |
| 1,3-Benzenediol, 5-methyl | – | √ | – | √ | – | – |
| 1,3-Benzenediol, 5-pentadecyl | – | – | – | √ | – | – |
| 13-Docosen-1-ol, (Z) | √ | – | – | – | – | – |
| 13-Tetradecen-1-ol acetate | √ | – | – | – | – | – |
| 17-Pentatriacontene | √ | √ | – | √ | – | – |
| 1-Chloro-2,4-dimethoxybenzene | – | – | √ | – | – | – |
| 1-Dodecanol, 3,7,11-trimethyl | – | – | – | √ | – | – |
| Eicosen-1-ol, cis-9 | – | – | – | √ | – | – |
| 1-Hentetracontanol | √ | – | √ | – | – | – |
| 1-Pentacosanol | √ | √ | – | – | – | – |
| 1-Tetracosanol | √ | √ | – | – | – | – |
| 1-Tricosanol | √ | – | – | – | – | – |
| 3-Methoxy-5-methylphenol | √ | √ | √ | √ | √ | – |
| 7,8-Dimethylbenzocyclooctene | – | √ | – | √ | – | – |
| 9,12,15-Octadecatrienoic acid, 2,3-dihydroxypropyl ester, (Z,Z,Z) | – | – | – | – | √ | – |
| 9,12-Octadecadienoic acid (Z,Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester | – | – | – | – | √ | – |
| Ascaridole | – | – | – | – | √ | – |
| Bacteriochlorophyll-c-stearyl | – | – | √ | √ | – | – |
| Benzene, 1,3-dimethoxy | – | – | – | √ | √ | – |
| Benzenemethanol, 3-hydroxy-5-methoxy | – | √ | – | √ | – | – |
| Benzene sulfonic acid, 4-amino-3-nitro | – | √ | √ | – | – | – |
| Benzenethiol, 4-chloro | – | – | √ | – | – | – |
| Bis(2-ethylhexyl) phthalate | √ | √ | – | – | – | √ |
| Borneol | – | – | √ | – | – | – |
| Campesterol | √ | √ | √ | – | √ | – |
| Camphor | – | – | √ | – | – | – |
| Cholest-4-ene-3,6-dione | – | – | – | √ | – | – |
| Stigmast-5-en-3-ol, (3β,24S) | √ | √ | √ | √ | √ | – |
| Di-butyl phthalate | – | – | – | – | – | √ |
| 1-Dodecane | – | – | – | – | √ | – |
| Dodecanoic acid, 3-hydroxy | – | – | – | √ | – | – |
| 1-Dotriacontane | – | – | √ | – | √ | √ |
| Ethanone, 1-(2,6-dihydroxy-4-methoxyphenyl) | – | – | √ | – | – | – |
| Ethanone, 1-(4-hydroxyphenyl) | – | – | – | – | √ | – |
| Ethyl linoleate | √ | √ | √ | – | √ | – |
| Eucalyptol | – | – | √ | – | – | – |
| α-D-Glucopyranoside, O-α-D-glucopyranosyl-(1.fwdarw.3)-β-D-fructofuranosyl | – | – | – | √ | – | – |
| 1-Hentetracontanol | – | √ | – | – | – | – |
| 1-Hentriacontane | √ | – | – | – | – | – |
| 1-Heptacosane | √ | – | – | – | – | – |
| Heptadecane, 9-hexyl | – | – | – | – | – | √ |
| Hexadecanoic acid | √ | √ | √ | √ | √ | – |
| Hexadecanoic acid, methyl ester | – | – | – | – | √ | √ |
| Hexanedioic acid, bis(2-ethylhexyl) ester | – | – | √ | – | – | – |
| Linalyl isovalerate | – | – | √ | – | – | – |
| 1,3-Dimethoxybenzene | – | – | √ | – | – | – |
| Methanol, (4-carboxymethoxy)benzoyl | – | – | √ | – | – | – |
| Octadecane, 1-chloro | – | – | – | – | √ | – |
| Oleic acid | – | – | √ | √ | – | – |
| 1-Pentatriacontane | √ | – | – | – | – | – |
| Phenol, 2,4-bis(1,1-dimethylethyl) | – | – | √ | √ | – | – |
| Phenol, 3,5-dimethoxy, acetate | – | – | – | √ | – | – |
| Stigmast-4-en-3-one | √ | √ | √ | √ | √ | – |
| Stigmast-3,5-dien-7-one | – | – | √ | – | – | – |
| Stigmastanol | √ | – | – | – | – | – |
| 1-Tetratetracontane | √ | – | – | – | – | – |
| Thujone | – | – | √ | – | – | – |
| Methyl tri-butyl ammonium chloride | – | √ | – | – | – | – |
| α-Tocopherol | √ | √ | – | – | √ | – |
| β-Fenchyl alcohol | – | – | √ | – | – | – |
| δ-Tocopherol | – | – | – | – | √ | – |
| α-Tocopherol-β-D-mannoside | √ | – | – | – | – | – |
√ Present; – Absent.
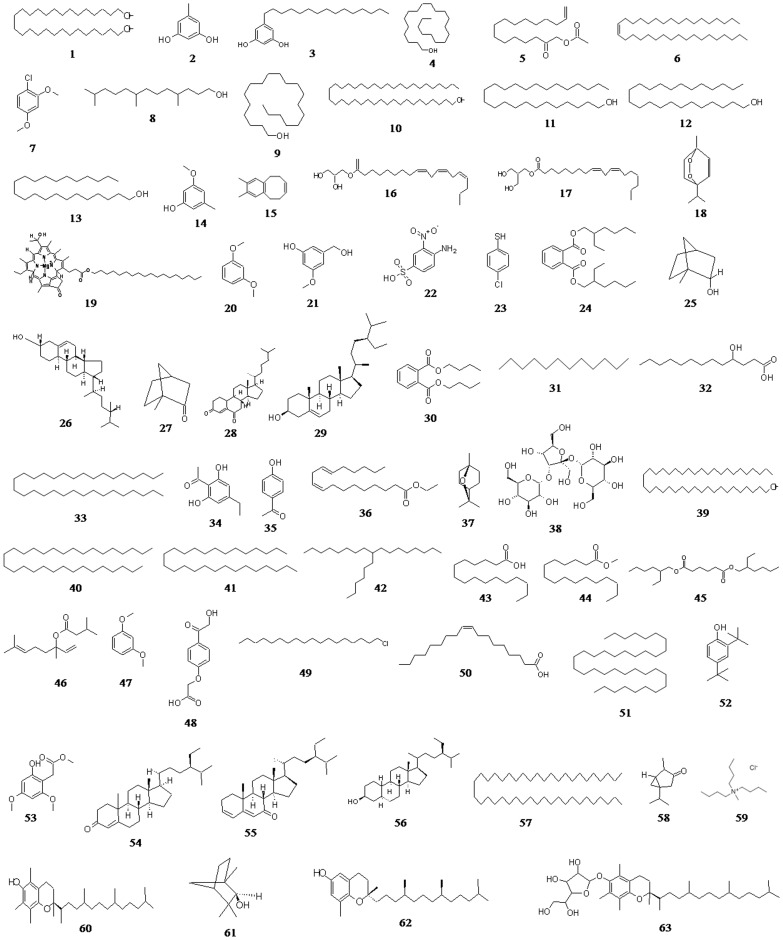
Figure 8. Phyto-chemotypes identified in different root extracts of R. imbricata.
1: 1,30-triacontanediol; 2: 1,3-benzenediol, 5-methyl; 3: 1,3-benzenediol, 5-pentadecyl; 4: 13-docosen-1-ol, (Z); 5: 13-tetradecen-1-ol acetate; 6: 17-pentatriacontene; 7: 1-chloro-2,4-dimethoxybenzene; 8: 1-dodecanol, 3,7,11-trimethyl; 9: eicosen-1-ol, cis-9; 10: 1-hentetracontanol; 11: 1-pentacosanol; 12: 1-tetracosanol; 13: 1-tricosanol; 14: 3-methoxy-5-methylphenol; 15: 7,8-dimethylbenzocyclooctene; 16: 9,12,15-octadecatrienoic acid, 2,3-dihydroxypropyl ester, (Z,Z,Z); 17: 9,12-octadecadienoic acid (Z,Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester; 18: ascaridole; 19: bacteriochlorophyll-c-stearyl; 20: benzene, 1,3-dimethoxy; 21: benzenemethanol, 3-hydroxy-5-methoxy; 22: benzene sulfonic acid, 4-amino-3-nitro; 23: benzenethiol, 4-chloro; 24: bis(2-ethylhexyl) phthalate; 25: borneol; 26: campesterol; 27: camphor; 28: cholest-4-ene-3,6-dione; 29: stigmast-5-en-3-ol, (3β,24S); 30: di-butyl phthalate; 31: 1-dodecane; 32: dodecanoic acid, 3-hydroxy; 33: 1-dotriacontane; 34: ethanone, 1-(2,6-dihydroxy-4-methoxyphenyl); 35: ethanone, 1-(4-hydroxyphenyl); 36: ethyl linoleate; 37: eucalyptol; 38: α-D-glucopyranoside, O-α-D-glucopyranosyl-(1.fwdarw.3)-β-D-fructofuranosyl; 39: 1-hentetracontanol; 40: 1-hentriacontane; 41: 1-heptacosane; 42: heptadecane, 9-hexyl; 43: hexadecanoic acid; 44: hexadecanoic acid, methyl ester; 45: hexanedioic acid, bis(2-ethylhexyl) ester; 46: linalyl isovalerate; 47: 1,3-dimethoxybenzene; 48: methanol, (4-carboxymethoxy)benzoyl; 49: octadecane, 1-chloro; 50: oleic acid; 51: 1-pentatriacontane; 52: phenol, 2,4-bis(1,1-dimethylethyl); 53: phenol, 3,5-dimethoxy acetate; 54: stigmast-4-en-3-one; 55: stigmast-3,5-dien-7-one; 56: stigmastanol; 57: 1-tetratetracontane; 58: thujone; 59: methyl tri-butyl ammonium chloride; 60: α-tocopherol; 61: β-fenchyl alcohol; 62: δ-tocopherol; 63: α-tocopherol-β-D-mannoside.
Table 8. Biological activities of active principles present in different root extracts of R. imbricate.
| Phyto-chemotypes | Biological activity |
| Eicosen-1-ol, cis-9 | Antimalarial, antifungal, antioxidant |
| 1-Tricosanol | Antibacterial, antifungal |
| 9,12,15-Octadecatrienoic acid, 2,3-dihydroxypropyl ester, (Z,Z,Z) | 5-Alpha-reductase inhibitor, antiMS, antiacne, antialopecic, antianaphylactic, antiandrogenic, antiarteriosclerotic, antiarthritc, anticoronary, antieczemic, antifibrinolytic, antigranular, antihistaminic, antiinflammatory, antileukotriene-D4, antimenorrhagic, antiprostatitic, cancer-preventive, carcinogenic, comedolytic, hepatoprotective, hypocholesterolemic, immunomodulator, insectifuge, metastatic, nematicide, propecic |
| 9,12-Octadecadienoic acid (Z,Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester | Antiinflammatory, hypocholesterolemic, cancer preventive, hepatoprotective, nematicide, insectifuge, antihistaminic, antieczemic, antiacne, 5-alpha reductase inhibitor antiandrogenic, antiarthritic, anticoronary, insectifuge |
| Ascaridole | Analgesic, ancylostomicide, anthelmintic, antiflatulent, antimalarial, carcinogenic, carminative, fungicide, nematicide, pesticide, plasmodicide, sedative, transdermal, trypanocide, vermifuge |
| Borneol | (-)-Chronotropic, (-)-inotropic, allelochemic, analgesic, antiacetylcholine, antibacterial, antibronchitic, antiescherichic, antifeedant, antiinflammatory, antiotitic, antipyretic, antisalmonella, antispasmodic, antistaphylococcic, antiyeast, CNS-stimulant, CNS-toxic, candidicide, choleretic, flavor; fungicide, hepatoprotective, herbicide, herbicide, inhalant, insect-repellent, insectifuge, irritant, myorelaxant, nematicide, perfumery, pesticide, sedative, tranquilizer |
| Campesterol | Antioxidant, hypocholesterolemic |
| Camphor | Allelopathic, analgesica, anesthetic, antiacne, antidiarrheic, antidysenteric, antiemetic, antifeedant, antifibrositic, antineuralgic, antipruritic, antiseptic, antispasmodic, CNS-stimulant, cancer preventive, carminative, convulsant, cosmetic, counterirritant, decongestant, deliriant, ecbolic, emetic, epileptigenic, expectorant, fungicide, herbicide, insect-repellent, insectifuge, irritant, nematicide, occuloirritant, P450-2B1-inhibitor, pesticide, respirainhibitor, respirastimulant, rubefacient, stimulant, transdermal, verrucolytic, vibriocide |
| Stigmast-5-en-3-ol, (3β,24S) | Androgenic, angiogenic, anorexic, antiadenomic, antiandrogenic, antibacterial, anticancer (breast), anticancer (cervix), anticancer (lung), antiedemic, antiestrogenic, antifeedant, antifertility, antigonadotrophic, antihyperlipoproteinaemic, antiinflammatory, antileukemic, antilymphomic, antimutagenic, antiophidic, antioxidant, antiprogestational, antiprostaglandin, antiprostatadenomic, antiprostatitic, antipyretic, antitumor (breast), antitumor (cervix), antitumor (lung), antiviral, apoptotic, artemicide, cancer-preventive, candidicide, caspase-8-inducer, estrogenic, febrifuge, gonadotrophic, hepatoprotective, hypocholesterolemic, hypoglycemic, hypolipidemic, pesticide, spermicide, ubiquiot, ulcerogenic |
| Di-butyl phthalate | Antimicrobial, Antifouling |
| Dodecanoic acid, 3-hydroxy | Flavor |
| Eucalyptol | Anesthetic, anthelmintic, antibacterial, antihalitosic, antiseptic, antitussive, decongestant, expectorant, hypotensive, insectifuge, irritant, pesticide, vermicide |
| α-D-Glucopyranoside, O-α-D-glucopyranosyl-(1.fwdarw.3)-β-D-fructofuranosyl | Preservative |
| Hexadecanoic acid | Antioxidant, hypocholesterolemic, nematicide, pesticide, lubricant, antiandrogenic, flavor, hemolytic 5-alpha reductase inhibitor |
| Hexadecanoic acid, methyl ester | Antioxidant, nematicide, pesticide, lubricant, antiandrogenic, flavor, hemolytic 5-alpha reductase inhibitor, hypocholesterolemic |
| Linalyl isovalerate | Fragrance |
| Oleic acid | 5-Alpha-reductase-inhibitor, allergenic, anemiagenic, antialopecic, antiandrogenic, antiinflammatory, antileukotriene-D4; cancer-preventive, choleretic, dermatitigenic, flavor, hypocholesterolemic, insectifuge, irritant, percutaneostimulant, perfumery, propecic |
| 1-Pentatriacontane | Herbistat |
| Stigmast-4-en-3-one | Antiprostatitic |
| Stigmast-3,5-dien-7-one | Antifertility |
| Thujone | Abortifacient, anthelmintic, antibacterial, antiseptic, antispasmodic, cerebrodepressant, convulsant, counterirritant, emmenagogue, epileptigenic, hallucinogenic, herbicide, neurotoxic, perfumery, pesticide, respirainhibitor, toxic |
| β-Fenchyl alcohol | Antimicrobial, antioxidant, flavor |
| δ-Tocopherol | 5-HETE-inhibitor, allergenic, analgesic, antiMD, antiMS, antiPMS, antiaggregant, antiaging, antialzheimeran, antianginal, antiarteriosclerotic, antiarthritic, antiatherosclerotic, antibronchitic, anticancer (breast), anticariogenic, anticataract, antichorea, antichoreic, anticonvulsant, anticoronary, antidecubitic, antidementia, antidermatitic, antidiabetic, antidysmenorrheic, antiepitheleomic, antifibrositic, antiglycosation, antiherpetic, antiinfertility, antiinflammatory, antiischemic, antileukemic, antileukotriene, antilithic, antilupus, antimaculitic, antimastalgic, antimelanomic, antimyoclonic, antineuritic, antineuropathic, antinitrosaminic, antiophthalmic, antiosteoarthritic, antioxidant, antiparkinsonian, antiproliferant, antiradicular, antiretinopathic, antirheumatic, antisenility, antisickling, antispasmodic, antisterility, antistroke, antisunburn, antisyndrome-X, antithalassemic, antithrombotic, antithromboxane-B2, antitoxemic, antitumor; antitumor (breast), antitumor (colorectal), antitumor (prostate), antitumor (stomach), antiulcerogenic, apoptotic, calcium-antagonist, cancer-preventive, cardioprotective, cerebroprotective, circulatory-stimulant, circulotonic, hepatoprotective, hypocholesterolemic, hypoglycemic, immunomodulator, immunostimulant, insulin-sparing, lipoxygenase-inhibitor, NO-inhibitor, ornithine-decarboxylase-inhibitor, P21-inducer, phospholipase-A2-inhibitor, protein-kinase-C-inhibitor, vasodilator |
Most of the pharmacological studies were conducted with the aqueous, ethanol, and hydro-alcoholic root extracts of this plant and it was found to have numerous biological activities such as anti-stress, adaptogenic, anti-hypoxic, immune-stimulatory, anti-cancer, cytoprotective, radioprotective, anti-hemolytic, anti-inflammatory, and wound healing potential [6]–[23]. Our investigations conclude that the compounds present in the ethanol and water extracts have the potential to perform these functions. Though, the root extracts of the plant obtained by polar solvent extraction have been investigated for their pharmacological actions in considerable detail, non polar root extracts were not studied till date. Hence, our primary objective in the present work was to find the bioactive constituents present in the non polar extraction of root of this herb. These findings will definitely usher in new directions in pharmacological and therapeutic investigations with the root extracts obtained from non polar solvent extraction such as n-hexane, chloroform, dichloroethane, and ethyl acetate.
Conclusion
In the present study, sixty three phyto-chemotypes have been identified from n-hexane, chloroform, dichloroethane, ethyl acetate, methanol, and 60% ethanol root extracts of R. imbricata by GC/MS analysis. It showed the existence of various bioactive principles that confirm the application of R. imbricata for various ailments in traditional system of medicine. However, isolation of individual phyto-chemotypes and subjecting them to biological activity will definitely give fruitful results to find a novel drug. It could be concluded that R. imbricata contains various bioactive phyto-chemotypes having phyto-pharmaceutical importance. However, further studies will need to be undertaken to ascertain its bioactivity, toxicity profile, effect on the ecosystem, and agricultural products.
Acknowledgments
Authors are thankful to Dr. Bassant Ballabh, STA ‘C’, Defence Institute of Bio-Energy Research, Defence Research & Development Organisation, Goraparao, PO-Arjunpur, Haldwani, PIN - 263 139, Uttarakhand, India, for critical review of the manuscript. Authors also acknowledge our colleague Mr. Ritendra Mishra for copyediting of the manuscript.
Funding Statement
Funding provided by Defence Research & Development Organization, Ministry of Defence, Government of India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gherman C, Culea M, Cozar O (2000) Comparative analysis of some active principles of herb plants by GC/MS. Talanta 53: 253–262. [DOI] [PubMed] [Google Scholar]
- 2. Carro N, Garcia CM, Cela R (1997) Microwave-assisted extraction of monoterpenols in must samples. Analyst 122: 325–329. [Google Scholar]
- 3. Robards K, Antolovich M (1997) Analytical chemistry of fruit bioflavonoids. Analyst 122: 11–34. [Google Scholar]
- 4. Pallado P, Tassinato G, D'Alpaos D, Traldi P (1997) Gas chromatography/mass spectrometry in aroma chemistry: a comparison of essential oils and flavours extracted by classical and supercritical techniques. Rapid Commun Mass Spectrom 11: 1335–1341. [Google Scholar]
- 5. Iordache A, Culea M, Gherman C, Cozar O (2009) Characterization of some plant extracts by GC–MS. Nuclear Instruments and Methods in Physics Research B 267: 338–342. [Google Scholar]
- 6. Mishra KP, Ganju L, Singh SB (2012) Anti-cellular and immunomodulatory potential of aqueous extract of Rhodiola imbricata rhizome. Immunopharmacol Immunotoxicol 34: 513–518. [DOI] [PubMed] [Google Scholar]
- 7. Tulsawani R, Meena DK, Shukla H, Sharma P, Meena RN, et al. (2011) Ninety days of repeated gavage administration of Rhodiola imbricata extract in rats. J Appl Toxicol doi:10.1002/jat.1739. [DOI] [PubMed] [Google Scholar]
- 8. Chawla R, Jaiswal S, Kumar R, Arora R, Sharma RK (2010) Himalayan bioresource Rhodiola imbricata as a promising radioprotector for nuclear and radiological emergencies. J Pharma and Bioallied Sci 2: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta V, Lahiri SS, Sultana S, Tulsawani RK, Kumar R (2010) Anti-oxidative effect of Rhodiola imbricata root extract in rats during cold, hypoxia and restraint (C-H-R) exposure and post-stress recovery. Food Chem Toxicol 48: 1019–1025. [DOI] [PubMed] [Google Scholar]
- 10. Mishra KP, Chanda S, Shukla K, Ganju L (2010) Adjuvant effect of aqueous extract of Rhodiola imbricata rhizome on the immune responses to tetanus toxoid and ovalbumin in rats. Immunopharmacol Immunotoxicol 32: 141–146. [DOI] [PubMed] [Google Scholar]
- 11. Kumar R, Phani Kumar G, Chaurasia OP (2010) In vitro antioxidant activity of methanolic extract of Rhodiola imbricata Edgew. Phcog J 2: 157–161. [Google Scholar]
- 12. Kumar R, Tayade A, Chaurasia OP, Hota S, Singh SB (2010) Evaluation of anti-oxidant activities and total phenol and flavonoid content of the hydro-alcoholic extracts of Rhodiola sp. Phcog J 2: 431–435. [Google Scholar]
- 13. Gupta V, Lahiri SS, Sultana S, Kumar R (2009) Mechanism of action of Rhodiola imbricata Edgew during exposure to cold, hypoxia and restraint (C-H-R) stress induced hypothermia and post stress recovery in rats. Food Chem Toxicol 47: 1239–1245. [DOI] [PubMed] [Google Scholar]
- 14. Mishra KP, Ganju L, Chanda S, Karan D, Sawhney RC (2009) Aqueous extract of Rhodiola imbricata rhizome stimulates Toll-like receptor 4, granzyme-B and Th1 cytokines in vitro . Immunobiology 214: 27–31. [DOI] [PubMed] [Google Scholar]
- 15. Gupta V, Saggu S, Tulsawani RK, Sawhney RC, Kumar R (2008) A dose dependent adaptogenic and safety evaluation of Rhodiola imbricata Edgew, a high altitude rhizome. Food Chem Toxicol 46: 1645–1652. [DOI] [PubMed] [Google Scholar]
- 16. Mishra KP, Padwad YS, Dutta A, Ganju L, Sairam M, et al. (2008) Aqueous extract of Rhodiola imbricata rhizome inhibits proliferation of an erythroleukemic cell line K-562 by inducing apoptosis and cell cycle arrest at G2/M phase. Immunobiology 213: 125–131. [DOI] [PubMed] [Google Scholar]
- 17. Arora R, Singh S, Sagar RK, Chawla R, Kumar R, et al. (2008) Radiomodulatory and free-radical scavenging activity of the fractionated aquo-alcoholic extract of the adaptogenic nutraceutical (Rhodiola imbricata)-A comparative in vitro assessment with ascorbate. J Diet Suppl 5: 147–163. [DOI] [PubMed] [Google Scholar]
- 18. Gupta A, Kumar R, Upadhyay NK, Pal K, Kumar R, et al. (2007) Effects of Rhodiola imbricata on dermal wound healing. Planta Med 73: 774–777. [DOI] [PubMed] [Google Scholar]
- 19. Kumar R, Gupta V, Saggu S, Tulswania RK, Divekar HM (2007) Adaptogenic and safety evaluation of Rhodiola imbricata root extract: A dose dependent study. Ind J Clin Biochem 22(Supl.)20S3.1: 171. [Google Scholar]
- 20. Mishra KP, Padwad YS, Jain M, Karan D, Ganju L, et al. (2006) Aqueous extract of Rhodiola imbricata rhizome stimulates proinflammatory mediators via phosphorylated IkappaB and transcription factor nuclear factor-kappaB. Immunopharmacol Immunotoxicol 28: 201–212. [DOI] [PubMed] [Google Scholar]
- 21. Goel HC, Bala M, Prasad J, Singh S, Agrawala PK, et al. (2006) Radioprotection by Rhodiola imbricata in mice against whole-body lethal irradiation. J Med Food 9: 154–160. [DOI] [PubMed] [Google Scholar]
- 22. Kanupriya, Prasad D, Sai Ram M, Kumar R, Sawhney RC, et al. (2005) Cytoprotective and antioxidant activity of Rhodiola imbricata against tert-butyl hydroperoxide induced oxidative injury in U-937 human macrophages. Mol Cell Biochem 275: 1–6. [DOI] [PubMed] [Google Scholar]
- 23. Arora R, Chawla R, Sagar R, Prasad J, Singh S, et al. (2005) Evaluation of radioprotective activities Rhodiola imbricata Edgew - a high altitude plant. Mol Cell Biochem 273: 209–223. [DOI] [PubMed] [Google Scholar]
- 24. Khanum F, Bawa AS, Singh B (2005) Rhodiola rosea: A Versatile Adaptogen. Comprehensive Reviews in Food Science and Food Safety 4: 55–62. [DOI] [PubMed] [Google Scholar]
- 25. Shellie RA, Poynter SD, Li J, Gathercole JL, Whittock SP, et al. (2009) Varietal characterization of hop (Humulus lupulus L.) by GC–MS analysis of hop cone extracts. J Sep Sci 32: 3720–3725. [DOI] [PubMed] [Google Scholar]
- 26. Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, et al. (2001) Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modifiedplant systems. Plant Cell 13: 11–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gullberg J, Jonsson P, Nordstrom A, Sjostrom M, Moritz T (2004) Design of experiments: an efficient strategy to identify factors influencing extraction and derivatization of Arabidopsis thaliana samples in metabolomic studies with gas chromatography/mass spectrometry. Anal Biochem 331: 283–295. [DOI] [PubMed] [Google Scholar]
- 28.Dr. Duke's Phytochemical and Ethnobotanical Databases. http://www.ars-grin.gov/duke/ Accessed 2012 Aug 10.