Abstract
The main pattern of cognitive impairments seen in early to moderate stages of Parkinson's disease (PD) includes deficits of executive functions. These nonmotor complications have a significant impact on the quality of life and day‐to‐day activities of PD patients and are not effectively managed by current therapies, a problem which is almost certainly due to the fact that the disease extends beyond the nigrostriatal system. To investigate the role of extrastriatal dopamine in executive function in PD, PD patients and a control group were studied with positron‐emission‐tomography using a high‐affinity dopamine D2/D3 receptor tracer, [11C]FLB‐457. All participants were scanned twice while performing an executive task and a control task. Patients were off medication for at least 12 h. The imaging analysis revealed that parkinsonian patients had lower [11C]FLB‐457 binding than control group independently of task conditions across different brain regions. Cognitive assessment measures were positively correlated with [11C]FLB‐457 binding in the bilateral dorsolateral prefrontal cortex and anterior cingulate cortex only in control group, but not in PD patients. Within the control group, during the executive task (as compared to control task), there was evidence of reduced [11C]FLB‐457 binding (indicative of increased dopamine release) in the right orbitofrontal cortex. In contrast, PD patients did not show any reduction in binding during the executive task (as compared with control task). These findings suggest that PD patients present significant abnormalities in extrastriatal dopamine associated with executive processing. These observations provide important insights on the pathophysiology of cognitive dysfunction in PD. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: FLB‐457, positron emission tomography, set‐shifting, cognition, mesocortical dopamine
INTRODUCTION
It has been reported that approximately 15–20% of patients with Parkinson's disease (PD) suffer some degree of cognitive impairment [Caviness et al., 2007; Muslimovic et al., 2005; Williams‐Gray et al., 2007a]. The main pattern of cognitive impairments seen in early to moderate stages of PD resembles that produced by frontal lobe damage and includes deficits of executive functions [Dubois and Pillon, 1997; Leh et al., 2010; Owen, 2004]. Although it is not yet possible to predict who will progress to functional impairment and how fast this might happen, early cognitive deficits may represent an important risk factor for dementia and have also been associated with shorter life expectancy [Caviness et al., 2007] and increased adverse effects from anti‐parkinsonian medications [Aarsland et al., 2001; Holroyd et al., 2001; Mayeux et al., 1992]. Thus, early recognition and understanding of the neurobiology of the executive deficits may be critical for the development of therapeutic strategies to combat them.
Neuroimaging studies have provided supporting evidence that disruption of both nigrostriatal [Owen et al., 1998] and mesocortical [Cools et al., 2002; Mattay et al., 2002; Monchi et al., 2004; 2007] pathway occurs in PD. While dopamine depletion within the caudate nucleus has been associated with cognitive disabilities [Carbon et al., 2004; Grahn et al., 2008; Lewis et al., 2003], recent neuroimaging reports have suggested that extrastriatal dopamine may also contribute to the cognitive deterioration in PD [Bruck et al., 2005; Klein et al., 2010; Rinne et al., 2000]. This observation is supported by recent studies reporting that a catechol O‐methyltransferase gene polymorphism (i.e., met/met) may cause abnormal dopamine levels in the prefrontal cortex (PFC) and decreases performance on executive tests [Williams‐Gray et al., 2007b]. Similarly, positron‐emission‐tomography (PET) experiments have suggested that there is a widespread decline of dopamine receptor availability in PD [Kaasinen et al., 2003; 2000a].
The recent development of new high‐affinity dopaminergic ligands, such as [11C]FLB‐457, permit an accurate assessment of dopaminergic function outside the striatum. Previous studies conducted with the Montreal‐card‐sorting‐task (MCST) [Ko et al., 2009] and related investigations [Aalto et al., 2005] have demonstrated in healthy controls significant release of dopamine in the PFC associate with executive processing. Similarly, in the anterior cingulate cortex (ACC) dopamine receptor availability has been shown to correlate with performance level on the Wisconsin‐card‐sorting‐task [Lumme et al., 2007]. Thus, while the extrastriatal dopamine has been shown to play an important role in executive functions in healthy subjects [Aalto et al., 2005; Ko et al., 2009; Lumme et al., 2007], much less clear is its contribution to executive dysfunction in PD patients.
In this study, we used [11C]FLB‐457 PET to image extrastriatal D2/D3 receptor binding in PD compared with controls during executive task performance. We tested as well whether cognitive function (as measured with the Montreal‐cognitive‐assessment [MoCA] scale) was correlated with dopamine receptor availability. Lastly, we investigated how executive task‐induced dopamine release was affected in PD compared with the control group in those prefrontal regions identified in our previous studies [Ko et al., 2009; Monchi et al., 2007].
METHOD
Subjects and Experimental Design
In this PET study, we recruited 16 participants who underwent imaging for a total of 32 acquisition scans. Eight patients with PD (56–81 years, 6 males, H&Y: 1.5–3) were identified and their demographic features are described in Table I. All PD patients were right‐handed and met the criteria for the diagnosis of idiopathic PD [Defer et al., 1999; Langston et al., 1992]; namely two of the three cardinal signs of PD (bradykinesia, tremor, rigidity), response to l‐dopa treatment and lack of evidence of other medical conditions associated with parkinsonism. PD patients withheld their antiparkinsonian medication for 12–18 h before the scanning session. Their mean score on the motor component (Part III) of the Unified Parkinson's Disease Rating Scale (UPDRS) prior to scanning (off medication) was 32.75 ± 4.60 out of a maximum of 108. Patients were screened for depression and dementia using the Beck Depression Inventory (BDI) and the MoCA, respectively (Table I). The MoCA has been proposed as an accurate assessment tool of cognitive function [Nasreddine et al., 2005], especially in addressing frontal and executive domain [Ismail et al., 2010], and has been validated for its superior sensitivity over the Mini‐Mental State Examination (MMSE) in PD [Hoops et al., 2009]. Parkinsonian patients were compared to a right‐handed control group (CG) (8 subjects; range, 53–82 y/o, 5 males) with no history of neurological or psychiatric disorders. The CG was also screened for dementia and depression using MoCA and BDI. The control subjects were recruited from the general population by advertising, and one patient's spouse also participated. None of the control subjects complained about memory problems. The patients and control subjects were not significantly different in age, handedness, MoCA (i.e., cognitive performance) and BDI (i.e., depression score). Handedness was assessed using the Edinburgh Handedness Inventory [Oldfield, 1971].
Table I.
Demographic figure of subjects
| PD (n = 8) | Controls (n = 8) | P‐value | |
|---|---|---|---|
| Age (years) | 66.3 ± 2.78 | 65.5 ± 3.12 | 0.86 |
| Male/Female | 6/2 | 5/3 | — |
| Disease duration (years) | 7.25 ± 2.12 | — | — |
| UPDRS III | 32.75 ± 4.60 | — | — |
| Hoehn and Yahr stage | 2.25 ± 0.19 | — | — |
| MoCA | 25.5 ± 0.82 | 26.75 ± 0.75 | 0.28 |
| BDI | 6.25 ± 1.85 | 4.75 ± 1.24 | 0.51 |
| Education | 17.25 ± 0.96 | 16.38 ± 1.00 | 0.54 |
| Total LEDD (mg/day) | 677.9 ± 182.5 | — | — |
| Dopamine agonist LEDD daily dose (mg/day) | 159.1 ± 48.5 | — | — |
| Injected mass of [11C]FLB‐457 (μg) | 1.36 ± 0.18 (active) | 1.89 ± 0.31 (active) | 0.16 |
| 1.45 ± 0.18 (control) | 1.98 ± 0.24 (control) | 0.10 |
UPDRS III, Unified Parkinson's Disease Rating Scale, motor score; MoCA, montreal cognitive assessment; BDI, beck depression inventory; LEDD, levodopa equivalent daily dose: l‐dopa dose + l‐dopa‐CR × 0.75 + pramipexole (mg) × 67 [Evans et al., 2004].
All participants were investigated with PET using the high‐affinity D2/D3 radiotracer, [11C]FLB‐457, while performing the MCST to measure extrastriatal dopaminergic receptor binding and changes in dopamine release. Each subject underwent a [11C]FLB‐457 PET scan on two separate days (at the same time) while they performed either the MCST (active task) or the control task (Fig. 1) [Ko et al., 2009]. Scan order was counterbalanced across subjects. All participants gave informed consent after reading the protocol, which was reviewed and approved by the local Research Ethics Committee.
Figure 1.

Study design. (a) Each subject underwent two [11C]FLB‐457 PET scans at the same time on two separate days while performing either the active task (retrieval with shift) or the control task (retrieval without shift) of the MCST. Scan order was counterbalanced across subjects. Participants started the MCST five minutes before the radio‐ligand injection and terminated 10 min before the end of the PET scanning with two‐minute breaks between blocks; (b) active task; (c) control task.
Cognitive Task
The tasks were displayed via a video eyewear (VR920; Vuzix Corporation, NY) placed on the plastic thermal mask. Details of the MCST have also been described in our previous studies [Ko et al., 2008a; 2009; Monchi et al., 2006]. In the retrieval with shift condition of the MCST (the active task, Fig. 1b), four reference cards were displayed in a row at the top of the screen in all trials. Each one of them encompasses three kinds of characteristics, i.e., number (one to four), shape (triangle, star, cross, and circle) and color (red, green, yellow, and blue). Their position changed pseudo‐randomly on every trial. A block of 20 classification trials was preceded by the brief presentation of a single cue card. The cue card did not reappear and had to be remembered throughout the block. On each classification trial, a new test card was presented below the reference cards and the subject had to match the test card to one of the four reference cards using one of four buttons with the right dominant hand. Matching each test card to one of the reference cards was based on a classification rule (color, shape, or number) determined by making a comparison between the previously viewed cue card and the current test card (Fig. 1b). The test card and the cue card shared only one characteristic among number, shape and color. The test cards on consecutive trials never shared the same attribute with the cue card, resulting in a pseudo‐random sequence which allowed for a set‐shift on each trial. Each selection of the reference card was followed by a three‐second positive (white) or negative (dark) feedback. Five blocks of 20 classification trials (total: 100 trials) were followed by a 2‐min break. A different cue card was presented before each block. At the end of each block, the subjects were asked if they remembered the cue card, and selected the cue card that they remember from four samples. The successfully remembered cue cards were counted as percentage of correct selection of the cue card (CUE). The control task was identical to the active task except that there was no set‐shift within a block (Fig. 1c). Subjects underwent a training session of the task before each PET session in order to minimize learning effect. Participants learnt how to perform the MCST within 30 min, i.e., reached >80% accuracy. Accuracy was counted as percentage of correct responses and was averaged for each scan. The performance time (PT) was measured from the presentation of new test card to the selection of the reference card. All values are presented as mean ± SE.
Positron‐Emission‐Tomography
PET scans were obtained with a high resolution PET‐CT, Siemens‐Biograph HiRez XVI (Siemens Molecular Imaging, Knoxville, TN) operating in 3D mode with an in‐plane resolution of ∼4.6 mm full width at half‐maximum (FWHM). To minimize subject's head movements in the PET scanner, we used a custom‐made thermoplastic facemask together with a head‐fixation system (Tru‐Scan Imaging, Annapolis). Before each emission scan, following the acquisition of a scout view for accurate positioning of the subject, a low dose (0.2 mSv) CT scan was acquired.
Upon completion of the acquisition, the emission list mode data was rebinned into a series of 3D sinogram. For each 3D sinogram, the data were normalized with attenuation and scatter correction before applying Fourier rebinning to convert the 3D sinograms into 2D sinograms [Defrise et al., 1997]. The 2D sinograms were then reconstructed into image space using a 2D filtered back projection algorithm, with a ramp filter at Nyquist cut‐off frequency.
[11C]FLB‐457 was injected into the left antecubital vein over 60 s and emission data were then acquired over a period of 90 min in 15 one‐minute frames and 15 five‐minute frames. For PD, the injected amount was 10.03 ± 0.16 mCi (specific activity: 3,034 ± 358 mCi/μmol) for the active condition and 9.66 ± 0.10 mCi (specific activity: 2,794 ± 392 mCi/μmol) for the control condition. For CG, the injected amount was 9.99 ± 0.21 mCi (specific activity: 2348 ± 288 mCi/μmol) for the active condition and 9.94 ± 0.38 mCi (specific activity: 2086 ± 289 mCi/μmol) for the control condition. There was no significant difference in the injected amount of tracer (one‐way ANOVA; f(3,28) = 0.500, P = 0.685) or the specific activity (one‐way ANOVA; f(3,28) = 1.466, P = 0.245).
High‐resolution MRI (GE Signa 1.5 T, T1‐weighted images, 1 mm slice thickness) of each subject's brain was acquired and transformed into standardized stereotaxic space [Talairach and Tournoux, 1988] using nonlinear automated feature‐matching to the MNI template [Collins et al., 1994; Robbins et al., 2004].
PET frames were smoothed, realigned, summed, registered to the corresponding MRI [Woods et al., 1993] and transformed into standardized stereotaxic space [Talairach and Tournoux, 1988] using the transformation parameters of the individual structural MRIs [Collins et al., 1994; Robbins et al., 2004]. All PET images were smoothed with an isotropic Gaussian of 6 mm FWHM to accommodate for intersubject anatomical variability. Voxelwise [11C]FLB‐457 binding potentials (BPND) was calculated using a simplified reference tissue (cerebellum) method [Gunn et al., 1997; Lammertsma and Hume, 1996; Sudo et al., 2001] to generate statistical parametric images of change in BPND [Aston et al., 2000]. This method uses the residuals of the least‐squares fit of the compartmental model to the data at each voxel to estimate the standard deviation of the BPND estimate, thus greatly increasing degrees of freedom without generating false‐positive results.
Statistical Analysis
The BPND‐maps were analyzed using a 2 × 2 factorial repeated measures ANOVA. First, the main effect of group independently of task conditions was analyzed (PD vs. CG); then, the interaction effect of task × group was investigated (PD vs. CG × Active vs. Control task). A post hoc analysis was carried out to study the simple effect of task (Active vs. Control) within each group. A threshold level of t > 4.2 was considered significant (P < 0.05, two‐tailed) corrected for multiple comparisons [Friston, 1997; Worsley et al., 1996] for those regions within our a priori hypothesis, i.e., the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), anterior cingulate cortex (ACC), and orbitofrontal cortex (OFC). A more stringent threshold (t > 4.8) was applied when the search was extended to the entire brain. Regions within our a priori hypothesis were extracted from automated anatomical labeling (AAL) using WFU PickAtlas (http://fmri.wfubmc.edu/software/PickAtlas) [Maldjian et al., 2003]. The reason for choosing these cortical regions as a priori areas was based on their consistent activations during card sorting tasks in previous [11C]FLB‐457 PET [Ko et al., 2009] and fMRI studies conducted by our and other groups [Buchsbaum et al., 2005; Konishi et al., 2002; Lie et al., 2006; Monchi et al., 2007].
Voxel‐based correlation analysis on [11C]FLB‐457 BPND maps from active and control scans was performed with MoCA score within each group using SPM5 (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). The t‐maps were thresholded at P < 0.001 uncorrected with an extent threshold of at least >20 contiguous voxels [Friston et al., 1996]. In this analysis, regions were considered significant at the threshold of P‐value<0.05 corrected at the cluster level.
To confirm individual changes in binding, BPND were extracted from volumes of interest (VOI, radius = 6 mm) centered at the statistical peak defined by the parametric maps.
The statistical analysis of the behavioral data and the VOI‐extracted BPND was performed with SPSS for Windows (Rel. 13.0 2004. Chicago: SPSS) by applying the 2 × 2 factorial repeated measures ANOVA and a post hoc t‐test when applicable. The Kolmogorov‐Smirnov test was used to test for a Gaussian normal distribution.
RESULTS
Behavioral Data
The patients and control subjects were not significantly different in age, handedness, MoCA (i.e., cognitive performance) and BDI (i.e., depression score) (Table I). The 2 × 2 factorial repeated measures ANOVA showed no significant main effect of group in all three behavioral measures of the MCST acquired during scanning (CUE, accuracy and PT; P > 0.05). For the main effect of task, only PT was significantly slower for active task compared to control task (P = 0.002), but no difference was found for CUE or accuracy (P > 0.05). There was no interaction effect of task and group in all three variables (P > 0.05). No correlation was observed between age and MoCA score within each group of subjects (CG: r = −0.625, P > 0.05; PD: r = −0.492, P > 0.05).
PET Data
The factorial repeated measures ANOVA with a 2 × 2 design showed a significant main effect of group (PD vs. CG) independently of task conditions with the CG having higher [11C]FLB‐457 binding than PD across different brain regions (P < 0.05 corrected, Fig. 2a,b). The voxel‐based correlation analysis showed that individuals in the CG with higher MoCA score (i.e., better cognitive performance) had higher [11C]FLB‐457 binding in the bilateral DLPFC (Left: BA 46/10, X = −36 Y = 48 Z = 20; Right: BA 46/9, X = 34 Y = 50 Z = 16; P < 0.05 corrected) and ACC (Left: BA32, X = −6 Y = 40 Z = 18; Right: BA32/24, X = 6 Y = 40 Z = 16; P < 0.05 corrected) (Fig. 3a,c). This correlation was not evident in PD patients (Fig. 3b,d). The significant difference in correlation (Fig. 3c,d: right DLPFC BA 46, X = 34 Y = 50 Z = 16) between PD and CG was confirmed by Fisher's Z transform (Z = 2.9998, P = 0.0024). Inclusion of UPDRS‐III and age as nuisance variables did not change the result.
Figure 2.
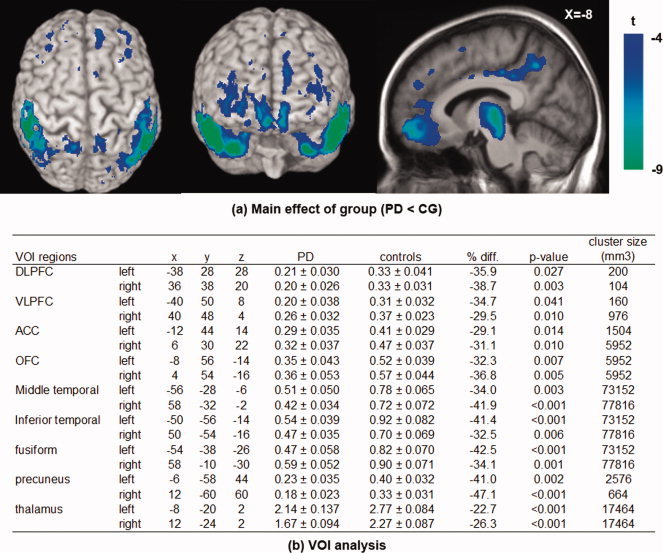
Main effect of group on [11C]FLB‐457 binding potential (BPND). (a) Voxel‐based analysis showed that the [11C]FLB‐457 BPND from both active and control scans were significantly lower in patients with Parkinson's disease (PD) than control group (CG) in various regions (P < 0.05 corrected). (b) Sample BPND (spherical VOI, radius 6 mm) was extracted from the statistical peak identified in the voxel‐based analysis, and averaged between control and active tasks. Independent t‐test between CG vs. PD in each VOI also confirmed the lower [11C]FLB‐457 BPND in all areas (*P < 0.05).
Figure 3.
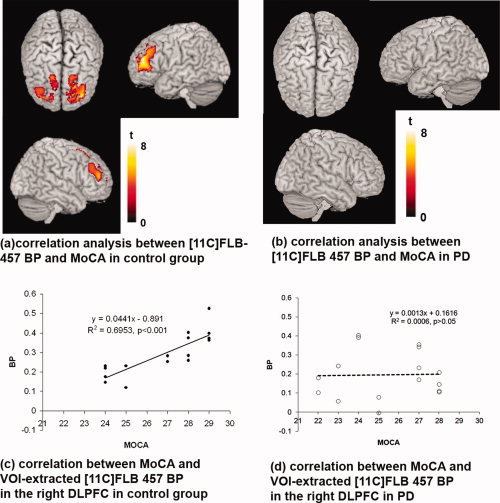
Correlation between MoCA and [11C]FLB‐457 BPND. (a) Voxel‐based analysis showed that the [11C]FLB‐457 BPND of active and control scans were positively correlated with MoCA only in control group in the bilateral DLPFC and ACC (P < 0.05 corrected at cluster level, thresholded at P < 0.001 uncorrected), (b) but not in PD (P > 0.05 corrected at cluster level, thresholded at P < 0.001 uncorrected). (c) Sample [11C]FLB‐457 BPND (spherical VOI, radius 6 mm) was extracted from the statistical peak identified in the voxel‐based analysis (right DLPFC, BA 46, X = 34 Y = 50 Z = 16). Significant correlation was confirmed with MoCA score only in control group (r = 0.834, P < 0.001), (d) but not in PD (r = 0.024, P > 0.05). The significant difference in correlations was confirmed by Fisher's Z transform (Z = 2.9998, P = 0.0024).
A significant interaction (group × task) effect was observed in the left (BA 11; X = −24, Y = 54, Z = −12; t = 4.5; P < 0.05 corrected) and right lateral (BA 10/11; X = 36, Y = 52, Z = −8; t = 4.8; P < 0.05 corrected) and medial orbitofrontal cortex (OFC, BA 11; X = 12, Y = 56, Z = −18; t = 5.8; P < 0.05 corrected) suggesting that task‐induced changes in [11C]FLB‐457 BPND were significantly different between PD and CG (Fig. 4).
Figure 4.
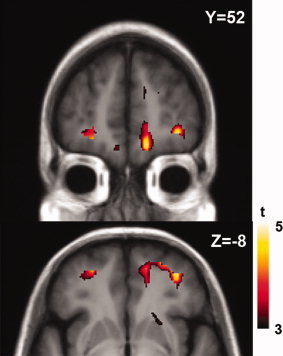
Interaction effect of group × task (PD_active – PD_control – CG_active + CG_control). Voxel‐based analysis showed that the [11C]FLB‐457 BPND was differentially affected by task performance between PD and CG in the OFC.
The simple effect of task (active vs. control condition) was analyzed within each group (Fig. 5). Within the CG, there was a significant decrease of [11C]FLB‐457 BPND in the right OFC (BA 11; t = 4.4, P < 0.05 corrected), left fusiform gyrus (BA 20; t = 5.0, P < 0.05 corrected) and uncus (BA 20; t = 5.0, P < 0.05 corrected). In contrast, in the PD group, no significant reduction in [11C]FLB‐457 BPND (i.e., release of dopamine) during active task was observed in any cortical areas. There was evidence of an increased BPND in different prefrontral areas in the PD group which was not observed in the CG. These areas included the ACC (BA32; X = 12 Y = 48 Z = 18; P < 0.05 corrected), VLPFC (BA46/47; X = 42 Y = 48 Z = −6; P < 0.05 corrected), medial prefrontal cortex (MPFC) (BA9; X = 12 Y = 48 Z = 34; P < 0.05 corrected) and OFC (BA 11; X = 10 Y = 58 Z = −18 and X = −28 Y = 50 Z = −10, P < 0.05 corrected).
Figure 5.

Summary of simple effect of task on [11C]FLB‐457 BPND within each group. (a) In control group, performing the active task compared to the control task decreased [11C]FLB‐457 BPND in the orbitofrontal cortex (OFC), uncus and fusiform gyrus. In PD, performing the active task compared with control task increased BPND in the anterior cingulate cortex (ACC), ventrolateral prefrontal cortex (VLPFC), medial prefrontal cortex (MPFC) and OFC. (b) The [11C]FLB‐457 BPND in the OFC (BA 11) was extracted from spherical VOI (X = 24, Y = 26, Z = −24; radius 6 mm) that was identified in the voxel‐based simple effect analysis of task (active vs. control) in control group. The significant reduction of [11C]FLB‐457 BPND during active task vs. control task was confirmed in control group (paired‐t test: t(7)=3.57, *P = 0.009), but not in PD (paired‐t test: t(7) = −1.136, P > 0.05). (c) The [11C]FLB‐457 BPND in the VLPFC (BA 46/47) was extracted from spherical VOI (X = 42, Y = 48, Z = −6; radius 6 mm) that was identified in the voxel‐based simple effect analysis of task (active vs. control) in PD. The significant increase of [11C]FLB‐457 BPND during active task vs. control task was confirmed in PD (paired‐t test: t (7) = 3.839, *P = 0.009), but not in control group (paired‐t test: t(7) = −0.186, P > 0.05).
DISCUSSION
This study identified a number of differences in dopaminergic function associated with executive function in the prefrontal cortex between PD patients and controls. The first main finding was that PD patients had an overall reduced D2/D3 receptor availability (i.e., less [11C]FLB‐457 binding) in different cortical areas compared to the control group. In particular, whole brain voxel‐based correlation analysis showed that, while in the CG, higher MoCA score (i.e., better cognitive performance) correlated with more receptor availability (i.e., higher [11C]FLB‐457 BPND) particularly in the DLPFC and ACC, this correlation was not observed in PD patients. Last, while individuals within the CG presented a significant decrease in [11C]FLB‐457 BPND in the OFC during the executive task, the PD group did not reveal any reduction in [11C]FLB‐457 BPND (i.e., release of dopamine) in any cortical area.
Reductions of D2/D3 Receptor Binding
In summarizing these findings, we should emphasize first that the behavioral analysis confirmed that the active task was more cognitively challenging than the control task as performance time was significantly slower. However, there was no significant main effect of groups or interactions suggesting that PD patients were not cognitively different from the CG (Table I). Nonetheless, patients with PD presented an overall decrease in [11C]FLB‐457 binding (i.e., D2/D3 receptor binding) compared to the control group in different brain regions (Fig. 2a,b). This observation confirms previous imaging reports with [11C]FLB‐457 PET [Kaasinen et al., 2003, 2000a] and postmortem studies [Scatton et al., 1982] conducted in PD patients. The reduction in D2/D3 receptor binding was observed in critical areas such as DLPFC, VLPFC, ACC, and OFC involved in different aspects of cognitive processing, particularly executive control. The interpretation of these findings remains open to question, as reduced postsynaptic receptor binding in these regions may reflect decreased receptor affinity, a primary reduction in D2/D3 receptor density, internalization of receptors in response to increased dopaminergic stimulation (e.g., chronic treatment [Thobois et al., 2004]) or as most likely secondary to competition from an increased release of endogenous DA triggered by well‐described compensatory mechanisms. This is not surprising in light of previous studies with [18F]FDOPA PET reporting an increase in presynaptic uptake in different frontal areas suggesting an up‐regulated mesocortical dopaminergic function in PD [Bruck et al., 2005; Kaasinen et al., 2001; Rakshi et al., 1999]. However, to our knowledge, no direct comparisons have been made to date between presynaptic and postsynaptic dopaminergic changes in PD at the cortical level.
Age‐related decline in [11C]FLB‐457 binding has been previously reported [Inoue et al., 2001; Kaasinen et al., 2002, 2000b; Narendran et al., 2009], thus this factor may have played a certain role in the PD‐related reduction of D2/D3 receptor availability. However, we believe that this possibility may be quite unlikely given that our groups of subjects were matched for age and the additional inclusion of age as nuisance variable in the analysis did not change our findings.
Of particular interest was the observation that voxel‐based correlation analysis showed that [11C]FLB‐457 BPND specifically at the level of the DLPFC and ACC was significantly correlated with the MoCA score in the CG (Fig. 3a,c). In other words, better cognitive performance (higher MoCA score) was associated in those prefrontal areas (i.e., DLPFC and ACC) with more receptor availability (i.e., higher [11C]FLB‐457 binding). This was not a surprise considering the observations of other reports showing that mesocortical dopaminergic function in those cortical areas plays an important role in cognitive processing and particularly in executive control [Ko et al., 2009; Lumme et al., 2007; MacDonald et al., 2009]. Interestingly, this correlation was absent in PD patients (Fig. 3b,d). The most likely explanation is that the reduced availability of D2/D3 receptors (i.e., reduced [11C]FLB‐457 binding) reported in Figure 2 along with postsynaptic compensatory mechanisms [Calne and Zigmond, 1991] may have played a role in disrupting this correlation. Another possible explanation for this lack of effect in the PD group was the presence of subjects likely responding to the criteria of mild cognitive impairment (MCI) and others that did not.
According to a proposed theory of dopamine function in PFC [Seamans and Yang, 2004], there exists a two‐state model of dopamine action with a balance between D1 and D2 receptor activation. As such, PFC networks would function in between the two‐states. A reduced D2‐receptor function would shift the system towards a state that would favor one mode of action at the expenses of response flexibility (i.e., impaired set‐shifting).
Disordered Dopaminergic Modulation
The interaction effect on [11C]FLB‐457 BPND demonstrated that the task‐induced changes of [11C]FLB‐457 BPND were significantly different between PD and CG particularly at the level of the OFC (Fig. 4). The post hoc simple effect analysis within the CG revealed that there was a significant reduction of [11C]FLB‐457 BPND at the level of the right OFC during the executive task (compared to the control task) (Fig. 5), indicating an increase in synaptic dopamine release. The traditional role of the OFC has been depicted as been involved mainly in decision making [Fellows, 2007; Kringelbach and Rolls, 2004] and reversal learning [Hampshire and Owen, 2006; Walker et al., 2009]. As the MCST requires a certain degree of disengagement from previously relevant sorting rule, it was not surprising to observe that there was an increase in dopamine release in the OFC, especially given that there were also positive prediction errors after the reversals (or set‐shifts). Recent PET studies have also suggested that [11C]FLB‐457 binding in the OFC is significantly correlated with the performance level of set‐shifting measured in a different type of sorting task (i.e., intra‐/extradimensional set‐shifting task) [MacDonald et al., 2009]. This is consistent with other fMRI studies which showed that performance of the MCST increases blood‐oxygen‐level‐dependency signal in the OFC [Monchi et al., 2007].
This task‐induced DA release was not observed in PD patients as no evidence of reduction of [11C]FLB‐457 BPND was seen during the active task (Fig. 5). While the interpretation of this finding remains open to question, there are a number of reasons that could explain this observation. Given that PD patients were studied off‐medication, one possible explanation is that there was not sufficient dopamine to be released in the prefrontal areas. However, another plausible explanation could be a possible “ceiling effect” linking this finding to our observation of reduced D2/D3 receptor binding reported above (Fig. 2). In other words, if the reduced D2/D3 receptor binding as suggested above is most likely secondary to a competition from a baseline increase in release of endogenous DA triggered by natural compensatory mechanisms [Bruck et al., 2005; Kaasinen et al., 2001; Rakshi et al., 1999], then it is conceivable that the lack of task‐induced release of dopamine is the result of an already maximized neuronal reserve (i.e., ceiling effect). Similarly, given that these patients were not naïve to dopaminergic medications, previous chronic treatment (with subsequent receptor changes) may also have played a role.
Although in these patients we did not observe any reduction in [11C]FLB‐457 BPND during the set‐shifting task, there was however a surprising task‐induced increase in [11C]FLB‐457 BPND in a number of prefrontal areas (Fig. 5), including VLPFC. The neurobiological interpretation of this finding is unclear, particularly because it was observed only in the PD patients and not in the control group. In fact, the majority of the studies conducted so far with this type of tracer have been performed in healthy subjects leaving largely unexplored their behavior in brain diseases. Nonetheless, based on current knowledge, the [11C]FLB‐457 BPND is determined by various factors such as dopamine receptor densities, radioligand affinity and synaptic dopamine concentration [Laruelle, 2000]. Traditionally, while acute reduction in [11C]FLB‐457 BPND is interpreted as an increase in synaptic dopamine concentration or internalization of dopamine receptors [Skinbjerg et al., 2010], acute increases in [11C]FLB‐457 BPND have been the subject of controversy with a leading interpretation being a possible reduction of synaptic dopamine concentration [Hagelberg et al., 2004]. In fact, while Fujita et al. [ 2000] reported that dopamine depletion may lead to increased cortical [123I]epidepride binding (i.e., a SPECT radiotracer equivalent to [11C]FLB‐457), the most recent attempts to demonstrate the cortical dopamine depletion effect in healthy controls on [11C]FLB‐457 and [18F]fallypride BPND failed, leaving this issue of induced reduction in release of dopamine in healthy subjects currently unresolved [Frankle et al., 2010; Riccardi et al., 2008].
On the basis of these preliminary reports, we suggest that the postsynaptic receptor changes observed in our patients may be a specific characteristic of the disease itself and may represent for PD patients (but not the CG) a postsynaptic compensatory mechanism secondary to the task‐demand. In fact, in support of our hypothesis, there have been a number of studies in experimental animals that have elegantly documented that dopamine receptor expression can be rapidly up‐regulated in response to an acute levodopa challenge [Hume et al., 1995; Murata and Kanazawa, 1993; Opacka‐Juffry et al., 1998]. Similarly in patients with early PD, Tedroff et al. [ 1996] previously demonstrated the possibility of having increased dopamine receptor binding in different brain areas in response to levodopa challenge. Svenningsson et al. [ 2000] showed that the levodopa‐induced c‐fos mRNA expression is not limited to the striatum but extended to extrastriatal regions including prefrontal and anterior cingulate areas. Although the underlying mechanisms of this acute receptor over‐expression is still unclear, it has been recently demonstrated that dopamine D2 receptor expression can be up‐regulated within an hour by facilitated recycling of internalized receptors [Ji et al., 2009]. Therefore, it is reasonable to suggest that our observation of task‐induced increased [11C]FLB‐457 BPND is not caused by a decrease in synaptic dopamine concentration [Frankle et al., 2010] but rather it could due to the increased (or up‐regulated) dopamine receptor expression or its affinity in PD.
Localization of Findings
A number of critical prefrontal areas linked to executive processing (i.e., DLPFC, VLPFC, ACC, OFC, and MPFC) were identified in our study. Lesion studies in primates and humans [Petrides, 2005; Stuss and Alexander, 2007] along with neuroimaging reports [Ko et al., 2008b; Leh et al., 2009; Monchi et al., 2001; Owen, 2000] have suggested that the DLPFC is engaged in high‐level executive control of monitoring and manipulation of working memory. Besides the DLPFC, the ACC has also been documented to be involved in different cognitive processing, especially motivation, error detection and conflict monitoring [Botvinick et al., 2004; Bush et al., 2000; Ko et al., 2009; Luu et al., 2000; MacDonald et al., 2000; Stuss and Alexander, 2007]. There is a close functional interaction between these two areas, i.e., DLPFC and ACC are often co‐activated when cognitive task difficulty is increased [Koski and Paus, 2000]. In particular, it has been proposed that the ACC monitors conflicts and signals to the DLPFC to implement a higher‐level cognitive control when behavioral adjustment is required [Botvinick et al., 2001]. The correlation of [11C]FLB‐457 binding with MoCA score we observed in CG particularly in those two areas, DLPFC and ACC (Fig. 3), strengthens the hypothesis that these structures are highly interconnected in cognitive processing and further suggests that postsynaptic dopaminergic receptor changes in this network plays an important role in higher‐level cognition.
Studies in primates have suggested that the VLPFC is involved with active encoding and retrieval of information [Petrides, 2005]. In humans, a more specific role of the VLPFC in card sorting task has been described with planning of set‐shifting [Monchi et al., 2004; 2007; 2001]. The fact that the behavioral performance of the tasks was not significantly different in PD vs. CG despite the overall differences in [11C]FLB‐457 binding (Fig. 2) strengthens the possibility that the observed increase in [11C]FLB‐457 binding in PD during the active task (Fig. 5) may represent a compensatory change in the absence of an increased synaptic dopamine transmission (i.e., task‐induced decreased [11C]FLB‐457 binding) in the prefrontal areas.
The role of the OFC in executive function has been often investigated with behavioral tasks associated with reversal learning [Hampshire and Owen, 2006; Walker et al., 2009]. However, activation of the OFC has been consistently reported during card sorting tasks [Monchi et al., 2004; 2007; 2001] partly due to the fact that the applied card sorting tasks also demand reversals of relevant sorting rule. Although, the temporal resolution of PET does not allow us to make strong claims about which specific cognitive function may be related to the increased DA release in our controls, it is possible that a composite effect of set‐shifting and reversals of relevant sorting rules may have contributed to this observation.
The MPFC is known to play a key‐role when refraining to react automatically to congruent stimuli, a prerequisite to gain time to settle on the appropriate response in complex decision making tasks and executive processing [Jaffard et al., 2007; 2008]. In our case, the task‐induced increase in [11C]FLB‐457 binding in the MPFC (Fig. 5) may suggest some dopaminergic compensatory changes secondary to the executive task‐demand.
Although all these cortical regions notoriously play a significant role in cognitive functions, it is important to acknowledge that other areas in the temporal lobe, precuneus and thalamus with lower [11C]FLB‐457 binding (i.e., D2/D3 receptor binding) were also observed in PD compared to the CG (Fig. 2a,b). PD‐related reduction of D2/D3 receptor binding in the temporal cortex and thalamus has been previously reported in the literature [Kaasinen et al., 2003], possibly due to the fact that these areas are particularly rich in D2 receptors [Olsson et al., 2004] therefore making them vulnerable to changes in receptor availability in PD. The functional role of temporal areas has been generally described to be associated with recognition memory (i.e., differentiating familiar and unfamiliar objects) [Milner, 1972]. While recent postmortem studies have shown D2 receptor‐loss in the temporal cortex in parkinsonism [Piggott et al., 2007], previous [11C]FLB‐457 PET studies provided evidence of hippocampus‐temporal dopaminergic influence on executive function via interaction with the PFC [Aalto et al., 2005; Takahashi et al., 2007; 2008]. The thalamus and precuneus have also been described to be involved with executive function including the active inhibition of automatic response to congruent stimuli [Jaffard et al., 2007; 2008]. Similarly, other fMRI investigations have confirmed activations in the temporal cortex, thalamus and precuneus during set‐shifting task suggesting a clear role in executive processing [Monchi et al., 2007]. The decrease in [11C]FLB‐457 binding in these areas, observed in the current and previous [11C]FLB‐457 PET studies [Kaasinen et al., 2003; 2000a] implies a global reduction in dopamine receptor availability in PD possibly reflecting, as suggested above, an overall upregulation of synaptic dopamine concentration.
Study Limitations
There are a number of intrinsic limitations in our study. First, while the high‐affinity dopaminergic ligand, [11C]FLB‐457, permits an accurate assessment of dopaminergic function at the cortical level, one disadvantage is that the BPND estimation is not possible at the striatal level. Unfortunately, this prevents to study simultaneously the nigrostriatal and mesocortical dopaminergic systems. Because of its high‐affinity, a much longer acquisition time (>3 h) is needed to obtain an appropriate estimation of BPND at the striatal level. This it is not possible with this radiotracer but is relatively feasible with another high‐affinity radiotracer, [18F]fallypride [Narendran et al., 2009]. This would however raise other practical issues related to limited use in PD patients due to the long acquisition time required.
Another potential limitation is the inclusion criteria of this study which may have a number of consequences. However, we felt that our inclusion criteria were justified as the most prudent way of generating meaningful results with a modest sample size. For example, limiting the study to patients who lacked severe cognitive deficits optimized the practicality of the experiment at the expense of their generalizability. Subsequent investigations on patients with more severe disease may well demonstrate greater differences compared to controls.
Along this line, the modest sample size may also represent a limiting factor, however we believe that the strict imaging approach proposed by Aston et al. [ 2000] utilized in our study may have prevented false‐positive observations.
Since our main interest was to understand the endogenous extrastriatal dopaminergic responses in the PFC during cognitive task performance, all patients were studied off‐medication. However, future studies should be designed to include drug interaction with behavioral tasks to investigate inverted‐U shape relationship between the PD severity and prefronto‐striatal activities [Rowe et al., 2008, 2010] along with the role of different gene polymorphisms [Williams‐Gray et al., 2007b]. Similarly, a number of reports have recently proposed that set‐shifting in PD may also be dependent upon the integrity of the noradrenergic system which may provide additional new insights in executive functions [Kehagia et al., 2009; 2010].
Acknowledgements
The authors thank all the staff of the CAMH‐research imaging centre for their assistance in carrying out the studies. They thank Dr. C. Marras for her assistance in recruiting some of the patients. They thank Mr. P. Bloomfield for his assistance in reconstructing the PET images.
REFERENCES
- Aalto S, Bruck A, Laine M, Nagren K, Rinne JO ( 2005): Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: A positron emission tomography study using the high‐affinity dopamine D2 receptor ligand [11C]FLB 457. J Neurosci 25: 2471–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh‐Sorensen P ( 2001): Risk of dementia in Parkinson's disease: A community‐based, prospective study. Neurology 56: 730–736. [DOI] [PubMed] [Google Scholar]
- Aston JA, Gunn RN, Worsley KJ, Ma Y, Evans AC, Dagher A ( 2000): A statistical method for the analysis of positron emission tomography neuroreceptor ligand data. Neuroimage 12: 245–256. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD ( 2001): Conflict monitoring and cognitive control. Psychol Rev 108: 624–652. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS ( 2004): Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci 8: 539–546. [DOI] [PubMed] [Google Scholar]
- Bruck A, Aalto S, Nurmi E, Bergman J, Rinne JO ( 2005): Cortical 6‐[18F]fluoro‐L‐dopa uptake and frontal cognitive functions in early Parkinson's disease. Neurobiol Aging 26: 891–898. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF ( 2005): Meta‐analysis of neuroimaging studies of the Wisconsin card‐sorting task and component processes. Hum Brain Mapp 25: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Calne DB, Zigmond MJ ( 1991): Compensatory mechanisms in degenerative neurologic diseases. Insights from parkinsonism. Arch Neurol 48: 361–363. [DOI] [PubMed] [Google Scholar]
- Carbon M, Ma Y, Barnes A, Dhawan V, Chaly T, Ghilardi MF, Eidelberg D ( 2004): Caudate nucleus: Influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. Neuroimage 21: 1497–1507. [DOI] [PubMed] [Google Scholar]
- Caviness JN, Driver‐Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, Evidente VG, Shill HA, Adler CH ( 2007): Defining mild cognitive impairment in Parkinson's disease. Mov Disord 22: 1272–1277. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC ( 1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM ( 2002): Dopaminergic modulation of high‐level cognition in Parkinson's disease: The role of the prefrontal cortex revealed by PET. Brain 125( Part 3): 584–594. [DOI] [PubMed] [Google Scholar]
- Defer GL, Widner H, Marie RM, Remy P, Levivier M ( 1999): Core assessment program for surgical interventional therapies in Parkinson's disease (CAPSIT‐PD). Mov Disord 14: 572–584. [DOI] [PubMed] [Google Scholar]
- Defrise M, Kinahan P, Townsend D, Michel C, Sibomana M, Newport D ( 1997): Exact and approximate rebinning algorithms for 3‐D PET data. IEEE Trans Med Imaging 16: 145–158. [DOI] [PubMed] [Google Scholar]
- Dubois B, Pillon B ( 1997): Cognitive deficits in Parkinson's disease. J Neurol 244: 2–8. [DOI] [PubMed] [Google Scholar]
- Fellows LK ( 2007): The role of orbitofrontal cortex in decision making: A component process account. Ann NY Acad Sci 1121: 421–430. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Mason NS, Rabiner EA, Ridler K, May MA, Asmonga D, Chen CM, Kendro S, Cooper TB, Mathis CA, and others ( 2010): No effect of dopamine depletion on the binding of the high‐affinity D(2/3) radiotracer [11C]FLB 457 in the human cortex. Synapse 64: 879–885. [DOI] [PubMed] [Google Scholar]
- Friston KJ ( 1997): Testing for anatomically specified regional effects. Hum Brain Mapp 5: 133–136. [DOI] [PubMed] [Google Scholar]
- Fujita M, Verhoeff NP, Varrone A, Zoghbi SS, Baldwin RM, Jatlow PA, Anderson GM, Seibyl JP, Innis RB ( 2000): Imaging extrastriatal dopamine D(2) receptor occupancy by endogenous dopamine in healthy humans. Eur J Pharmacol 387: 179–188. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM ( 2008): The cognitive functions of the caudate nucleus. Prog Neurobiol 86: 141–155. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ ( 1997): Parametric imaging of ligand‐receptor binding in PET using a simplified reference region model. Neuroimage 6: 279–287. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Aalto S, Kajander J, Oikonen V, Hinkka S, Nagren K, Hietala J, Scheinin H ( 2004): Alfentanil increases cortical dopamine D2/D3 receptor binding in healthy subjects. Pain 109( 1–2): 86–93. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Owen AM ( 2006): Fractionating attentional control using event‐related fMRI. Cereb Cortex 16: 1679–1689. [DOI] [PubMed] [Google Scholar]
- Holroyd S, Currie L, Wooten GF ( 2001): Prospective study of hallucinations and delusions in Parkinson's disease. J Neurol Neurosurg Psychiatry 70: 734–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, Weintraub D ( 2009): Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 73: 1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume SP, Opacka‐Juffry J, Myers R, Ahier RG, Ashworth S, Brooks DJ, Lammertsma AA ( 1995): Effect of L‐dopa and 6‐hydroxydopamine lesioning on [11C]raclopride binding in rat striatum, quantified using PET. Synapse 21: 45–53. [DOI] [PubMed] [Google Scholar]
- Inoue M, Suhara T, Sudo Y, Okubo Y, Yasuno F, Kishimoto T, Yoshikawa K, Tanada S ( 2001): Age‐related reduction of extrastriatal dopamine D2 receptor measured by PET. Life Sci 69: 1079–1084. [DOI] [PubMed] [Google Scholar]
- Ismail Z, Rajji TK, Shulman KI ( 2010): Brief cognitive screening instruments: An update. Int J Geriatr Psychiatry 25: 111–120. [DOI] [PubMed] [Google Scholar]
- Jaffard M, Benraiss A, Longcamp M, Velay JL, Boulinguez P ( 2007): Cueing method biases in visual detection studies. Brain Res 1179: 106–118. [DOI] [PubMed] [Google Scholar]
- Jaffard M, Longcamp M, Velay JL, Anton JL, Roth M, Nazarian B, Boulinguez P ( 2008): Proactive inhibitory control of movement assessed by event‐related fMRI. Neuroimage 42: 1196–1206. [DOI] [PubMed] [Google Scholar]
- Ji Y, Yang F, Papaleo F, Wang HX, Gao WJ, Weinberger DR, Lu B ( 2009): Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc Natl Acad Sci USA 106: 19593–19598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Aalto S, K NA, Hietala J, Sonninen P, Rinne JO ( 2003): Extrastriatal dopamine D(2) receptors in Parkinson's disease: A longitudinal study. J Neural Transm 110: 591–601. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Kemppainen N, Nagren K, Helenius H, Kurki T, Rinne JO ( 2002): Age‐related loss of extrastriatal dopamine D(2) ‐like receptors in women. J Neurochem 81: 1005–1010. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nagren K, Hietala J, Oikonen V, Vilkman H, Farde L, Halldin C, Rinne JO ( 2000a): Extrastriatal dopamine D2 and D3 receptors in early and advanced Parkinson's disease. Neurology 54: 1482–1487. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nurmi E, Bruck A, Eskola O, Bergman J, Solin O, Rinne JO ( 2001): Increased frontal [(18)F]fluorodopa uptake in early Parkinson's disease: Sex differences in the prefrontal cortex. Brain 124( Part 6): 1125–1130. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, Farde L, Rinne J ( 2000b): Age‐related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging 21: 683–688. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Cools R, Barker RA, Robbins TW ( 2009): Switching between abstract rules reflects disease severity but not dopaminergic status in Parkinson's disease. Neuropsychologia 47: 1117–1127. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW ( 2010): Learning and cognitive flexibility: Frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol 20: 199–204. [DOI] [PubMed] [Google Scholar]
- Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, Baudrexel S, Diederich NJ, Heiss WD, Hilker R ( 2010): Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 74: 885–892. [DOI] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Bloomfield P, Houle S, Strafella AP ( 2008a): Theta burst stimulation‐induced inhibition of dorsolateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine release during a set‐shifting task: A TMS‐[(11)C]raclopride PET study. Eur J Neurosci 28: 2147–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Petrides M, Strafella AP ( 2008b): Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex affects performance of the wisconsin card sorting task during provision of feedback. Int J Biomed Imaging 2008: 143238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Ptito A, Monchi O, Cho SS, Van Eimeren T, Pellecchia G, Ballanger B, Rusjan P, Houle S, Strafella AP ( 2009): Increased dopamine release in the right anterior cingulate cortex during the performance of a sorting task: A [11C]FLB 457 PET study. Neuroimage 46: 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Hayashi T, Uchida I, Kikyo H, Takahashi E, Miyashita Y ( 2002): Hemispheric asymmetry in human lateral prefrontal cortex during cognitive set shifting. Proc Natl Acad Sci USA 99: 7803–7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski L, Paus T ( 2000): Functional connectivity of the anterior cingulate cortex within the human frontal lobe: A brain‐mapping meta‐analysis. Exp Brain Res 133: 55–65. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET ( 2004): The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog Neurobiol 72: 341–372. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP ( 1996): Simplified reference tissue model for PET receptor studies. Neuroimage 4( 3 Part 1): 153–158. [DOI] [PubMed] [Google Scholar]
- Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, Watts R ( 1992): Core assessment program for intracerebral transplantations (CAPIT). Mov Disord 7: 2–13. [DOI] [PubMed] [Google Scholar]
- Laruelle M ( 2000): Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. J Cereb Blood Flow Metab 20: 423–451. [DOI] [PubMed] [Google Scholar]
- Leh SE, Petrides M, Strafella AP ( 2010): The neural circuitry of executive functions in healthy subjects and Parkinson's disease. Neuropsychopharmacology 35: 70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM ( 2003): Cognitive impairments in early Parkinson's disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci 23: 6351–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie CH, Specht K, Marshall JC, Fink GR ( 2006): Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage 30: 1038–1049. [DOI] [PubMed] [Google Scholar]
- Lumme V, Aalto S, Ilonen T, Nagren K, Hietala J ( 2007): Dopamine D2/D3 receptor binding in the anterior cingulate cortex and executive functioning. Psychiatry Res 156: 69–74. [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker DM ( 2000): Medial frontal cortex in action monitoring. J Neurosci 20: 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW III, Cohen JD, Stenger VA, Carter CS ( 2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Cervenka S, Farde L, Nyberg L, Backman L ( 2009): Extrastriatal dopamine D2 receptor binding modulates intraindividual variability in episodic recognition and executive functioning. Neuropsychologia 47: 2299–2304. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, Hyde TM, Weinberger DR ( 2002): Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann Neurol 51: 156–164. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Denaro J, Hemenegildo N, Marder K, Tang MX, Cote LJ, Stern Y ( 1992): A population‐based investigation of Parkinson's disease with and without dementia. Relationship to age and gender. Arch Neurol 49: 492–497. [DOI] [PubMed] [Google Scholar]
- Milner B ( 1972): Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg 19: 421–446. [DOI] [PubMed] [Google Scholar]
- Monchi O, Ko JH, Strafella AP ( 2006): Striatal dopamine release during performance of executive functions: A [(11)C] raclopride PET study. Neuroimage 33: 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A ( 2004): Neural bases of set‐shifting deficits in Parkinson's disease. J Neurosci 24: 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Mejia‐Constain B, Strafella AP ( 2007): Cortical activity in Parkinson's disease during executive processing depends on striatal involvement. Brain 130( Part 1): 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A ( 2001): Wisconsin Card Sorting revisited: Distinct neural circuits participating in different stages of the task identified by event‐related functional magnetic resonance imaging. J Neurosci 21: 7733–7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Kanazawa I ( 1993): Repeated L‐dopa administration reduces the ability of dopamine storage and abolishes the supersensitivity of dopamine receptors in the striatum of intact rat. Neurosci Res 16: 15–23. [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B ( 2005): Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65: 1239–1245. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, Vora S, Litschge M, Kendro S, Cooper TB, et al. ( 2009): Positron emission tomography imaging of amphetamine‐induced dopamine release in the human cortex: A comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse 63: 447–461. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H ( 2005): The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695–699. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Olsson H, Halldin C, Farde L ( 2004): Differentiation of extrastriatal dopamine D2 receptor density and affinity in the human brain using PET. Neuroimage 22: 794–803. [DOI] [PubMed] [Google Scholar]
- Opacka‐Juffry J, Ashworth S, Ahier RG, Hume SP ( 1998): Modulatory effects of L‐DOPA on D2 dopamine receptors in rat striatum, measured using in vivo microdialysis and PET. J Neural Transm 105( 4–5): 349–364. [DOI] [PubMed] [Google Scholar]
- Owen AM ( 2000): The role of the lateral frontal cortex in mnemonic processing: The contribution of functional neuroimaging. Exp Brain Res 133: 33–43. [DOI] [PubMed] [Google Scholar]
- Owen AM ( 2004): Cognitive dysfunction in Parkinson's disease: The role of frontostriatal circuitry. Neuroscientist 10: 525–537. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC ( 1998): Abnormal basal ganglia outflow in Parkinson's disease identified with PET. Implications for higher cortical functions. Brain 121 ( Part 5): 949–965. [DOI] [PubMed] [Google Scholar]
- Petrides M ( 2005): Lateral prefrontal cortex: Architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci 360: 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott MA, Ballard CG, Rowan E, Holmes C, McKeith IG, Jaros E, Perry RH, Perry EK ( 2007): Selective loss of dopamine D2 receptors in temporal cortex in dementia with Lewy bodies, association with cognitive decline. Synapse 61: 903–911. [DOI] [PubMed] [Google Scholar]
- Rakshi JS, Uema T, Ito K, Bailey DL, Morrish PK, Ashburner J, Dagher A, Jenkins IH, Friston KJ, Brooks DJ ( 1999): Frontal, midbrain and striatal dopaminergic function in early and advanced Parkinson's disease A 3D [(18)F]dopa‐PET study. Brain 122 ( Part 9): 1637–1650. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Baldwin R, Salomon R, Anderson S, Ansari MS, Li R, Dawant B, Bauernfeind A, Schmidt D, Kessler R ( 2008): Estimation of baseline dopamine D2 receptor occupancy in striatum and extrastriatal regions in humans with positron emission tomography with [18F] fallypride. Biol Psychiatry 63: 241–244. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Portin R, Ruottinen H, Nurmi E, Bergman J, Haaparanta M, Solin O ( 2000): Cognitive impairment and the brain dopaminergic system in Parkinson disease: [18F]fluorodopa positron emission tomographic study. Arch Neurol 57: 470–475. [DOI] [PubMed] [Google Scholar]
- Robbins S, Evans AC, Collins DL, Whitesides S ( 2004): Tuning and comparing spatial normalization methods. Med Image Anal 8: 311–323. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Ghosh BC, Eckstein D, Williams‐Gray CH, Fallon S, Barker RA, Owen AM ( 2008): Parkinson's disease and dopaminergic therapy—Differential effects on movement, reward and cognition. Brain 131( Part 8): 2094–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Hughes LE, Barker RA, Owen AM ( 2010): Dynamic causal modelling of effective connectivity from fMRI: Are results reproducible and sensitive to Parkinson's disease and its treatment? Neuroimage 52: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatton B, Rouquier L, Javoy‐Agid F, Agid Y ( 1982): Dopamine deficiency in the cerebral cortex in Parkinson disease. Neurology 32: 1039–1040. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR ( 2004): The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74: 1–58. [DOI] [PubMed] [Google Scholar]
- Skinbjerg M, Liow JS, Seneca N, Hong J, Lu S, Thorsell A, Heilig M, Pike VW, Halldin C, Sibley DR, et al. ( 2010): D2 dopamine receptor internalization prolongs the decrease of radioligand binding after amphetamine: A PET study in a receptor internalization‐deficient mouse model. Neuroimage 50: 1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP ( 2007): Is there a dysexecutive syndrome? Philos Trans R Soc Lond B Biol Sci 362: 901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo Y, Suhara T, Inoue M, Ito H, Suzuki K, Saijo T, Halldin C, Farde L ( 2001): Reproducibility of [11 C]FLB 457 binding in extrastriatal regions. Nucl Med Commun 22: 1215–1221. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Gunne L, Andren PE ( 2000): L‐DOPA produces strong induction of c‐fos messenger RNA in dopamine‐denervated cortical and striatal areas of the common marmoset. Neuroscience 99: 457–468. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Hayashi M, Okubo Y, Takano A, Ito H, Suhara T ( 2007): Memory and frontal lobe functions; possible relations with dopamine D2 receptors in the hippocampus. Neuroimage 34: 1643–1649. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Takano H, Arakawa R, Okumura M, Otsuka T, Kodaka F, Hayashi M, Okubo Y, Ito H, et al. ( 2008): Differential contributions of prefrontal and hippocampal dopamine D(1) and D(2) receptors in human cognitive functions. J Neurosci 28: 12032–12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain: 3‐Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme Medical Publishers; 122 p. [Google Scholar]
- Tedroff J, Pedersen M, Aquilonius SM, Hartvig P, Jacobsson G, Langstrom B ( 1996): Levodopa‐induced changes in synaptic dopamine in patients with Parkinson's disease as measured by [11C]raclopride displacement and PET. Neurology 46: 1430–1436. [DOI] [PubMed] [Google Scholar]
- Thobois S, Vingerhoets F, Fraix V, Xie‐Brustolin J, Mollion H, Costes N, Mertens P, Benabid AL, Pollak P, Broussolle E ( 2004): Role of dopaminergic treatment in dopamine receptor down‐regulation in advanced Parkinson disease: A positron emission tomographic study. Arch Neurol 61: 1705–1709. [DOI] [PubMed] [Google Scholar]
- Walker SC, Robbins TW, Roberts AC ( 2009): Differential contributions of dopamine and serotonin to orbitofrontal cortex function in the marmoset. Cereb Cortex 19: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams‐Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA ( 2007a): Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain 130( Part 7): 1787–1798. [DOI] [PubMed] [Google Scholar]
- Williams‐Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA ( 2007b): Catechol O‐methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci 27: 4832–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR ( 1993): MRI‐PET registration with automated algorithm. J Comput Assist Tomogr 17: 536–546. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC ( 1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]


