Abstract
Background
Understanding how sex and tobacco exposure may modify lifetime risks for cancer mortality is important for effective communication of risk in targeted public health messages.
Objective
To determine lifetime risk estimates for cancer death associated with sex and smoking status in the United States.
Methods
A pooled cohort design using ten well-defined epidemiologic cohorts including middle-aged and older individuals was used to estimate the lifetime risk for cancer death at selected index ages, with death from non-cancer causes as the competing risk, by sex and smoking status.
Results
There were a total of 11,317 cancer-related deaths. At age 45 years, the lifetime risk of cancer death for male smokers is 27.7% (95% CI 24.0% to 31.4%) compared to 15.8% (95% CI 12.7% to 18.9%) for male non-smokers. At age 45 years, the lifetime risk of cancer death for female smokers is 21.7% (95% CI 18.8% to 24.6%) compared to 13.2% (95% CI 11.0% to 15.4%) for female non-smokers. Remaining lifetime risk for cancer death declined with age, and men have a greater risk for cancer death compared to women. Adjustment for competing risk of death, particularly representing cardiovascular mortality, yielded a greater change in lifetime risk estimates for men and smokers compared to women and non-smokers.
Conclusions
At the population level the lifetime risk for cancer death remains significantly higher for smokers compared to non-smokers, regardless of sex. These estimates may provide clinicians with useful information for counseling individual patients and highlight the need for continued public health efforts related to smoking cessation.
Keywords: Tobacco, Smoking, Cancer, Lifetime Risk, Cancer mortality, Sex
INTRODUCTION
Recent decades have witnessed a substantial decrease in cancer death rates for both men and women from 1990 to 2005 [1]. Despite these encouraging statistics, absolute numbers of cancer cases and deaths are expected to increase due to an aging population. Cancer confers a substantial public health burden and accounts for approximately 25% of all deaths in the United States [1]. Continued focus and effective communication to the public about modifiable risk factors for cancer and cancer death are therefore imperative. Smoking remains one of the more ubiquitous and preventable contributors to increased risk of cancer and cancer death [2]. Tobacco smoke has been causally associated with multiple cancer types [3, 4]. Much of these data come from the British Doctor’s cohort, which has reported on >50 years of follow up on 34,439 male physicians with detailed smoking habits [4–7].
There have been a variety of pharmacologic, public health, policy, legislative, and legal strategies employed to decrease tobacco use and exposure [8–13]. However, large differences exist in tobacco use by education status, race, and ethnicity, [12] which may contribute to sex and ethnic specific differences in cancer incidence and mortality [14]. Also concerning is that the smoking rate among youth (grades 9–12) is ~20% and has not changed considerably since 2004 [15]. These trends should prompt continued efforts at reducing tobacco use and the development of more effective methods at communicating the risks associated with smoking [12].
Lifetime risk (LTR) estimation is a concept that considers the cumulative risk of an individual developing or dying from a condition during his/her remaining lifetime. LTR estimates provide insight into the public health burden of disease and allow for targeted initiatives for those age groups at greatest risk of disease or death [16]. An example of this is the widely publicized data on the LTR for breast cancer (1 in 8 women at age 40 years) [17]. Wide dissemination of this knowledge likely contributed to increased screening rates for breast cancer in the early 1990s [18]. The concept of LTR also allows for comparison of the public health burden between different diseases. It has been utilized to convey risk for cardiovascular diseases and has shed insight into the cumulative effect of risk factors [19–26]. Another advantage of estimating LTR is the ability to account for competing risks and perhaps provide more clinically appropriate estimates for patient counseling and risk prediction [19].
Lifetime risk estimates for cancer death in the US have previously been generated for individual cancer types [17, 27–29] and are available from the Surveillance Epidemiology and End Results (SEER) program of the National Cancer Institute [30]. However, these studies and the SEER database have limited data on individual risk factor and health behaviors. The purpose of this study was to determine lifetime risk estimates for cancer death associated with sex and smoking status at selected ages using pooled data from multiple US cohorts with detailed risk factor information, including tobacco use.
METHODS
Study Sample
Data for this study came from the Cardiovascular Lifetime Risk Pooling Project of which detailed methods have been previously published [31]. Data from 18 US epidemiologic cohort studies were pooled to create the Cardiovascular Lifetime Risk Pooling Project. All cohorts had to meet several criteria: 1) community- or population-based sampling or large volunteer cohort; 2) availability of at least one baseline examination at which participants provided demographic, personal and medical history information and underwent direct measurement of physiologic and/or anthropometric variables (e.g., blood pressure, weight); 3) longitudinal follow-up of at least 10 years with complete or near-complete ascertainment of vital status; and 4) availability of cause-specific or cardiovascular mortality data with or without ascertainment of non-fatal cardiovascular events. Datasets with cause-specific cancer mortality were included in this study. These cohorts consist of the Atherosclerosis Risk in Communities (ARIC) Study, Chicago Heart Association Detection Project in Industry (CHA), Framingham Heart Study (FHS), Framingham Offspring Study (FHSOFF), Honolulu Heart Program (HHP), the Hispanic EPESE (HISEP), the First National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study (NHEF), NHANES II Mortality Study (NHEFII), Puerto Rico Heart Health Project (PRHHP). The foregoing data were obtained from limited access datasets available from the National Heart, Lung and Blood Institute. Data from the WHI-OS were obtained directly from the study by agreement with the investigators. All data were appropriately de-identified and all study protocols and procedures were approved by the IRB at Northwestern University.
Smoking status measures and event ascertainment
Participants were stratified according to self-reported smoking status levels as measured within three years of each index age. For example, self-reported smoking status measured between ages 42 and 48 were included in the analyses for age 45. Smoking status was categorized as current smoker (typically within one year) vs. former or nonsmoker. Events were ascertained using several strategies selected by each cohort’s investigator group. For death events, many cohorts used linkage to the National Death Index for underlying cause of death from death certificate data, whereas others used adjudicated cause of death by study investigators after review of all available medical records and/or autopsy data. For the present analyses, all-cause mortality and deaths due to cancer (as adjudicated, or indicated by ICD-9 codes 140 to 208, or equivalent codes from ICD-8 or 10) were included. Prior studies suggest high concordance between underlying cause of death as adjudicated by trained physician adjudicators and cause of death obtained from death certificates, especially for cancer as the underlying cause of death [32].
Statistical Analyses
To calculate lifetime risk, a modified technique of survival analysis was used, as described previously [22, 33]. Participants contribute information on the incidences of cancer death and non-cancer death for each age they attained during follow-up. Participants could enter the sample at any age and contribute person-time to follow-up if smoking status was known at the index age. Age-specific hazards, incidence rates, cumulative incidence, and survival probabilities were calculated as in a Kaplan-Meier analysis [34]. Because the Kaplan-Meier cumulative incidence does not reflect the competing risk for death from causes other than cancer, adjustment was made for this competing risk to yield a true remaining lifetime risk for cancer death [35]. Each subject in the study sample was followed up from entry until the occurrence of death, attainment of 95 years of age, or the last clinic examination or medical contact for which follow-up data were available. Remaining LTR estimates for cancer death were stratified by smoking status and were calculated separately for men and women beginning at index ages 45, 55, 65, and 75 years. Given the uncertainty associated with small numbers, LTR estimates are only reported to ages where weighted person-time exceeded 100-person years. Statistical analyses were performed using SAS statistical software, version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Baseline characteristics
The mean age at entry in the 10 cohort studies ranged from 36.9 yrs to 74.6 yrs, and average follow up time ranged from 5.4 years to 35.5 years (Table 1). Number of participants and total follow up time varied by index age: for example, for index age 55 years, there were 13,901 men and 4786 women followed for a total of 203,993 person-years, during which time 2583 cancer deaths and 8815 total deaths occurred. The proportion of participants with death due to cancer ranged from 0.4% (ARIC; a study with younger participants and shorter follow up) to 20.4% (FHS). Site-specific cancer death information was only available from 3 cohorts – HHP, HISEP, and NHEF. This included 2143 cancer deaths attributable mainly to the gastrointestinal (n=785, 36.6%) and respiratory (n=421, 19.6%) systems.
Table 1.
Cohort characteristics and proportion of participants with death from cancer during observed follow up
| ARIC | CHA | FHS | FHSOFF | HHP | HISEP | NHEF | NHEFII | PRHHP | WHI-OS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total N | 15371 | 33902 | 5079 | 4739 | 8006 | 3042 | 11399 | 12504 | 9475 | 91294 |
| Cancer deaths (%) | 61 (0.4) | 3987 (10.5) | 1034 (20.4) | 260 (5.5) | 1532 (19.1) | 106 (3.5) | 982 (8.7) | 568 (4.5) | 212 (2.2) | 2575 (2.8) |
| Entry age, mean (y) | 54.2 | 40.4 | 44.2 | 36.9 | 54.4 | 74.6 | 49.8 | 46.2 | 54.9 | 63.6 |
| Entry age, range (y) | 45–64 | 18–90 | 28–64 | 20–62 | 45–68 | 65–99 | 25–74 | 18–74 | 47–77 | 50–79 |
| Follow up yrs, mean | 10.8 | 29 | 35.5 | 24.1 | 21.3 | 5.4 | 16.2 | 13.2 | 14.2 | 7.7 |
| Follow up yrs, range | 0.0–13.1 | 0.1–37.0 | 24.0–54.8 | 0.1–31.6 | 7.0–33.1 | 0.0–7.9 | 0–22.1 | 0.0–16.0 | 14.0–20.0 | 0.1–10.6 |
| POOLED COHORTS | ||||||||||
| Index Age (y) | Men, N | Women, N |
Total Follow Up (Person-Years) |
Cancer Deaths, N | Total Deaths, N | |||||
| 45 | 8409 | 4319 | 164,212 | 1375 | 3942 | |||||
| 55 | 13,901 | 4786 | 203,993 | 2583 | 8815 | |||||
| 65 | 7544 | 3872 | 108,590 | 1678 | 6990 | |||||
| 75 | 1312 | 1906 | 24,088 | 463 | 2356 | |||||
Abbreviations: ARIC = Atherosclerosis Risk in Communities, CHA = Chicago Heart Association, FHS = Framingham Heart Study, FHOSFF = Framingham Heart Study Offspring Cohort, HHP = Healthy Heart Program, HISEP = Hispanic Established Populations for Epidemiologic Studies of the Elderly, NHEF = National Health and Nutrition Examination Survey, NHEFII = National Health and Nutrition Examination Survey II, PRHHP = Puerto Rico Heart Health Program, WHI = Women’s Health Initiative Observational Study
Lifetime risk for cancer death by sex and age
The lifetime risk for cancer death to age 90, adjusted for the competing risk of death from other causes, is shown in Table 2 for selected index ages. The lifetime risks for cancer death to age 90 as estimated from the NCI SEER database (http://seer.cancer.gov/) are also shown. For most cohorts, there is remarkable concordance between the nationally-representative SEER estimate and the cohort-specific estimates. For example, LTR estimates for cancer death for a 45 year old male from our cohorts ranged from 15.6% to 26.8%; estimates provided by SEER for this same index age were 22.3%. A 45 year old female had LTR estimates ranging from 13.0% to 18.5%, comparable to the 17.8% estimate from SEER.
Table 2.
Lifetime risks for death from cancer at selected index ages to age 90 (% and 95% CI), by individual cohort and compared to SEER national surveillance data
| Male | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Index age | CHA | FHS | HHP | HISEP | NHEF | NHEFII | PRHHP | WHI-OS | SEER |
| 45 | 22.7 (21.7–23.6) | 22.8 (21.0–24.5) | 26.8 (25.6–28.0) | n/a | 15.6 (13.9–17.4) | 21.0 (18.4–23.6) | n/a | n/a | 22.3 |
| 55 | 22.7 (21.5–23.8) | 22.8 (21.0–24.6) | 26.8 (25.5–28.0) | n/a | 14.7 (12.9–16.5) | 20.5 (17.8–23.2) | 12.3 (8.4–16.3) | n/a | 22.3 |
| 65 | 21.3 (19.3–23.4) | 22.4 (20.5–24.4) | 25.5 (24.1–26.9) | 7.6 (5.0–10.2) | 12.2 (10.4–14.0) | 18.6 (15.8–21.4) | 11.9 (7.7–16.1) | n/a | 20.9 |
| 75 | 20.8 (12.3–29.4) | 19.9 (17.6–22.3) | 23.8 (21.6–26.0) | 6.8 (4.1–9.5) | 7.7 (5.5–10.0) | 15.9 (11.9–20.0) | 8.3 (3.1–13.5) | n/a | 16.8 |
| Female | |||||||||
| Index age | CHA | FHS | HHP | HISEP | NHEF | NHEFII | PRHHP | WHI-OS | SEER |
| 45 | 18.3 (17.3–19.2) | 18.5 (17.0–20.0) | n/a | n/a | 17.4 (15.7–19.0) | 13.0 (11.3–14.8) | n/a | n/a | 17.8 |
| 55 | 17.1 (16.0–18.2) | 18.0 (16.5–19.5) | n/a | n/a | 12.1 (10.6–13.6) | 11.8 (10.1–13.5) | n/a | 20.7 (11.5–30.0) | 17.2 |
| 65 | 16.4 (14.4–18.5) | 15.9 (14.4–17.4) | n/a | 11.0 (8.3–13.7) | 8.9 (7.4–10.3) | 9.5 (7.9–11.1) | n/a | 19.6 (10.1–29.1) | 15.4 |
| 75 | 10.1 (4.7–15.5) | 12.2 (10.6–13.8) | n/a | 10.0 (7.1–13.0) | 6.2 (4.3–8.0) | 7.0 (4.9–9.2) | n/a | 17.3 (7.0–27.6) | 11.6 |
Abbreviations: CHA = Chicago Heart Association, FHS = Framingham Heart Study, HHP = Healthy Heart Program, HISEP = Hispanic Established Populations for Epidemiologic Studies of the Elderly, NHEF = National Health and Nutrition Examination Survey, NHEFII = National Health and Nutrition Examination Survey II, PRHHP = Puerto Rico Heart Health Program, WHI = Women’s Health Initiative Observational Study, SEER = Surveillance Epidemiology and End Results (http://seer.cancer.gov)
Remaining LTR for death from cancer declined with advancing index age, especially at older ages (Table 2). Except for the NHEF cohort at age 45, the LTR of death from cancer is consistently greater for men than women. Among men, the HHP cohort of Japanese-American men had the highest LTR for cancer death, 26.8%, at age 45 years. The Hispanic cohort (HISEP) generally had the lowest LTR for cancer death, especially for males.
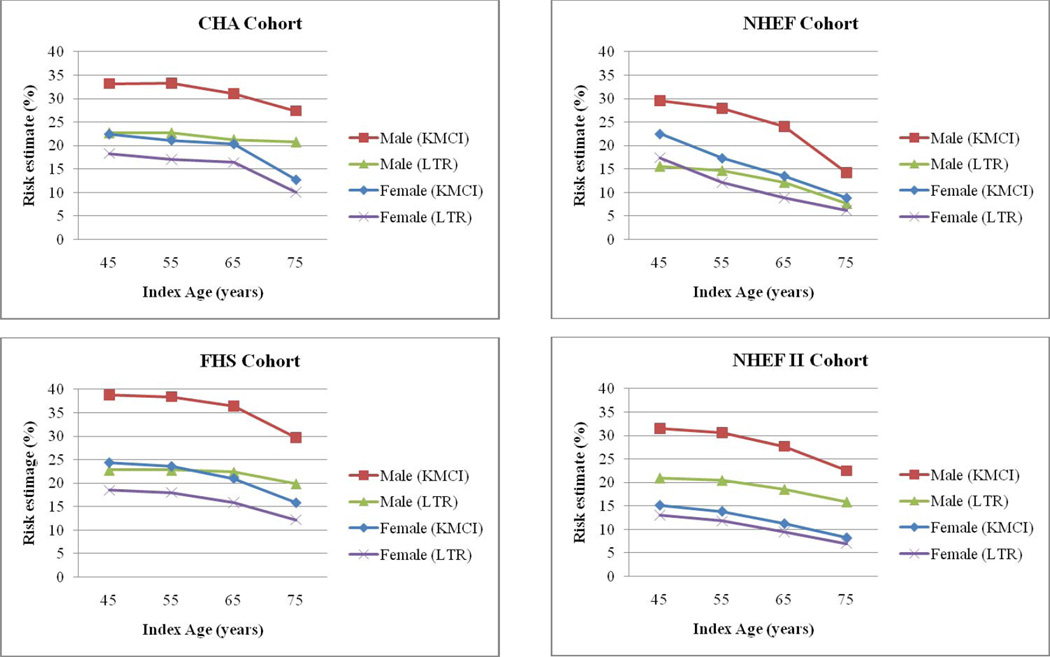
Figure 1 illustrates both the unadjusted Kaplan-Meier cumulative incidence (KMCI) and lifetime risk (LTR) for cancer death from four representative cohorts (CHA, FHS, NHEF, and NHEFII). The difference between the KMCI and LTR curves within each sex represents the contribution of competing risk of death from other causes. As reflected in Figure 1there is a larger contribution of competing risk of death for men compared to women at ages 45, 55, and 65 years. The competing risk of death appears to remain similar across all index ages for women.
Fig. 1.
KMCI and LTR stratified by cohort
Pooled risk estimates - effect of sex and smoking
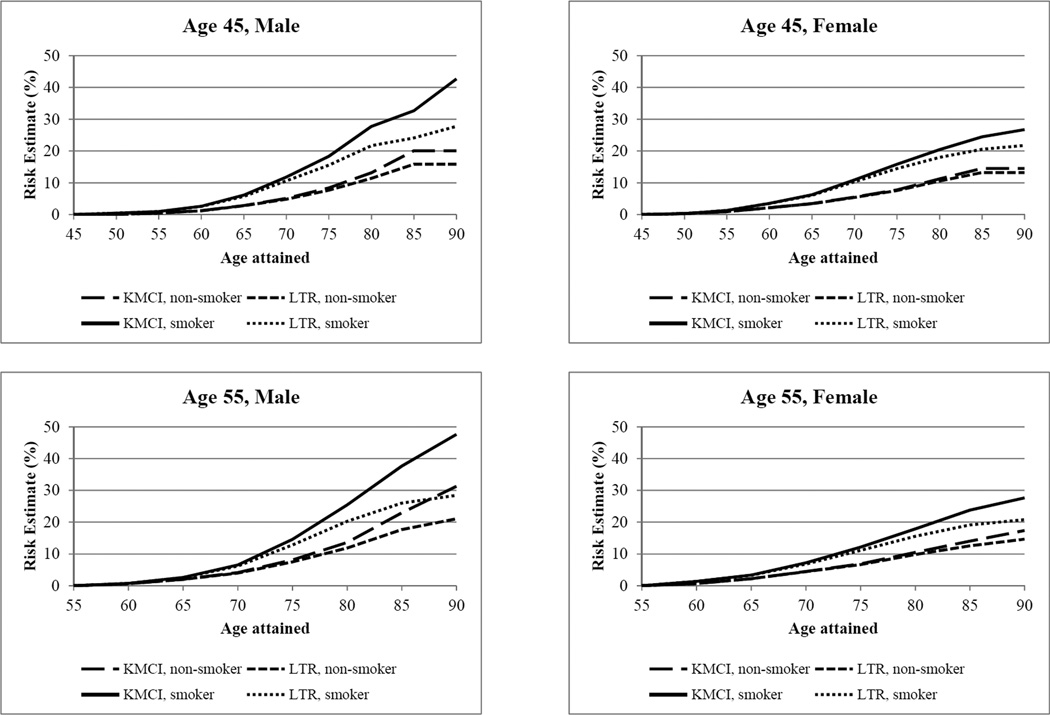
Table 3 displays the pooled lifetime risk for cancer death (adjusted for competing risk of non-cancer death) stratified by sex and smoking status. Current smoking (compared with non-smoking status) is associated with greater LTR for cancer death for both sexes at all index ages, although to a greater extent in males. Adjustment for competing risk of death in LTR estimates (compared with KMCI estimates), results in a greater difference for men and smokers than for women and non-smokers (Figure 2). The competing risk of death from non-cancer causes, such as cardiovascular disease, visually represented by differences in the KMCI and LTR curves, contributes a greater proportion of risk to male smokers across all index ages. For both men and women, smoking is associated with an accelerated lifetime risk for cancer death. Table 4 displays the age at which men and women attain a 10% cumulative risk for cancer death depending upon smoking status. At age 45, male and female smokers will attain a 10% lifetime risk of cancer death at age 70, compared to age 79 for non-smokers, indicating that cancer deaths occur at substantially younger ages among smokers, causing greater life-years lost.
Table 3.
Lifetime Risk Estimates by Sex and Smoking status at selected index ages to age 90 (% and 95% CI)
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Index Age | Non-smoker | Smoker | p valuea | Non-smoker | Smoker | p valuea |
| 45 | 15.8 (12.7–18.9) | 27.7 (24.0–31.4) | <0.0001 | 13.2 (11.0–15.4) | 21.7 (18.8–24.6) | <0.0001 |
| 55 | 21.1 (19.6–22.5) | 28.4 (26.8–30.1) | <0.0001 | 14.6 (13.2–16.0) | 20.7 (18.7–22.8) | <0.0001 |
| 65 | 17.6 (16.2–19.1) | 24.2 (22.3–26.1) | <0.0001 | 13.3 (11.9–14.8) | 18.2 (15.6–20.8) | 0.001 |
| 75 | 16.9 (13.9–19.8) | 23.0 (19.0–27.1) | 0.02 | 9.7 (8.1–11.3) | 17.5 (13.4–21.6) | 0.0005 |
χ2 test
Fig. 2.
KMCI and LTR by age, smoking status, and gender
Table 4.
Age (years) at which lifetime risk for cancer death exceeds 10% by sex and smoking status
| Male | Female | |||
|---|---|---|---|---|
| Index Age | Non-smoker | Smoker | Non-smoker | Smoker |
| 45 | 79 | 70 | 79 | 70 |
| 55 | 79 | 73 | 81 | 74 |
| 65 | 82 | 78 | 84 | 80 |
| 75 | 84 | 80 | 91 | 83 |
DISCUSSION
Principal Findings
Prior research on lifetime risk for cancer death has focused on specific cohorts, individual cancer sites [17, 27, 28, 36], and non-US populations [4, 37]. This study determined lifetime risk estimates for cancer death in the US stratified by sex and smoking status using a pooled cohort study design. The estimates obtained were remarkably similar to those generated by the nationally-representative SEER database. Unlike SEER, our pooled cohort provides the ability to convey estimates stratified by individual risk factors, such as sex and smoking status. To our knowledge, this study is the first of its kind in a US population.
As highlighted in Figure 2, the lifetime risk for cancer death decreases with advancing index age across all cohorts. Since these curves mirror the unadjusted death rates (KMCI), this decrease appears predominantly due to depletion of individuals susceptible to death from cancer through middle age, in addition to increase in competing risk of death from other causes with aging. The overall greater competing risk of death in men compared to women across all index ages, as estimated by the difference in the KMCI and LTR plots, is likely due to an increased burden of cardiovascular morbidity and mortality [21, 22]. Previous work in single cohorts has shown that the lifetime risk for cardiovascular death is highly associated with cumulative cardiovascular risk factor (hypertension, hyperlipidemia, diabetes, and smoking) burden [20]. Of interest, in those studies, the risk for non-cardiovascular disease death was also dependent on the aggregate burden of cardiovascular risk factors [20]. Our results are consistent with these previous results showing a greater competing risk of death in men.
Regardless of sex, smokers have a substantially greater lifetime risk of cancer death and this difference persists across all index ages. This contrasts with lifetime risk data for cardiovascular disease, for which smokers and non-smokers tend to have similar risks [22]. This contrast is likely due to mechanistic differences in disease development as tobacco use appears to contribute to earlier risk of cardiovascular death compared to an overall increase in the risk of cancer death. Non-smokers tend to live substantially longer than smokers and develop cardiovascular events substantially later in life than smokers, resulting in a similar overall lifetime risk for cardiovascular disease, but substantially later events, longer years lived free of disease, and compression of morbidity for non-smokers. The fact that non-smokers have a decreased lifetime risk for cancer death across all ages implies that any decrease in tobacco exposure at the population level could lead to persistent decreases in cancer deaths and improved longevity.
Although there has been a marked decrease in smoking prevalence during the decades of observation during which the diverse cohort studies followed their participants (as well as a decline in cardiovascular disease death rates), we observed little evidence of birth cohort effects across our cohorts in lifetime risks for cancer death, whether considered overall or stratified by smoking status [31]. It appears that once a risk factor like smoking is present, it is associated with fairly constant relative and absolute risks, regardless of birth cohort, as is the case for the major cardiovascular risk factors. We did observe some evidence of racial/ethnic differences in cancer death rates, as indicated by the modest differences in lifetime risk for cancer death we observed between cohorts in our study.
Potential Limitations
Current smoking status was assigned at each index age and our data did not allow us to examine the effect of smoking cessation at later ages during follow up. However, inclusion of individuals who later stopped smoking at a given index age would, if anything, tend to under-estimate lifetime risks for cancer death among smokers. Likewise, current smoking status at a given age is the information available to a clinician and patient during a clinical encounter, and later smoking cessation is difficult to predict. Our study utilized a pooled cohort study design for analyses of lifetime risk by smoking status. Limitations of this approach may include different follow up periods and number of cancer deaths for each cohort, and potential for birth cohort effects. Site specific cancer death information was only available for a small number of cohorts and thus was not included in the analysis. By design, the individual cohorts were also composed of different sex, ethnic, and racial proportions. This could limit the generalizability of our results. However, our LTR estimates for cancer death were remarkably similar to nationally-representative data from SEER, the effect of smoking was remarkably similar across the diverse cohorts, and our pooled design tends to diminish birth cohort effects and secular trends, since individuals from multiple birth cohorts and subgroups contribute information for each age attained during follow up [31].
Clinical implications
Conveying risk information to patients and the public can be difficult. The sheer volume of risk information for multiple chronic diseases can overwhelm any meaningful public health message. Numerous variables can affect whether or not risk is conveyed in an effective way to impact health outcomes including sex, health beliefs, and format of the risk message [38–40]. For example, Timmermans et al. found that visual representation of population figures (as opposed to percentages and frequencies) had the highest affective impact and perceived risk in hypothetical health scenarios [40]. Our results could contribute a novel approach of estimating overall lifetime risk with individual risk factor information, and thus the ability to tailor risk estimates.
An interesting comparison is information conveyed in 10 year vs. lifetime risk estimates. Significant limitations with 10 year risk estimation have been discussed in detail in the cardiovascular literature [19]. Younger individuals tend not to exceed 10 year risk estimates of cardiovascular disease (CVD) of greater than 10%, even for individuals with substantial burden of risk factors [41]. However, the lifetime risk for developing atherosclerotic cardiovascular disease at 50 years of age is 68.9% for men and 50.2% for women with 2 or more major risk factors [22]. Individuals with optimal levels of all risk factors at age 50 years have a dramatically lower remaining lifetime risk for CVD (5.2% for men, 8.2% for women), indicating that primordial prevention strategies could significantly impact disease burden.
Similar limitations are seen with risk estimates related to cancer. Based on 10 year risk data for a 45 year old male, 9/1000 smokers will die of lung or colon cancer compared to 2/1000 non-smokers [29], an absolute difference of 0.7%. In contrast, our results show that a 45 year old male smoker has a 28% lifetime risk of cancer death compared to 16% for a non-smoker, representing a 75% increase in lifetime risk. In terms of patient counseling, the risks conveyed in 10 year risk estimates to younger age groups may not fully convey the long term hazards of behaviors such as smoking. Physician visits are often limited in scope, and delivery of understandable information in a timely manner is paramount to effective patient care. Statistical risk models, such as the Gail model used in breast cancer, are not perfect but can provide clinicians with accurate estimates of risk to help guide patient care, surveillance, and clinical decision making [42–44]. Our results could provide the foundation for a risk calculator or integrated alert in an electronic medical record that clinicians could use to deliver lifetime risk and longevity estimates to guide smoking cessation efforts.
Public Health Implications
With the aging of the population, cancer cases and deaths are expected to increase in total number [45, 46]. Public health messages to promote cancer prevention strategies should focus on modifiable risk factors with the potential to attenuate this trend. A substantial portion of the US population continues to use tobacco and rates have plateaued. Prior research has shown that smoking cessation contributes to real declines in morbidity and mortality from a variety of causes, including cancer [47–49]. Incorporating lifetime risk information in public health messages and anti-smoking campaigns could result in a more effective message similar to the message of lifetime risk for breast cancer (1 in 8 for women at age 40 years) that was publicized to increase mammography rates [17, 18] These estimates could also help predict future population disease burden and decisions regarding distribution of resources for smoking cessation and prevention. Our data provide researchers and health policy experts with evidence that continued efforts at decreasing tobacco use could lead to further declines in cancer death burden and significant life-years gained, especially if this message is targeted at younger age groups.
Supplementary Material
ACKNOWLEDGEMENTS
This work and Dr. Lloyd-Jones were supported by a grant from the National Heart, Lung, and Blood Institute (R21 HL085375).
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. We acknowledge the following for their contributions to the WHI study: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Abbreviations
- LTR
lifetime risk
- KMCI
Kaplan-Meier Cumulative Incidence
- CVD
cardiovascular disease
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun M. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Adami HO, Day NE, Trichopoulos D, Willett WC. Primary and secondary prevention in the reduction of cancer morbidity and mortality. Eur J Cancer. 2001;37(Suppl 8):S118–S127. doi: 10.1016/s0959-8049(01)00262-3. [DOI] [PubMed] [Google Scholar]
- 3.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(Suppl 2):S3–S9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- 4.Doll R, Peto R, Boreham J, Sutherland I. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br J Cancer. 2005;92:426–429. doi: 10.1038/sj.bjc.6602359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doll R, Hill AB. Mortality in relation to smoking: ten years observations of British doctors. Br Med J. 1964;1:1399–1410. doi: 10.1136/bmj.1.5395.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doll R, Peto R. Mortality in relation to smoking: 20 years' observations on male British doctors. Br Med J. 1976;2:1525–1536. doi: 10.1136/bmj.2.6051.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years' observations on male British doctors. BMJ. 1994;309:901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones WJ, Silvestri GA. The Master Settlement Agreement and its impact on tobacco use 10 years later: lessons for physicians about health policy making. Chest. 2010;137:692–700. doi: 10.1378/chest.09-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodu B, Cole P. Impact of the American anti-smoking campaign on lung cancer mortality. Int J Cancer. 2002;97:804–806. doi: 10.1002/ijc.10108. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins DP, Razi S, Leeks KD, Priya Kalra G, Chattopadhyay SK, Soler RE. Smokefree policies to reduce tobacco use. A systematic review. Am J Prev Med. 2010;38:S275–S289. doi: 10.1016/j.amepre.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Chandler MA, Rennard SI. Smoking cessation. Chest. 2010;137:428–435. doi: 10.1378/chest.09-0124. [DOI] [PubMed] [Google Scholar]
- 12.Cokkinides V, Bandi P, McMahon C, Jemal A, Glynn T, Ward E. Tobacco control in the United States--recent progress and opportunities. CA Cancer J Clin. 2009;59:352–365. doi: 10.3322/caac.20037. [DOI] [PubMed] [Google Scholar]
- 13.Roeseler A, Burns D. The quarter that changed the world. Tob Control. 2010;19(Suppl 1):i3–15. doi: 10.1136/tc.2009.030809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leistikow B. Lung cancer rates as an index of tobacco smoke exposures: validation against black male approximate non-lung cancer death rates, 1969–2000. Prev Med. 2004;38:511–515. doi: 10.1016/j.ypmed.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Eaton DK, Kann L, Kinchen S, et al. Youth risk behavior surveillance - United States, 2009. MMWR Surveill Summ. 2010;59:1–142. [PubMed] [Google Scholar]
- 16.Merrill RM, Weed DL. Measuring the public health burden of cancer in the United States through lifetime and age-conditional risk estimates. Ann Epidemiol. 2001;11:547–553. doi: 10.1016/s1047-2797(01)00254-x. [DOI] [PubMed] [Google Scholar]
- 17.Feuer EJ, Wun LM, Boring CC, Flanders WD, Timmel MJ, Tong T. The lifetime risk of developing breast cancer. J Natl Cancer Inst. 1993;85:892–897. doi: 10.1093/jnci/85.11.892. [DOI] [PubMed] [Google Scholar]
- 18.Grunbaum JA, Kann L, Kinchen SA, et al. Youth Risk Behavior Surveillance--National Alternative High School Youth Risk Behavior Survey, United States, 1998. MMWR CDC Surveill Summ. 1999;48:1–44. [PubMed] [Google Scholar]
- 19.Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121:1768–1777. doi: 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd-Jones DM, Dyer AR, Wang R, Daviglus ML, Greenland P. Risk factor burden in middle age and lifetime risks for cardiovascular and non-cardiovascular death (Chicago Heart Association Detection Project in Industry) Am J Cardiol. 2007;99:535–540. doi: 10.1016/j.amjcard.2006.09.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd-Jones DM, Wilson PW, Larson MG, et al. Lifetime risk of coronary heart disease by cholesterol levels at selected ages. Arch Intern Med. 2003;163:1966–1972. doi: 10.1001/archinte.163.16.1966. [DOI] [PubMed] [Google Scholar]
- 25.Marma AK, Berry JD, Ning H, Persell SD, Lloyd-Jones DM. Distribution of 10-year and lifetime predicted risks for cardiovascular disease in US adults: findings from the National Health and Nutrition Examination Survey 2003 to 2006. Circ Cardiovasc Qual Outcomes. 2010;3:8–14. doi: 10.1161/CIRCOUTCOMES.109.869727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persell SD, Zei C, Cameron KA, Zielinski M, Lloyd-Jones DM. Potential use of 10-year and lifetime coronary risk information for preventive cardiology prescribing decisions: a primary care physician survey. Arch Intern Med. 2010;170:470–477. doi: 10.1001/archinternmed.2009.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller BA, Scoppa SM, Feuer EJ. Racial/ethnic patterns in lifetime and age-conditional risk estimates for selected cancers. Cancer. 2006;106:670–682. doi: 10.1002/cncr.21647. [DOI] [PubMed] [Google Scholar]
- 28.Kim HL, Puymon MR, Qin M, Guru K, Mohler JL. A method for using life tables to estimate lifetime risk for prostate cancer death. J Natl Compr Canc Netw. 2010;8:148–154. doi: 10.6004/jnccn.2010.0011. [DOI] [PubMed] [Google Scholar]
- 29.Woloshin S, Schwartz LM, Welch HG. The risk of death by age, sex, and smoking status in the United States: putting health risks in context. J Natl Cancer Inst. 2008;100:845–853. doi: 10.1093/jnci/djn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altekruse S, Kosary C, Krapcho M, et al. Bethesda, MD: National Cancer Institute; 2009. SEER Cancer Statistics Review, 1975–2007 based on November 2009 SEER data submission, posted to the SEER web site, 2010. [Google Scholar]
- 31.Berry J, Dyer A, Cai X, et al. Remaining Lifetime Risks For Cardiovascular Disease by Age and Risk Factor Burden: The Cardiovascular Lifetime Risk Pooling Project. N Engl J Med. 2011 (In Press) [Google Scholar]
- 32.Lloyd-Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129:1020–1026. doi: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]
- 33.Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. Jama. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–81. [Google Scholar]
- 35.Gaynor J, Feuer E, Tan C, et al. On the use of cause-specific failure and conditional failure probabilities. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
- 36.Merrill RM, Kessler LG, Udler JM, Rasband GC, Feuer EJ. Comparison of risk estimates for selected diseases and causes of death. Prev Med. 1999;28:179–193. doi: 10.1006/pmed.1998.0399. [DOI] [PubMed] [Google Scholar]
- 37.Vollset SE, Tverdal A, Gjessing HK. Smoking and deaths between 40 and 70 years of age in women and men. Ann Intern Med. 2006;144:381–389. doi: 10.7326/0003-4819-144-6-200603210-00004. [DOI] [PubMed] [Google Scholar]
- 38.Kurz-Milcke E, Gigerenzer G, Martignon L. Transparency in risk communication: graphical and analog tools. Ann N Y Acad Sci. 2008;1128:18–28. doi: 10.1196/annals.1399.004. [DOI] [PubMed] [Google Scholar]
- 39.Sach TH, Whynes DK. Men and women: beliefs about cancer and about screening. BMC Public Health. 2009;9:431. doi: 10.1186/1471-2458-9-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Timmermans DR, Ockhuysen-Vermey CF, Henneman L. Presenting health risk information in different formats: the effect on participants' cognitive and emotional evaluation and decisions. Patient Educ Couns. 2008;73:443–447. doi: 10.1016/j.pec.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Marma AK, Lloyd-Jones DM. Systematic examination of the updated Framingham heart study general cardiovascular risk profile. Circulation. 2009;120:384–390. doi: 10.1161/CIRCULATIONAHA.108.835470. [DOI] [PubMed] [Google Scholar]
- 42.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 43.Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91:1829–1846. doi: 10.1093/jnci/91.21.1829. [DOI] [PubMed] [Google Scholar]
- 44.Schonfeld SJ, Pee D, Greenlee RT, et al. Effect of changing breast cancer incidence rates on the calibration of the Gail model. J Clin Oncol. 2010;28:2411–2417. doi: 10.1200/JCO.2009.25.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 46.Thun M, Jemal A, Desantis C, Blackard B, Ward E. An overview of the cancer burden for primary care physicians. Prim Care. 2009;36:439–454. doi: 10.1016/j.pop.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Taylor DH, Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. Am J Public Health. 2002;92:990–996. doi: 10.2105/ajph.92.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008;299:2037–2047. doi: 10.1001/jama.299.17.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bosetti C, Gallus S, Peto R, et al. Tobacco smoking, smoking cessation, and cumulative risk of upper aerodigestive tract cancers. Am J Epidemiol. 2008;167:468–473. doi: 10.1093/aje/kwm318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.