Abstract
We analyzed 72 items related to serum biochemistry and hematology in 85 specific pathogen-free (SPF) Seoul National University (SNU) miniature pigs aged 1- to 36-months which originated from a Minnesota miniature pig. Almost all examined items were similar between male and female pigs. However, some items such as Cr level, B/C ratio, C.R.F, LDH, LAP and T4 were significantly different between male and female pigs (P<0.05). Thirty four examined items showed age-related changes, and the significant changes were observed in animals less than six months old. The values for BUN, K, uric acid, Ca, Ca++, and Pi were significantly higher in pigs younger than six months of age, which might reflect poor kidney function in young pigs. Additionally, TIBC, UIBC and RDW were significantly higher in young pigs, and RBC, Hb, HCT, MCHC and MCV were significantly lower in young pigs, thus indicating a similar physiology of iron deficiency anemia. These age-related specific phenotypes seemed to be normal, but it should be considered in the long-term experiment using the young pigs. In conclusion, in this study, we defined the normal reference intervals for SPF SNU miniature pigs, and we also determined that there are some physiological differences between the pig genders and ages. This study provides fundamental data for use in experiments involving SPF SNU miniature pigs.
Keywords: Hematology, miniature pig, serum biochemistry, specific pathogen free
We analyzed the blood chemistries of Seoul National University (SNU) miniature pigs to understand their normal physiology and physiological status [1,2]. Serum biochemistry and hematology were studied to assess additives, meals and welfare [3] and to diagnose health status. Normal hematology and serum biochemistry values have been reported for laboratory mice, rats, rabbits and other animals [4].
Previous reports have provided normal pig blood biochemistry values for domestic and miniature pig [5-8]. However, to our knowledge, there have been few reports about the blood biochemistry for specific pathogen-free (SPF) miniature pigs, although simple hematology tests have been conducted in germ-free Minnesota miniature pigs [9,10]. In addition, normal values for blood biochemistry may be age-dependent, and only age-related changes in platelet activity have previously been reported [11,12].
The origin of the SNU miniature pig is a Minnesota miniature pig. After acquisition, the pigs were housed in a specific pathogen-free environment and bred in a closed colony for 30 years at the Chicago Medical School miniature pig facility [13]. In 2004, 24 miniature pigs were transported in a sustained germ-free isolator to an SPF pig facility at Seoul National University. Only two maternal origins were defined, and the pigs were bred as closed colonies. The animals have been maintained as SPF grade and have been used for various studies, such as xenotransplantation and toxicology.
In this study, the normal hematology and serum biochemistry values for each item were assessed to determine the reference intervals. Additionally, age-related changes were analyzed. These data are fundamental for use of SNU miniature pigs as a laboratory animal.
Materials and Methods
Animals
We performed serum-biochemistry and hematology analyses on 85 miniature pigs (47 males and 38 females), aged 1 to 36 months. They were all conditioned in the same animal room at 24±2℃ with a 12/12 (light/dark) cycle. All air and water was filtered, and all equipment was sterilized before use. We used irradiated pig feed for weaning and growing pigs (Purina Korea, Seongnam, Korea). No antibiotics or vaccines were used. The pigs were maintained under specific pathogen-free conditions and were free from viral and bacterial pathogens [14]. This study was approved as an animal use protocol by the Institutional Animal Care and Use Committee at Seoul National University.
Blood collection and analysis
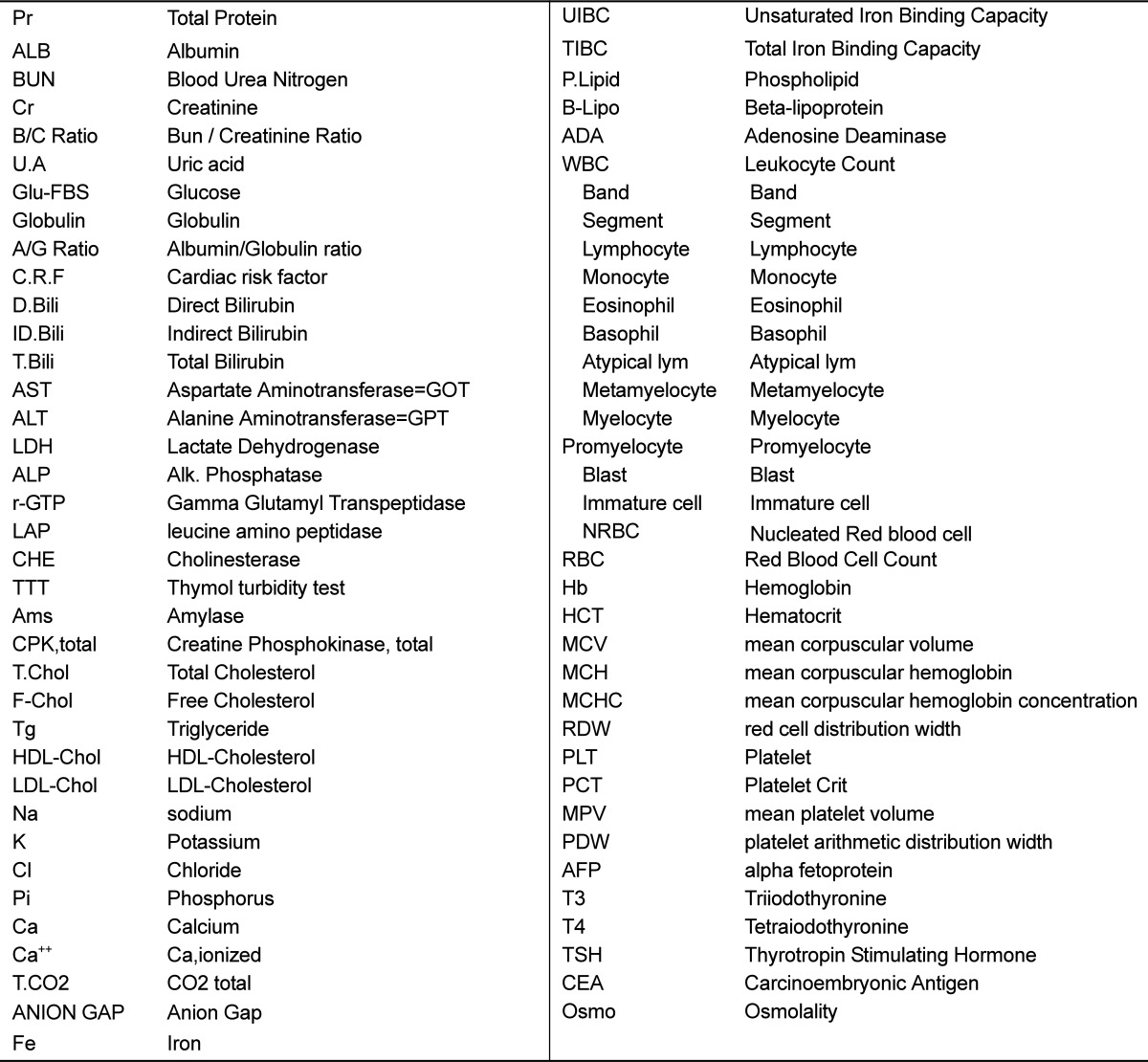
Fresh whole blood was collected by cardiac puncture into heparin blood collection tubes and plain tubes (BD bioscience, San Jose, CA, USA). Serum was separated from the blood in plain tubes via centrifugation at 3,000 rpm for 20 min. Blood biochemistry tests were conducted at the Seoul Medical Science Institute using 5 mL of fresh whole blood and 2 mL of serum. In total, we examined 72 items (Table s1).
Table s1.
Full name of the blood chemistry items analyzed in this study
Analysis of the reference interval
To determine normal values for SNU miniature pigs, we calculated the mean and standard deviation of each item. Significant differences between male and female pigs were analyzed using an independent sample T-test (SPSS ver. 17.0, SPSS Inc., Chicago, IL, USA). Further, a reference interval was calculated using the Clinical and Laboratory Standard Institute (CLSI) guidelines C28-A2 and C28-A3 [15]. The 95% reference intervals for whole pigs, male pigs and female pigs were acquired using MedCalc version 11.1.1 (MedCalc Software, Mariakerke, Belgium). When examined values showed a normal distribution in the Kolmogorov-Smirnov test, we acquired a 95% interval as the reference interval. When examined values did not show a normal distribution, a robust method was used for acquiring the reference interval.
Analysis of the age-related changes
The results were grouped according to age as follows: 1) 15 pigs under six months of age; 2) 15 pigs between 6and 12 months of age; 3) 15 pigs between 12 and 18 months of age; 4) ten pigs between 18 and 24 months of age; 5) 12 pigs between 24 and 30 months of age; 6) nine pigs between 30 and 36 months of age; and 7) nine pigs over three years of age. The mean values for each age group were calculated, and an age-related analysis was conducted. Significant differences were confirmed using the one-way ANOVA with Tukey's post test using the Graphpad Prizm ver. 5.0 (Graphpad Software Inc., CA, USA).
Results
Normal ranges for serum biochemistry and hematology of the SNU miniature pig
Almost all examined items were similar between male and female pigs. However, some values such as Cr level, B/C ratio, C.R.F, LDH, LAP and T4 were significantly different between male and female pigs (P<0.05); Cr and LAP were high in males, and B/C ratio, C.R.F, LDH and T4 were high in females.
Cr and LAP are normally higher in males than in females. In addition, the values for B/C ratio, C.R.F, LDH and T4 were significantly higher in females than males.
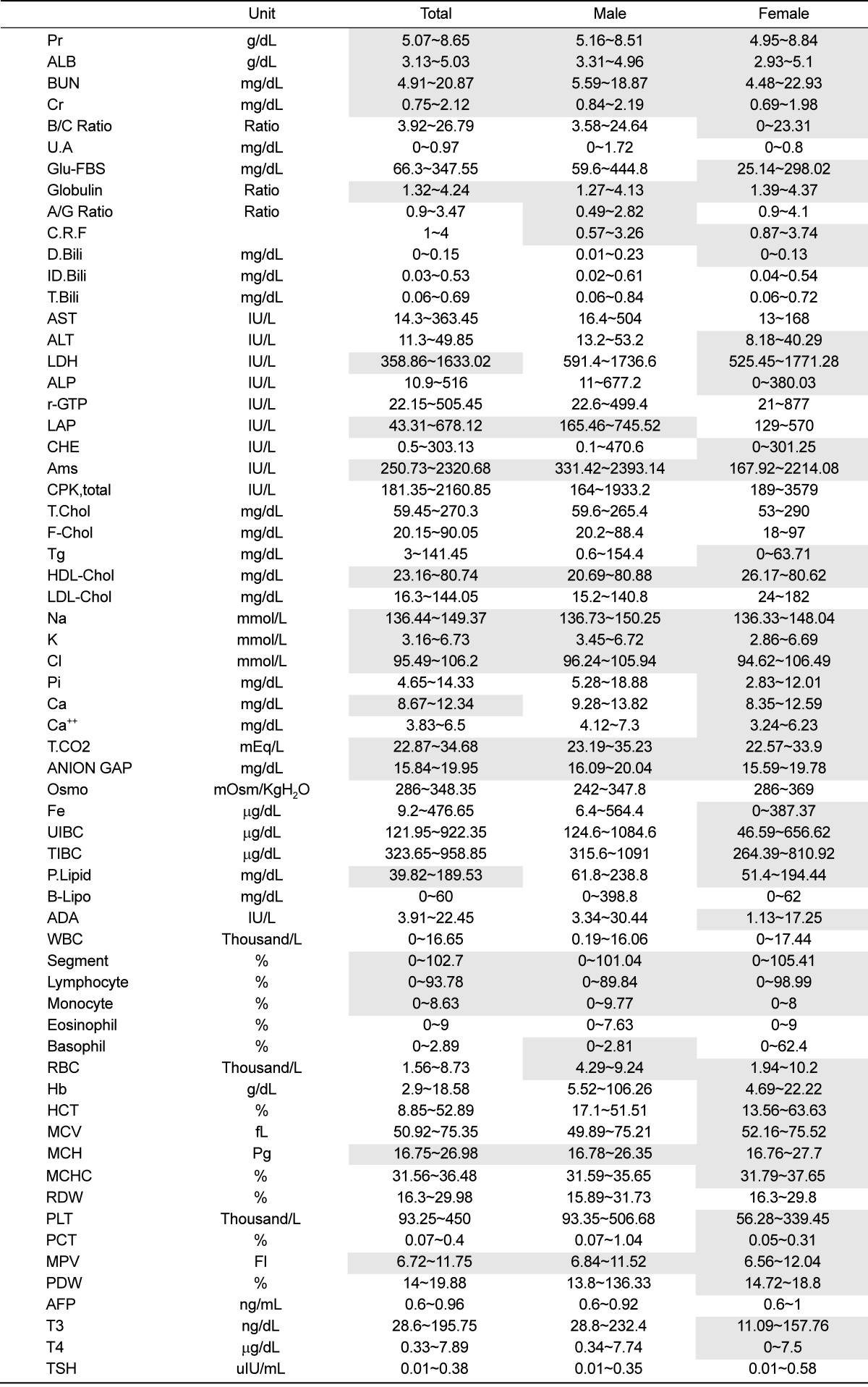
The reference intervals were calculated for all pigs, male pigs, and female pigs, respectively. Among the 72 examined items, male and female pigs had normal distributions for 31 and 48 items, respectively, and only 21 examined items showed normal distributions in all examined pigs. For B/C ratio, LDH and T4, the examined values were significantly higher in females, but the reference intervals were relatively lower and narrower in females compared to those of the males (Table 1).
Table 1.
Normal values and reference intervals for serum biochemistry and hematology in Seoul National University (SNU) miniature pigs
Grey boxes indicate items with a normal distribution.
Age-related changes were found in some items
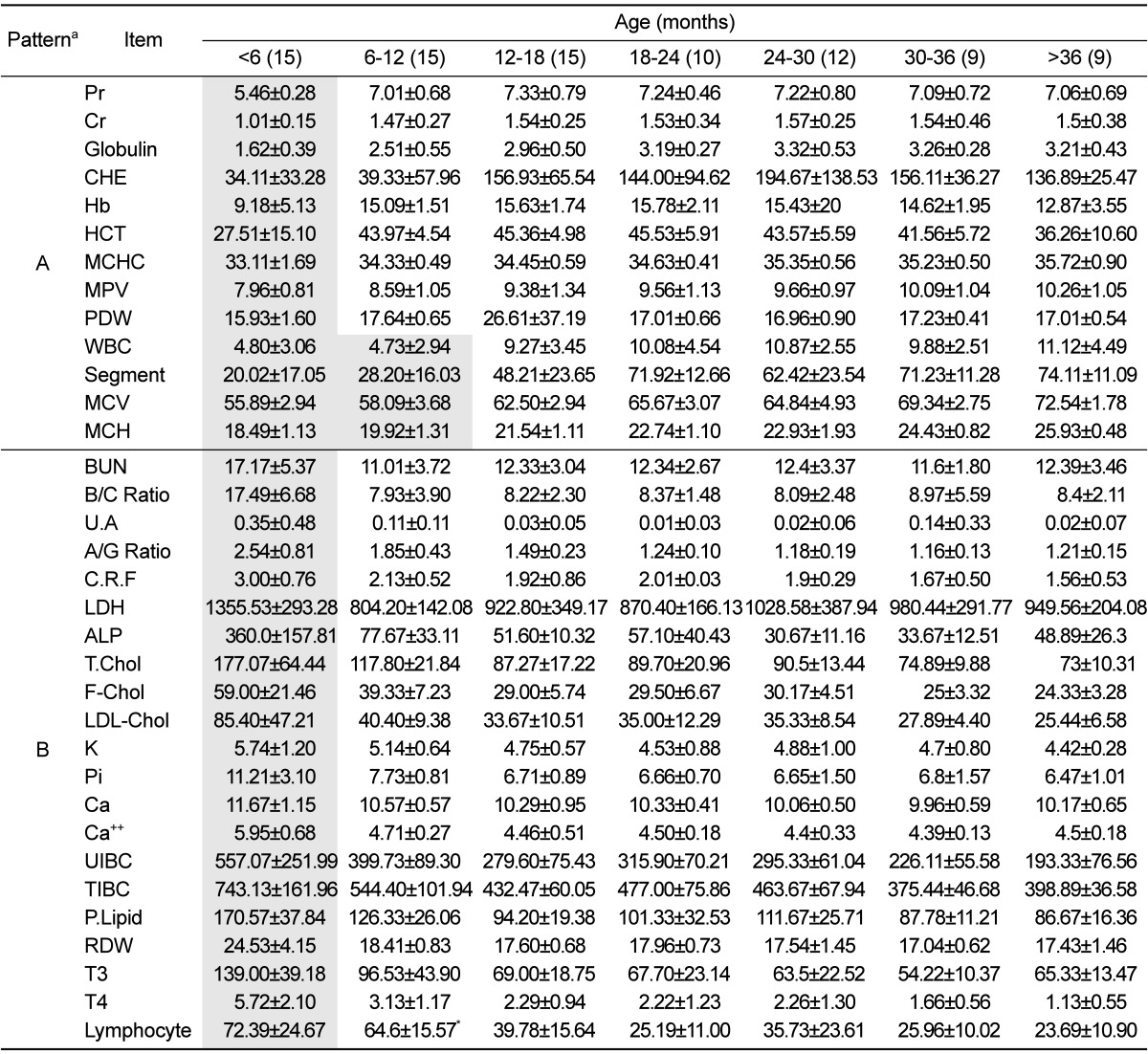
Although, all values were included in normal reference interval of SNU miniature pig, some significant changes were observed in pigs between 6 and 12 months of age. Among the examined 72 items, 34 showed age-related changes, resulting in two age-related patterns as shown in Table 2. One pattern (pattern A) represented significantly low values, and the other pattern (pattern B) represented significantly high values for pigs younger than 6 to 12 months. The levels of Pr, Cr, globuline, CHE, Hb, HCT, MCHC, MPV, PDW, WBC, segment lymphocyte, MCV and MCH showed pattern A, and BUN, B/C ratio, U.A, A/G ratio, CRF, LDH, ALP, T-Chol, F-Chol, LDL-Chol, K, Pi, Ca, UIBC, TIBC, P-Lipid, RDW, T3 and T4 showed pattern B. There were no significant changes in older pigs. Among the 14 items following pattern A, the values for WBC, segment, MCV and MHC significantly increased after 12 months of age.
Table 2.
Age-related patterns in hematology and serum biochemistry
Grey boxes indicate significant differences in pigs of different ages in a one-way ANOVA with Turkey's post test using the Graphpad Prizm ver. 5.0 (Graphpad software Inc). Data are shown as mean±standard deviation
aA indicates significantly lower value in pigs younger than six months of age and B indicates a significantly higher value in pigs younger than six months of age.
Discussion
The miniature pig has physiological and anatomical similarity with human, and has been used for laboratory animal in such field of cardiovascular system, renal disease, transplantation and toxicology [16]. Thus, basic information about serum biochemistry and hematology were essential for understanding the data of experiment. This study was conducted especially for SPF SNU miniature pigs, which were originated from the Minnesota miniature pigs [17]. Hematology analysis for SPF pig colony was valuable for understanding normal miniature pig physiology.
Various aged 85 SNU miniature pigs were used to establish the reference intervals (Table 1). There were some items that had significantly different reference intervals between male and female pigs. The values for Cr and LAP were significantly higher in males, consistent with previous reports that Cr and LAP are normally higher in males than in females, and the low muscle volume of females may be the basis for this difference [18]. In addition, the values for B/C ratio, C.R.F, LDH and T4 were significantly higher in females than they were in males. The history of pregnancy and lactation in females might be the reason for the high LDH and T4 values [19,20], but the cause of the high C.R.F values in females is unclear.
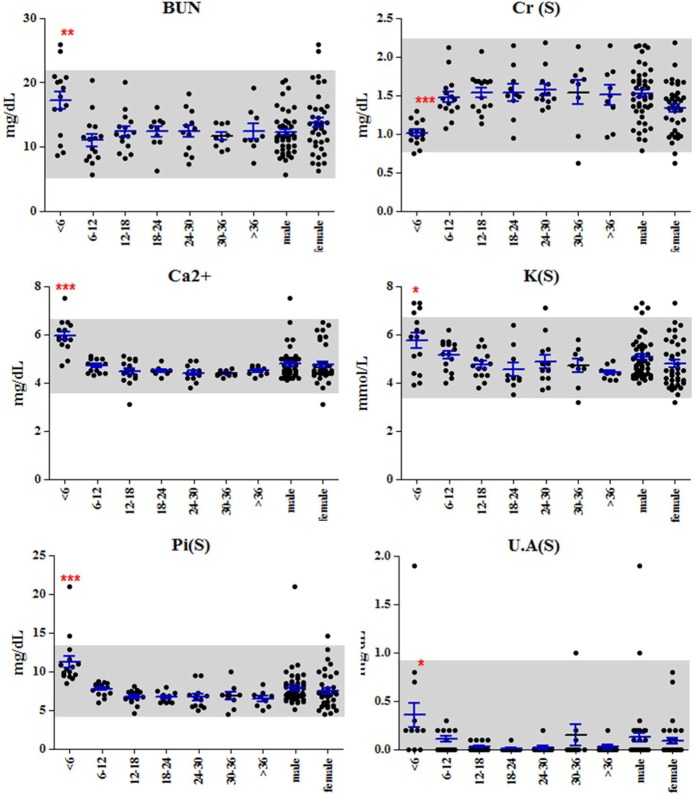
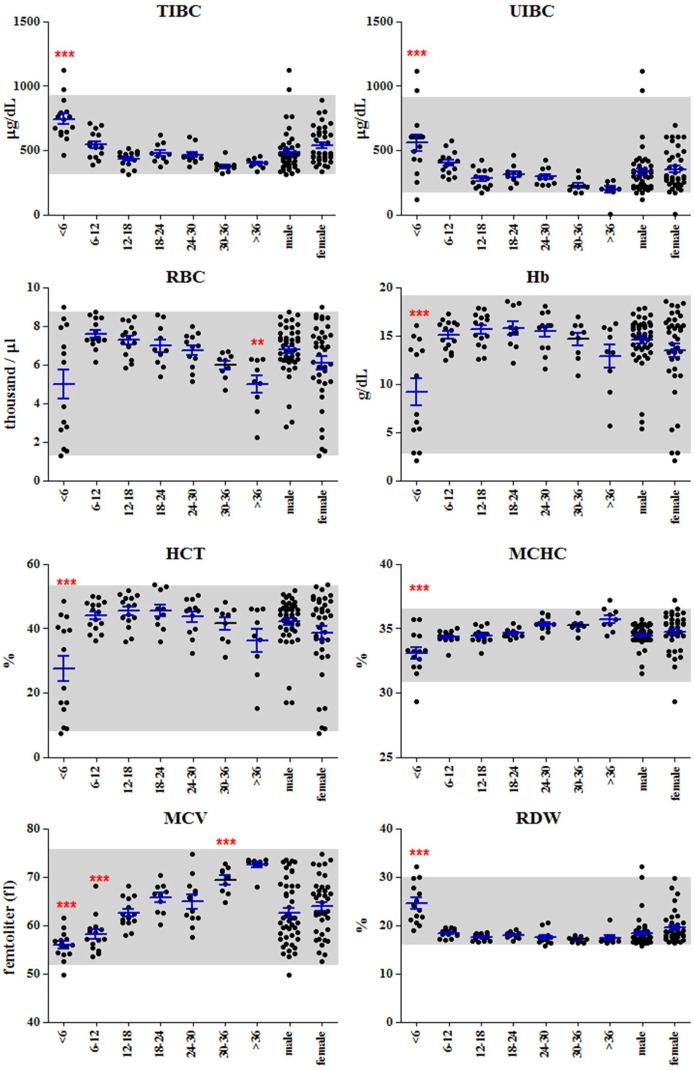
Although, all values were included in normal reference interval of SNU miniature pig, some significant changes were observed in pigs between 6 and 12 months of age. Based on the significant changes in the young pigs, there were several possible specific conditions of poor kidney function, symptom of iron deficiency anemia, hypercholesterolemia and deficiency of protein intake. The values for BUN, K, uric acid, Ca, Ca++, and Pi were significantly higher in pigs less than six months of age, a possible indication of poor kidney function (Figure 1). However, the level of Cr, an indicator of kidney function, was significantly lower in young pigs, possibly due to the low muscle volume [21]. Additionally, TIBC, UIBC and RDW were significantly higher in young pigs, but RBC, Hb, HCT, MCHC and MCV were significantly lower in young pigs (Figure 2). This might indicate a similar symptom of a iron deficiency anemia [22]. Furthermore, although the levels of triglyceride and B-lipid were low, high levels of T chol, LDL-chol, F chol and P lipid may indicate hypercholesterolemia. In addition, the levels of Pr, albumin and globulin were lower in young pigs, possibly due to a deficiency in protein intake or a immature digestive system [23]. These age-related specific phenotypes seemed to be normal, but it should be considered in the long-term experiment using the young pigs.
Figure 1.
Indicators of kidney function. Levels of BUN, Ca++, K Pi and UA were high in young pigs, indicating poor kidney function. Grey boxes indicate the normal value range for the SNU miniature pig. One-way ANOVAs with Tukey's post test were conducted using Graphpad Prizm ver. 5.0 (Graphpad software). "*", "**" and "***" indicate significant (0.01<P value< 0.05), very significant (0.01 < P value < 0.001), and extremely significant (P < 0.001), respectively.
Figure 2.
Indicators of anemia. Levels of TIBC, UIBC and RDW were high in young pigs; however, levels of Hb, HCT, MHCH and MCV were low. These levels indicate iron deficiency anemia in young pigs. "***" indicate extremely significant (P< 0.001). Grey boxes indicate the normal value ranges for SNU miniature pigs.
The SNU miniature pig is used as a laboratory animal and xenograft donor animal. Thus, understanding its normal physiological phenotype is fundamental. Hematology and serum biochemistry represent the functioning of organs or systems [24], and related data could be used to determine health status. In this study, we defined the normal reference intervals for SNU miniature pigs and discovered that there are some gender-specific differences and age-related difference in these intervals. This study could provide fundamental data for the experimental use of SPF SNU miniature pigs.
Acknowledgments
This study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (Project no. A040004). Miniature pigs were supported by the BCARD, Bio-max institute, SNU.
References
- 1.Smith VG, Leman AD, Seaman WJ, VanRavenswaay F. Pig weaning weight and changes in hematology and blood chemistry of sows injected with recombinant porcine somatotropin during lactation. J Anim Sci. 1991;69(9):3501–3510. doi: 10.2527/1991.6993501x. [DOI] [PubMed] [Google Scholar]
- 2.Carter DB, Lai L, Park KW, Samuel M, Lattimer JC, Jordan KR, Estes DM, Besch-Williford C, Prather RS. Phenotyping of transgenic cloned piglets. Cloning Stem Cells. 2002;4(2):131–145. doi: 10.1089/153623002320253319. [DOI] [PubMed] [Google Scholar]
- 3.Adenkola A, Sackey, Adelaiye Haematological and serum biochemical changes in pigs administered with ascorbic acid and transported by road for four hours during the harmattan season. J Cell Anim Biol. 2009;3(2):21–28. [Google Scholar]
- 4.Kiberd BA. A patient centered approach to the treatment of renal vascular disease to prevent end stage renal failure. Geriatr Nephrol Urol. 1997;7(2):61–66. doi: 10.1023/a:1008283607039. [DOI] [PubMed] [Google Scholar]
- 5.Dungan LJ, Wiest DB, Fyfe DA, Smith AC, Swindle MM. Normal hematology, serology, and serum protein electrophoresis values in fetal Yucatan miniature swine. Lab Anim Sci. 1995;45(3):285–289. [PubMed] [Google Scholar]
- 6.Trávnícek J, Mandel L. Haematology of conventional and germfree miniature Minnesota piglets. II. Serum proteins and immunoglobulins. Z Versuchstierkd. 1982;24(5-6):308–317. [PubMed] [Google Scholar]
- 7.Friendship RM, Lumsden JH, McMillan I, Wilson MR. Hematology and biochemistry reference values for Ontario swine. Can J Comp Med. 1984;48(4):390–393. [PMC free article] [PubMed] [Google Scholar]
- 8.Brockus CW, Mahaffey EA, Bush SE, KruppDespain W. Hematologic and serum biochemical reference intervals for Vietnamese potbellied pigs (Sus scrofa) Comp Clin Path. 2005;13(4):152–165. [Google Scholar]
- 9.Trávnícek J, Mandel L, Trebichavský I, Talafantov M. Immunological state of adult germfree miniature Minnesota pigs. Folia Microbiol (Praha) 1989;34(2):157–164. doi: 10.1007/BF02823696. [DOI] [PubMed] [Google Scholar]
- 10.Mandel L, Trvncek J. Haematology of conventional and germfree miniature Minnesota piglets. I. Blood picture. Z Versuchstierkd. 1982;24(5-6):299–307. [PubMed] [Google Scholar]
- 11.Faustini M, Bronzo V, Maffeo G, Russo V, Munari E, Vigo D. Reference intervals and age-related changes for platelet count, mean platelet volume and plateletcrit in healthy pre-weaning piglets in Italy. J Vet Med A Physiol Pathol Clin Med. 2003;50(9):466–469. doi: 10.1046/j.1439-0442.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- 12.Kitagaki M, Yamaguchi M, Nakamura M, Sakurada K, Suwa T, Sasa H. Age-related changes in haematology and serum chemistry of Weiser-Maples guineapigs (Cavia porcellus) Lab Anim. 2005;39(3):321–330. doi: 10.1258/0023677054307042. [DOI] [PubMed] [Google Scholar]
- 13.Setcavage TM, Kim YB. Variability of the immunological state of germfree colostrum-deprived Minnesota miniature piglets. Infect Immun. 1976;13(2):600–607. doi: 10.1128/iai.13.2.600-607.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin SM, Shin JS, Kim KS, Gong CH, Park SK, Kim JS, Yeom SC, Hwang ES, Lee CT, Kim SJ, Park CG. Islet isolation from adult designated pathogen-free pigs: use of the newer bovine nervous tissue-free enzymes and a revised donor selection strategy would improve the islet graft function. Xenotransplantation. 2011;18(6):369–379. doi: 10.1111/j.1399-3089.2011.00677.x. [DOI] [PubMed] [Google Scholar]
- 15.CLSI. Defining, establishing, and verifying reference interval in the clinical laboratory; Approved guideline. 3rd. Wayne, Pensylvania: CLSI; 2008. pp. C28–A3. [Google Scholar]
- 16.Smith AC, Swindle MM. Preparation of swine for the laboratory. ILAR J. 2006;47(4):358–363. doi: 10.1093/ilar.47.4.358. [DOI] [PubMed] [Google Scholar]
- 17.Dettmers AE, Rempel WE, Hacker DE. Response to recurrent mass selection for small size in swine. J Anim Sci. 1971;33(6):1212–1215. doi: 10.2527/jas1971.3361212x. [DOI] [PubMed] [Google Scholar]
- 18.Ghisolfi J, Landetcheverry O, Olives JP, Thouvenot JP, Nguyen VB, Vaux J. [Evaluation of the lean body and/or muscle mass in children receiving artificial nutrition. Comparative value of anthropometric data and the urinary excretion of creatinine and 3-methylhistidine] Arch Fr Pediatr. 1984;41(6):385–389. [PubMed] [Google Scholar]
- 19.He S, Bremme K, Kallner A, Blombck M. Increased concentrations of lactate dehydrogenase in pregnancy with preeclampsia: a predictor for the birth of small-for-gestational-age infants. Gynecol Obstet Invest. 1995;39(4):234–238. doi: 10.1159/000292417. [DOI] [PubMed] [Google Scholar]
- 20.Riis PM, Madsen A. Thyroxine concentrations and secretion rates in relation to pregnancy, lactation and energy balance in goats. J Endocrinol. 1985;107(3):421–427. doi: 10.1677/joe.0.1070421. [DOI] [PubMed] [Google Scholar]
- 21.Afting EG, Bernhardt W, Janzen RW, Rthig HJ. Quantitative importance of non-skeletal-muscle N tau-methylhistidine and creatine in human urine. Biochem J. 1981;200(2):449–452. doi: 10.1042/bj2000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hobbs DJ, Steinke JM, Chung JY, Barletta GM, Bunchman TE. Rasburicase improves hyperuricemia in infants with acute kidney injury. Pediatr Nephrol. 2010;25(2):305–309. doi: 10.1007/s00467-009-1352-1. [DOI] [PubMed] [Google Scholar]
- 23.Kaysen GA, Jones H, Jr, Martin V, Hutchison FN. A low-protein diet restricts albumin synthesis in nephrotic rats. J Clin Invest. 1989;83(5):1623–1629. doi: 10.1172/JCI114060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adenkola AY, Durotoye LA. 2004. Haematological study during prepartum and postpartum periods in brown savanna dose in Zaria, Nigeria; Proceedings 38th Annual Conference Agricultural Society; Nigeria. 2004. pp. 538–540. [Google Scholar]