Abstract
Objective:
To examine the pattern of association between microstructure of temporal lobe connections and the breakdown of episodic memory that is a core feature of mild cognitive impairment (MCI).
Methods:
Twenty-five individuals with MCI and 20 matched controls underwent diffusion MRI and cognitive assessment. Three temporal pathways were reconstructed by tractography: fornix, parahippocampal cingulum (PHC), and uncinate fasciculus. Tissue volume fraction—a tract-specific measure of atrophy—and microstructural measures were derived for each tract. To test specificity of associations, a comparison tract (corticospinal tract) and control cognitive domains were also examined.
Results:
In MCI, tissue volume fraction was reduced in the fornix. Axial and radial diffusivity were increased in uncinate and PHC implying more subtle microstructural change. In controls, tissue volume fraction in the fornix was the predominant correlate of free recall. In contrast, in MCI, the strongest relationship was with left PHC. Microstructure of uncinate and PHC also correlated with recognition memory, and recognition confidence, in MCI.
Conclusions:
Episodic memory in MCI is related to the structure of multiple temporal association pathways. These associations are not confined to the fornix, as they are in healthy young and older adults. In MCI, because of a compromised fornix, alternative pathways may contribute disproportionally to episodic memory performance.
Breakdown of episodic memory is a core feature of mild cognitive impairment (MCI).1 Previous studies of MCI have focused on changes in the medial temporal lobe (MTL). However, the MTL is one node of wider networks for memory that include frontal and parietal lobes.2 The “extended hippocampal system”3 incorporates the fornix—a large fiber tract composed mostly of connections associated with the hippocampal formation.4 Alternatively, MTL-cortical interactions can be mediated by parahippocampal tracts including the uncinate fasciculus and the temporal portion of the cingulum bundle (parahippocampal cingulum [PHC]).
In healthy older adults, the microstructure of the fornix is an established correlate of episodic memory performance. Fornix microstructure accounts for both age-related and age-independent variations in free recall.5 Recent diffusion MRI studies have found that fornix microstructure is compromised in MCI and Alzheimer disease.6,7 Early damage might lead to engagement of alternative interactions,8 so that episodic memory becomes disproportionately associated with microstructure of tracts other than the fornix. To test this hypothesis, diffusion MRI tractography was used to reconstruct the fornix, PHC, and uncinate in MCI and controls. Intrinsic microstructure and subtle atrophy were quantified separately9,10 dealing with an important confound in MCI and aging.11 Microstructure was first correlated with free recall, allowing a direct comparison of pattern of correlation between groups. Relationships between tract microstructure and recognition memory were then assessed in more detail in MCI.
METHODS
Participants with MCI
Patients were recruited from the Cardiff Memory Clinic. Standardized assessment included clinical history, ascertainment of vascular risk factors, neurologic examination, basic hematology and biochemistry, neuroimaging with CT or MRI, and screening with the Addenbrooke's Cognitive Examination.12 Diagnosis of MCI was based on current criteria.13 Objective memory impairment was confirmed by a score of >1.5 SDs below age-matched controls on either the Addenbrooke's verbal memory subscore12 or the visual memory test from the Repeatable Battery for the Assessment of Neurological Status.14 All patients had a Mini-Mental State Examination score of ≥24 (mean = 26; SD = 1.7) and a Clinical Dementia Rating of 0.5.15 Seven patients had additional evidence of executive dysfunction (multidomain MCI); all others had pure amnestic MCI. Exclusion criteria included the following: previous moderate to severe head injury; prior or current alcohol and/or drug abuse (as defined by DSM-IV-TR); previous large-artery or disabling stroke or cerebral hemorrhage; known peripheral, cervical, or coronary artery disease; structural heart disease or heart failure; and contraindications to MRI. In addition, no patient met diagnostic criteria or had characteristic cognitive or behavioral features to suggest other degenerative disorders. Consecutive patients, who were eligible and willing to take part, were recruited and assessed by a single neurologist (M.J.O.).
Healthy control participants
The 20 healthy control participants were a subgroup of a sample of 46 individuals between the ages of 53 and 93 years, recruited for an aging study.5 The subgroup was selected to provide optimal matching with the MCI group. Those older than age 65 years (the MCI group were all older than 65) and with a verbal IQ not exceeding 2 SDs above the average patient IQ in the National Adult Reading Test–Revised (NART-R)16 were included (n = 20; see table 1). Subgroup selection was based entirely on demographic variables and verbal intelligence, and was blind to memory scores and imaging data. Exclusion criteria for the healthy participants were identical to those for the MCI group with the addition of no significant previous symptoms related to memory.
Table 1.
Demographic information and performance in the episodic memory tasks for MCI and control groupsa
Diffusion MRI
Diffusion-weighted MRI data were acquired using a 3T GE HDx MRI system (General Electric, Waukesha, WI) with a twice-refocused, spin-echo, echo-planar imaging sequence providing whole-brain coverage (60 slices, 2.4-mm thickness, field of view 23 cm, acquisition matrix 96 × 96). Acquisition was peripherally gated to the cardiac cycle. Echo delay time was 87 milliseconds, and parallel imaging (ASSET factor 2) was used. The b value was 1,200 s/mm2. Data were acquired with diffusion encoded along 30 isotropically distributed orientations and 3 non–diffusion-weighted scans according to an optimized gradient vector scheme.17 Acquisition time was approximately 13 minutes. Images were corrected for distortions, introduced by the diffusion-weighting gradients, and for subject motion with appropriate reorienting of the encoding vectors.18 Image voxels around the fornix are particularly susceptible to CSF contamination and hence partial volume artifacts.10 The free water elimination approach9,10 was used to correct for atrophy-related partial volume effects before fitting a tensor model to the data in each voxel.
Tractography and tract-specific measures
Tractography19 was performed according to previously published methods5 using ExploreDTI (www.exploreDTI.com). Tractography based on the tensor model is less successful in reconstructing the fornix because of its close proximity to other white matter tracts (e.g., anterior commissure). Deterministic tracking based on constrained spherical deconvolution20 has proven to be a more appropriate technique for fornix tractography.5 The deterministic tracking algorithm estimated the principal diffusion orientation at each seed point and propagated in 0.5-mm steps along this direction. The fiber orientation(s) was then estimated at the new location and the tracking moved a further 0.5 mm along the direction that subtended the smallest angle to the current trajectory. A trajectory was traced through the data until the scaled height of the fiber orientation density function peak fell below 0.1 or the direction of the pathway changed through an angle greater than 60°.
Initial whole-brain tractography was performed using every voxel as a seed point. Three-dimensional reconstructions of the 4 tracts (see figure e-1 on the Neurology® Web site at www.neurology.org) were then extracted by applying multiple waypoint regions of interest (ROIs) and Boolean logical operations (for example, fibers that traversed ROI-1 and ROI-2 but not ROI-3). ROIs were manually drawn in native space on color-coded fiber orientation maps by a single blinded operator (C.M.-B.) using established landmark techniques published previously5 and reproduced in e-Methods. The mean fractional anisotropy (FA), mean diffusivity, axial diffusivity (AD), and radial diffusivity (RD) were calculated from the tissue tensors, as previously described,21 providing tract-specific indices of white matter microstructure. Tissue volume fraction was also derived for each tract. The free water elimination approach involves fitting a bi-tensor model to diffusion data in each voxel. This includes a compartment that models the contribution of free water (isotropic, with diffusion coefficient fixed to that of free water at body temperature) and a separate tensor that models tissue (gray or white matter). In addition to fitting these tensors, the model fits a term f, which represents the volume fraction of tissue within the voxel (essentially setting the relative contribution of each compartment).9 For an individual tract, tissue volume fraction could be defined as the total amount of tissue (white matter) expressed as a proportion of the volume of all voxels visited by tractography.
Pilot studies were performed in subsets of data including both healthy volunteers and MCI to assess, in a blinded manner, intrarater reproducibility of tract measurements. Reproducibility was very good for all tracts and measures: fornix FA (n = 5) intraclass correlation coefficient (ICC) 0.85, coefficient of variation (CoV) 5.4%; uncinate (n = 12, left and right in 6 subjects) ICC >0.99, CoV <1% for both FA and mean diffusivity; and PHC (n = 12, left and right in 6 subjects) ICC >0.99, CoV <1% for both FA and mean diffusivity.
Cognitive testing
Neuropsychological assessment was performed over two 1.5-hour testing sessions. Verbal intelligence was estimated with the NART-R. Episodic memory was assessed with instruments selected to gain access to different aspects of memory processing. Both free recall (recollection) and recognition memory were assessed.
Episodic memory measures
Free recall was assessed with the Free and Cued Selective Reminding Test22 as in our previous study.5 To assess episodic memory in more detail in MCI, it was important to select measures that minimized the risk of patients performing at floor level. The visual recognition test from the Doors and People Test23 and the face recognition test from the Camden Recognition Memory Test (CRMT)24 have proven utility in amnestic patients25 and were therefore chosen. Recollection- vs familiarity-based recognition processes were probed further in the CRMT with the “remember-know” procedure.26 “Know” responses primarily reflect familiarity-based processes in the absence of specific recollections. Participants were also asked to indicate if they felt “sure” (confident) or “not sure” (not confident) about their recognition decision. Thus, correct responses in the CRMT were categorized into Hits Remembered, Hits Known, Hits Sure, and Hits Not Sure.
Nonepisodic memory “control” measures
Visual short-term span was assessed with the Visual Pattern Test.27 Cognitive processing speed was measured as the mean response speed in baseline conditions of executive function tasks (which are not further reported here) that involved the generation of the alphabet, counting upward in 1s, and reading a list of color words as quickly and accurately as possible.
Statistical analyses
Statistical analyses were performed using SPSS 16.0 (SPSS, Inc., Chicago, IL). To allow the effects of healthy aging and disease to be evaluated separately, analyses were based on raw rather than age-scaled scores. Between-group comparisons were controlled for multiple comparisons with the Bonferroni method: individual comparisons had to reach a significance level of p ≤ 0.004 for cognitive measures and p ≤ 0.001 for microstructural indices to comply with experiment-wise corrected significance level of 0.05.
Pearson product-moment correlations were calculated between white matter microstructural indices and cognitive measures. In this setting, where multiple comparisons are performed across correlated measures that are not independent, the Bonferroni method is unduly conservative. Type I error was therefore controlled with the false discovery rate (FDR) at a stringent level of 1% (q < 0.01), with the q-value software.28 Correlations that reached 1% FDR–corrected significance are highlighted in bold in tables 3 and 4; those that reached a 5% FDR threshold are referred to as trends and are highlighted in italics.
Table 3.
Pearson product-moment correlations between mean tissue volume fraction (f) in the 3 temporal association tracts and the comparison tract and verbal free recall for the healthy older control and MCI groupsa
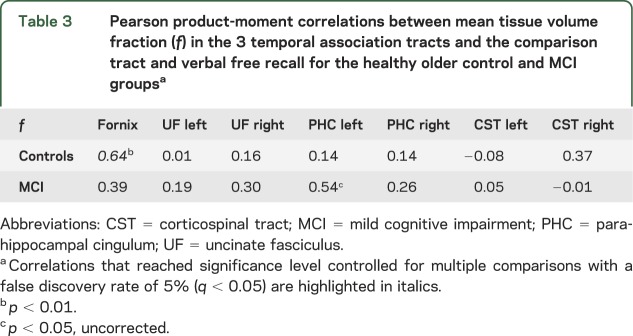
Table 4.
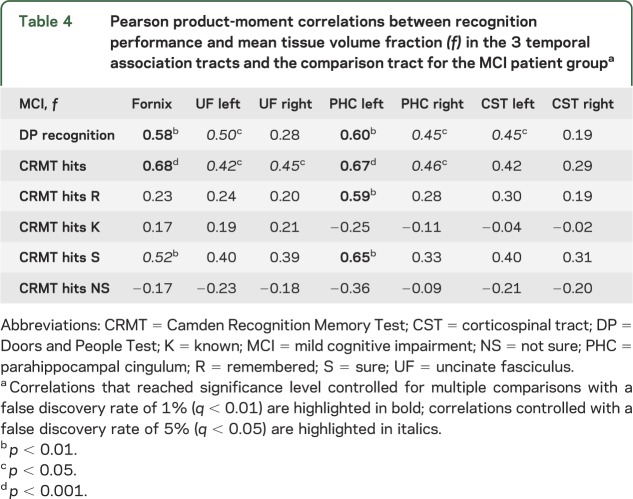
Pearson product-moment correlations between recognition performance and mean tissue volume fraction (f) in the 3 temporal association tracts and the comparison tract for the MCI patient groupa
Scatter plots were visually inspected for outliers: one patient score at floor level in the Doors and People visual recognition task and one control participant's tissue volume fraction index in the right corticospinal tract were thereby excluded.
Standard protocol approvals, registrations, and patient consents
Ethical approval for the study was provided by the South East Wales Research Ethics Committee (panel C). All participants provided informed, written consent.
RESULTS
Group comparisons
The MCI group was impaired across a range of episodic memory measures (table 1).
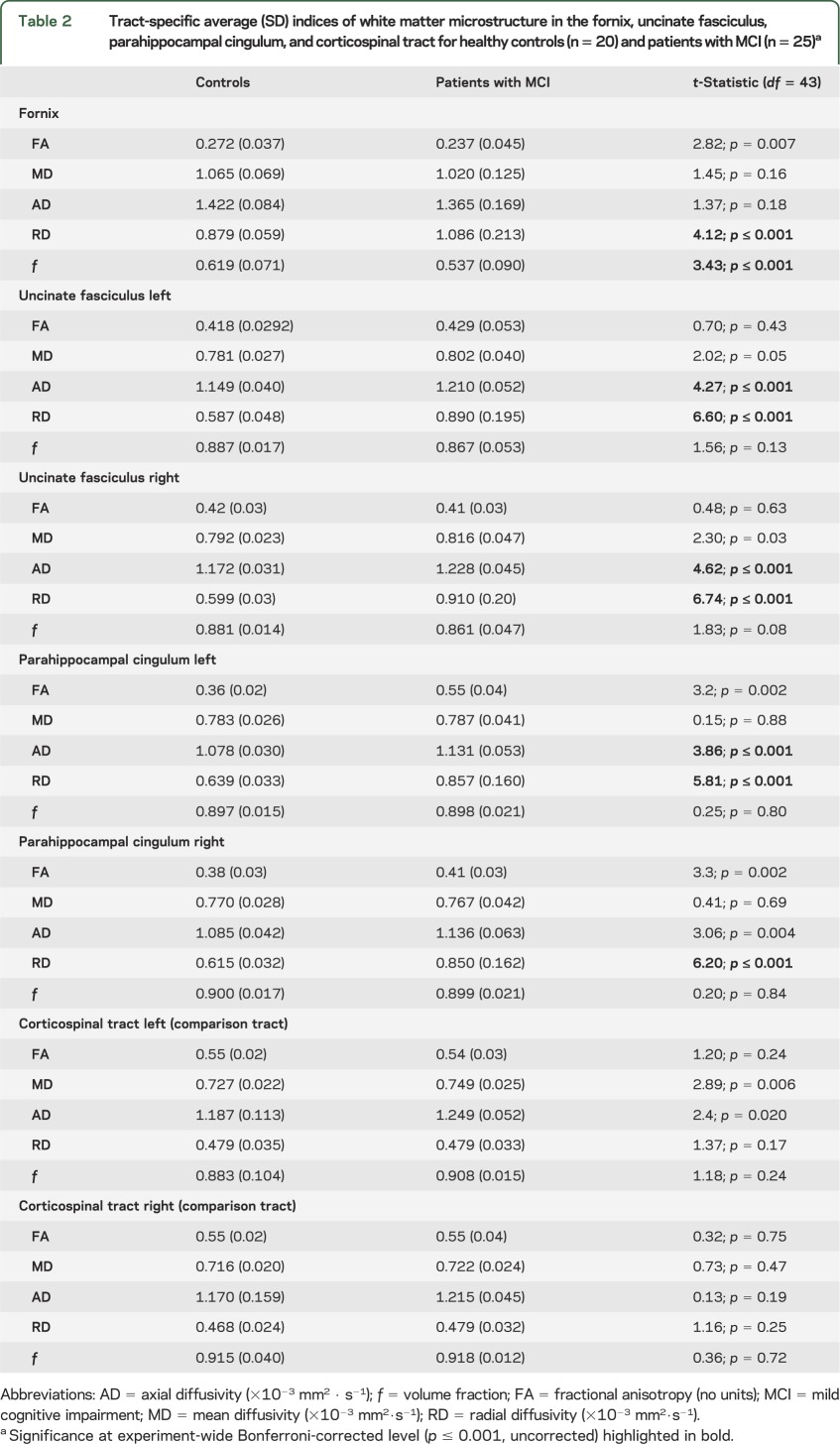
In MCI, tissue volume fraction was reduced in the fornix but not other temporal tracts (table 2). RD was increased in all 3 tracts and AD in the PHC and the uncinate fasciculus. No MCI-related changes were found in the corticospinal tract.
Table 2.
Tract-specific average (SD) indices of white matter microstructure in the fornix, uncinate fasciculus, parahippocampal cingulum, and corticospinal tract for healthy controls (n = 20) and patients with MCI (n = 25)a
Memory performance and white matter microstructure
Correlations were limited to indices that demonstrated sensitivity to disease effects in MCI, i.e., AD, RD, and tissue volume fraction.
In the control group, free recall correlated with structure of the fornix but not other tracts (table 3). In contrast, in MCI, the strongest relationship with free recall was found for the left PHC.
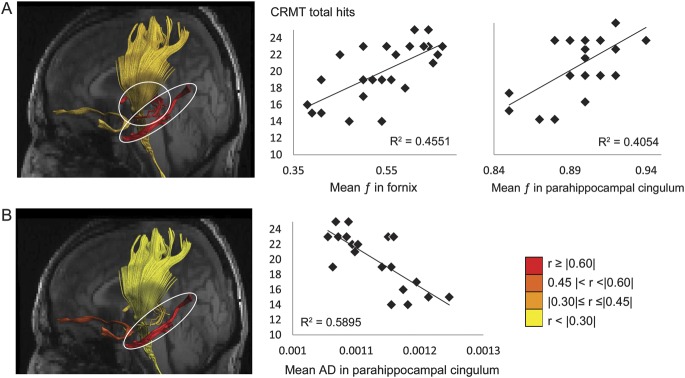
Correlational analyses between white matter microstructure and recognition memory performance were appropriate only for the MCI group as controls performed at ceiling level. Visual recognition correlated with tissue volume fraction in the fornix and the left PHC (table 4, figure 1A). In addition, tissue volume fraction in the left PHC correlated with high confidence judgments and with “remember” responses in the CRMT.
Figure 1. Reconstructions of the fornix, uncinate fasciculus, parahippocampal cingulum, and corticospinal tract in the native space of one individual with mild cognitive impairment coregistered with that individual's T1-weighted structural MRI showing the left side of the brain.
The tracts are color-coded to visualize the strength of the correlations between (A) tissue volume fraction f and (B) axial diffusivity (AD) and face recognition performance in the Camden Recognition Memory Test (CRMT) for the patient group. The correlations between recognition performance and volume fraction in the fornix, volume fraction in the parahippocampal cingulum, and AD in the parahippocampal cingulum reached false discovery rate–corrected significance level with q < 0.01 and are highlighted by white ovals and illustrated further in the scatter plots.
Similarly, for AD, there was a negative association between AD in the left PHC and total recognition performance in the CRMT (r = −0.77, p < 0.001, FDR q < 0.01) and trends for response confidence level in the left PHC (r = −0.60, p < 0.01, FDR q < 0.05) and uncinate fasciculus (r = −0.56, p < 0.01, FDR q < 0.05) (table e-1).
No association between performance in control cognitive tasks and microstructure of temporal pathways was evident (table e-2).
DISCUSSION
Patients with MCI displayed evidence of structural compromise of the fornix compared with matched controls, based on a tract-specific measure of atrophy, tissue volume fraction. In addition, there was evidence of microstructural alteration in the residual fornix and in the other temporal association tracts, the uncinate, and PHC. The role of the fornix as the predominant correlate of recall performance—found in young29 and healthy older5 adults—was not reproduced in patients with MCI. In patients, the strongest correlate of verbal free recall was left PHC structure. Furthermore, the importance of the PHC in this group was underlined by the significant associations with recognition, which provided a more meaningful measure of residual memory performance. In MCI, both tissue fraction and microstructure of the uncinate and PHC were associated with recognition memory. An additional novel finding, consistent with current theories on the neurobiological basis of memory confidence, was an association between left PHC microstructure and confident recognition judgments.
Aside from the fornix, MTL-cortical interactions can be mediated by several pathways. The uncinate fasciculus links the anterior temporal lobe and parahippocampal region with the medial and orbitofrontal prefrontal cortices.30 The temporal (parahippocampal) portion of the cingulum (PHC)31 contains fibers that arise and project to parahippocampal cortices, and provides an indirect route to and from the prefrontal cortex in addition to direct parietal connections.32
Previous diffusion tensor imaging studies, investigating one or the other tract in isolation, have shown correlations with episodic memory.33,34 The present study extends these findings substantially both by demonstrating specificity of association—by comparison with both a control tract and control cognitive domains—and by assessing associations with multiple pathways of temporal interaction simultaneously. This is important because lesion studies in monkeys show that the significance of some connections for memory can be appreciated only when other pathways of interaction with the temporal lobe have been disconnected.35 In particular, lesions of the uncinate and temporal stem white matter in monkeys have little impact on memory when the fornix is left intact, but in combination with fornix section contribute to a severe, amnesic-like syndrome.36 The contrast in pattern of correlations between controls and MCI raises the possibility that this is also true of the breakdown of human memory in disease.
The specific association between fornix microstructure and recollection-based episodic memory was predicted by earlier MRI analyses5,29 and by the impact of lesions that selectively disrupt fornical fibers.25 One interpretation of the present results is a disproportionate recruitment of nonfornical pathways to support recognition memory. As expected,5,29 successful episodic memory in the healthy brain relied primarily on an intact fornix. In contrast, in MCI, episodic memory cannot depend primarily on fornix contributions so relies on other temporal association tracts, albeit unsuccessfully in behavioral terms.
The recruitment of nonfornical connections may be linked to a shift in memory strategies from recollection to more familiarity-based processes.37 Whereas recollection is underpinned by the extended hippocampal system, familiarity is linked to the parahippocampal region, most notably the perirhinal cortex.3 The majority of fibers in the fornix connect the hippocampus and subiculum (linked to recollection), rather than the parahippocampal region (linked to familiarity).4 The assumption is that fibers from the parahippocampal region, which predominantly contribute to the uncinate and PHC rather than the fornix, now become more critical. This interpretation is consistent with studies that have reported preserved familiarity but impaired recollection-based memory in MCI.37
The fornix was not exclusively affected in MCI: subtle microstructural alterations were observed across all reconstructed temporal association pathways. Unlike other tracts, a decrease in volume fraction was observed in the fornix. This evidence of localized atrophy may indicate a more advanced stage of structural degeneration in this tract and adds to previous diffusion tensor imaging studies that have not distinguished atrophy from true alterations in microstructure.6,7 In PHC and uncinate, AD and RD were the measures most sensitive to presumed pathology in MCI. It has been argued that these measures reflect axonal and myelin-related pathology, respectively. The pattern of results illustrates a limitation of FA as a general measure of tract “integrity.” In situations whereby both AD and RD increase, FA changes are likely to be minimal; in fact, in pathologic states that preferentially influence AD (presumed axonal degeneration), FA would be expected to increase. The observation that FA is a relatively insensitive measure in MCI corroborates previous diffusion MRI studies.38 In the presence of axonal degeneration, the whole diffusion profile needs to be considered to capture relevant microstructural change.
Functional MRI studies have linked the posterior parietal cortex with meta-memorial processes signaling confidence levels for recognition memory.39 The PHC connects these regions to the temporal lobe. Therefore, the relationship between high recognition confidence (“sure” responses) and AD in the PHC is intriguing and suggests that parietal modulation of MTL regions could be communicated directly via this tract.39 The role of the PHC can also be understood in the context of increasingly recognized contributions of posteromedial cortex to memory.40 The contribution of the uncinate to recognition memory is less clear. In healthy older adults, there was evidence of a role for the uncinate in strategic aspects of memory.5 One possible role of the uncinate, therefore, is in mediating executive contributions to memory, which may be particularly relevant in the subset of individuals with additional executive difficulties. It is likely that there are a variety of subprocesses that support recognition in MCI, each supported by particular connections. Further studies that distinguish neuropsychologically between various aspects of recognition should begin to tease apart these differential contributions.
This study confirms that structural damage of the fornix occurs in MCI, along with a more subtle microstructural change of the uncinate and PHC. In the presence of a compromised fornix, these extrafornical tracts have a disproportionate influence on memory performance. One possible interpretation is a shift toward reliance on familiarity-based memory processes. Furthermore, we propose that the PHC has a crucial role in the modulation of confidence in memory judgments. Understanding patterns of residual memory is important in developing rehabilitative strategies. Prospective studies are now needed to examine directly how relationships between white matter tract structure and episodic memory evolve with memory decline.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the staff of the Llandough Memory Clinic, Cardiff, for their assistance with patient recruitment.
Glossary
- AD
axial diffusivity
- CoV
coefficient of variation
- CRMT
Camden Recognition Memory Test
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision
- FA
fractional anisotropy
- FDR
false discovery rate
- ICC
intraclass correlation coefficient
- MCI
mild cognitive impairment
- MTL
medial temporal lobe
- NART-R
National Adult Reading Test–Revised
- PHC
parahippocampal cingulum
- RD
radial diffusivity
- ROI
region of interest
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
C.M.-B.: design, data collection, data analysis and interpretation, drafting the manuscript. S.H.: data collection, data analysis and interpretation. D.K.J.: conceptualization, design, data analysis and interpretation, drafting the manuscript. A.L.: data analysis and interpretation. J.P.A.: conceptualization, design, data analysis and interpretation, drafting the manuscript. M.J.O.: conceptualization, design, data analysis and interpretation, drafting the manuscript.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308 [DOI] [PubMed] [Google Scholar]
- 2.Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 1990;28:597–613 [DOI] [PubMed] [Google Scholar]
- 3.Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends Cogn Sci 2006;10:455–463 [DOI] [PubMed] [Google Scholar]
- 4.Saunders RC, Aggleton JP. Origin and topography of fibers contributing to the fornix in macaque monkeys. Hippocampus 2007;17:396–411 [DOI] [PubMed] [Google Scholar]
- 5.Metzler-Baddeley C, Jones DK, Belaroussi B, Aggleton JP, O'Sullivan MJ. Frontotemporal connections in episodic memory and aging: a diffusion MRI tractography study. J Neurosci 2011;31:13236–13245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozoki AC, Korolev IO, Davis NC, Hoisington LA, Berger KL. Disruption of limbic white matter pathways in mild cognitive impairment and Alzheimer's disease: a DTI/FDG-PET study. Hum Brain Mapp 2012;33:1792–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mielke MM, Kozauer NA, Chan KC, et al. Regionally-specific diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. Neuroimage 2009;46:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry 2003;74:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med 2009;62:717–730 [DOI] [PubMed] [Google Scholar]
- 10.Metzler-Baddeley C, O'Sullivan MJ, Bells S, Pasternak O, Jones DK. How and how not to correct for CSF-contamination in diffusion MRI. Neuroimage 2012;59:1394–1403 [DOI] [PubMed] [Google Scholar]
- 11.Serra L, Perri R, Cercignani M, et al. Are the behavioral symptoms of Alzheimer's disease directly associated with neurodegeneration? J Alzheimers Dis 2010;21:627–639 [DOI] [PubMed] [Google Scholar]
- 12.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke’s Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 2006;21:1078–1085 [DOI] [PubMed] [Google Scholar]
- 13.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 1998;20:310–319 [DOI] [PubMed] [Google Scholar]
- 15.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 16.Nelson HE, Willison J. National Adult Reading Test (NART) Test Manual, 2nd ed Windsor, UK: NFER–Nelson; 1991 [Google Scholar]
- 17.Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 1999;42:515–525 [PubMed] [Google Scholar]
- 18.Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 2009;61:1336–1349 [DOI] [PubMed] [Google Scholar]
- 19.Jeurissen B, Leemans A, Jones DK, Tournier JD, Sijbers J. Probabilistic fiber tracking using the residual bootstrap with constrained spherical deconvolution. Hum Brain Mapp 2011;32:461–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tournier JD, Calamante F, Gadian DG, Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage 2004;23:1176–1185 [DOI] [PubMed] [Google Scholar]
- 21.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 1996;36:893–906 [DOI] [PubMed] [Google Scholar]
- 22.Grober E, Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol 1987;3:13–36 [Google Scholar]
- 23.Baddeley AD, Emslie H, Nimmo-Smith I. Doors and People Test. Bury St Edmunds, UK: Thames Valley Test Company; 1994 [Google Scholar]
- 24.Warrington EK. The Camden Memory Test. Hove, UK: Psychology Press; 1996 [Google Scholar]
- 25.Vann SD, Tsivilis D, Denby CE, et al. Impaired recollection but spared familiarity in patients with extended hippocampal system damage revealed by 3 convergent methods. Proc Natl Acad Sci USA 2009;106:5442–5447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tulving E. Elements of Episodic Memory. New York: Oxford University Press; 1983 [Google Scholar]
- 27.Della Salla S, Gray C, Baddeley A, Wilson L. Visual Patterns Test. London: Harcourt Assessment; 1997 [Google Scholar]
- 28.Storey JD. A direct approach to false discovery rates. J R Stat Soc Ser B 2002;64:479–498 [Google Scholar]
- 29.Rudebeck SR, Scholz J, Millington R, Rohenkohl G, Johansen-Berg H, Lee AC. Fornix microstructure correlates with recollection but not familiarity memory. J Neurosci 2009;29:14987–14992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York: Oxford University Press; 2006 [Google Scholar]
- 31.Catani MR, Howard J, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 2002;17:77–94 [DOI] [PubMed] [Google Scholar]
- 32.Mufson EJ, Pandya DN. Some observations on the course and composition of the cingulum bundle in the rhesus monkey. J Comp Neurol 1984;225:31–43 [DOI] [PubMed] [Google Scholar]
- 33.Fujie S, Namiki C, Nishi H, et al. The role of the uncinate fasciculus in memory and emotional recognition in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord 2008;26:432–439 [DOI] [PubMed] [Google Scholar]
- 34.Jhoo JH, Lee DY, Choo IH, et al. Discrimination of normal aging, MCI and AD with multimodal imaging measures on the medial temporal lobe. Psychiatr Res 2010;183:237–243 [DOI] [PubMed] [Google Scholar]
- 35.Bachevalier J, Parkinson JK, Mishkin M. Visual recognition in monkeys: effects of separate vs. combined transection of the fornix and amygdalofugal pathways. Exp Brain Res 1985;57:554–561 [DOI] [PubMed] [Google Scholar]
- 36.Gaffan D, Parker A, Easton A. Dense amnesia in the monkey after transection of fornix, amygdala and anterior temporal stem. Neuropsychologia 2001;39:52–70 [DOI] [PubMed] [Google Scholar]
- 37.Anderson ND, Ebert PL, Jennings JM, Grady CL, Cabeza R, Graham SJ. Recollection- and familiarity-based memory in healthy aging and amnestic mild cognitive impairment. Neuropsychology 2008;22:177–187 [DOI] [PubMed] [Google Scholar]
- 38.Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain 2010;133:529–539 [DOI] [PubMed] [Google Scholar]
- 39.Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. Neuroimage 2006;29:1150–1160 [DOI] [PubMed] [Google Scholar]
- 40.Vannini P, O'Brien J, O'Keefe K, Pihlajamäki M, Laviolette P, Sperling RA. What goes down must come up: role of the posteromedial cortices in encoding and retrieval. Cereb Cortex 2011;21:22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.