Abstract
Adiponectin has been receiving a great deal of attention due to its potential therapeutic use for metabolic and cardiovascular disorders. Adiponectin expression levels and multimerization are down-regulated in obesity and up-regulated by insulin sensitizers such as thiazolidinediones (TZDs), metformin, sulfonylurea and resveratrol (RSV). The precise mechanisms underlying adiponectin up- and down-regulation remain largely unknown, but recent studies indicate that the cellular and plasma levels of adiponectin could be regulated at both transcriptional and post-transcriptional levels. At the post-translational level, TZDs and resveratrol promote adiponectin levels and multimerization via up-regulation of disulfidebond-A oxidoreductase-like protein (DsbA-L). Adiponectin levels are also stimulated by FOXO1 and AMP-activated protein kinase (AMPK), and are suppressed by PKA or silencing mediator of retinoid andthyroid hormone receptors (SMRT). Since multimerization is important not only for adiponectin function but also for stability, increasing adiponectin multimerization has become a promising drug target for the treatment of metabolic diseases and other related disorders.
Keywords: Adiponectin, TZDs, Resveratrol, Metformin, DsbA-L
1. Introduction
Adiponectin, a 30 KD adipokine predominantly secreted from adipose tissue, exerts multiple protective properties against obesity[1–4], insulin resistance [5,6], inflammation [7–9], and cardiovascular diseases [10–12]. Adiponectin exists in cells and in the plasma in three major forms: trimers, hexamers, and the high-molecular-weight (HMW) forms, and the HMW form of adiponectin has been shown to be the most bioactive with respect to insulin action[4,13]. Reduction of the HMW form, rather than the total levels of adiponectin, has been shown to be associated with various metabolic disease states [14,15], suggesting that enhancing adiponectin multimerization could be an effective approach for the treatment of insulin resistance and related metabolic diseases.
Adiponectin expression, multimerization and secretion are increased by agonists of the nuclear receptor peroxisome proliferator-activated receptors γ (PPARγ) such as thiazolidinediones (TZDs) [15–18] and TZD structurally related compounds such as vitamin E vitamers α- and γ-tocopherol [19]. Adiponectin multimerization and cellular levels are also induced by MRL24, a PPARγ ligand that has been shown to protect adiponectin from obesity-induced down-regulation [20]. In addition to these PPARγ activators, several other small molecules such as resveratrol (RSV), a stilbenoid natural compound with an anti-diabetic property[21–23], and metformin [24] are also reported to stimulate adiponectin expression and secretion.
In this review, we have summarized our current understanding on the mechanisms by which the above-mentioned PPARγ activators and small molecules that promote adiponectin expression levels and multimerization. We will also discuss how phosphorylation and interaction with other nuclear receptors (NRs) positively or negatively regulate PPARγ activity and subsequent adiponectin expression.
2. Transcriptional and post-transcriptional regulation of adiponectin by PPARγ
The stimulatory effect of TZDs on adiponectin cellular levels[15–17] and multimerization [25,26] are well established. One possible mechanism underlying the promoting effect of TZDs is to promote PPARγ binding to a putative PPARγ-responsive element (PPRE) on the adiponectin gene promoter, thus leading to increased adiponectin gene transcription [15]. Adiponectin transcription is also stimulated by TZD structurally relevant compounds such as vitamin E vitamers, α- and γ-tocopherol [27], as well as PPARγ ligand anandamide [28]. However, the underlying mechanisms by which adiponectin gene is stimulated by these molecules remain to be further determined.
In addition to promoting adiponectin gene transcription, some recent studies suggest that activation of PPARγ can up-regulate adiponectin levels and secretion via a post-translational mechanism. Several recent studies indicate that activation of PPARγ induces the transcription of genes coding for proteins in the endoplasmic reticulum (ER) involved in the post-translational process such as adiponectin multimerization (Fig. 1) [18,29,30]. It has been shown that treating 3T3-L1 adipocytes with troglitazone had little effect on the mRNA levels of adiponectin [29]. On the other hand, this PPARγ agonist significantly enhanced the cellular levels of ER oxidoreductase Ero1-Lα, leading to increased secretion of the HMW complex of adiponectin [29]. Adiponectin levels and multimerization are also stimulated by a different TZD compound, rosiglitazone [18]. Interestingly, the promoting effects of rosiglitazone on adiponectin multimerization and cellular levels are greatly reduced in 3T3-L1 adipocytes in which the expression levels of disulfide-bond A oxidoreductase-like protein (DsbA-L) are suppressed by siRNA. In addition, overexpression of DsbA-L enhances adiponectin multimerization [18]. Rosiglitazone markedly repressed the transcription of another ER chaperone, ERp44, leading to decreased retention of adiponectin in the ER and elevated assembly and secretion of the HMW adiponectin complexes [30,31]. Taken together, these findings demonstrate that activation of PPARγ could enhance adiponectin levels and multimerization via post-transcription-dependent mechanisms involving ER chaperons such as Ero1-Lα, DsbA-L or ERp44 (Fig. 1) [18,29,30].
Fig. 1.
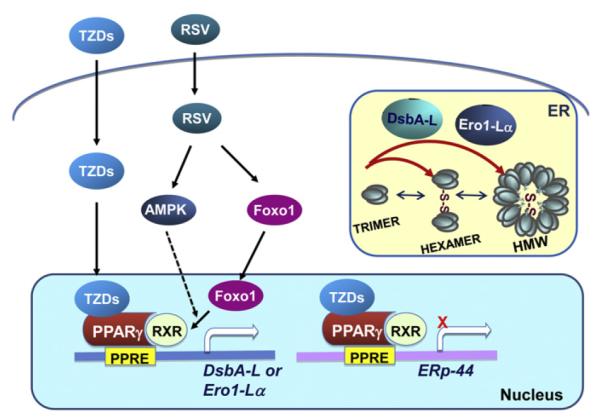
TZDs and resveratrol promote adiponectin multimerization through regulation of ER chaperones. TZD-activated PPARγ binds to the promoter of DsbA-L, Ero1-Lα or ERp44, leading to enhanced transcriptions of DsbA-L and Ero1-Lα, but repression of ERp44 transcription. Elevated cellular levels of DsbA-L and Ero1-Lα promote adiponectin multimerization in the ER. Resveratrol enhances adiponectin production and secretion mainly through increasing the expression levels of DsbA-L, which leads to elevated adiponectin multimerization and stability. Activation of AMPK or Foxo1 mediates the stimulatory effect of resveratrol on DsbA-L expression, but the underlying mechanism remains to be established.
3. Up- and down-regulation of adiponectin levels by targeting PPARγ
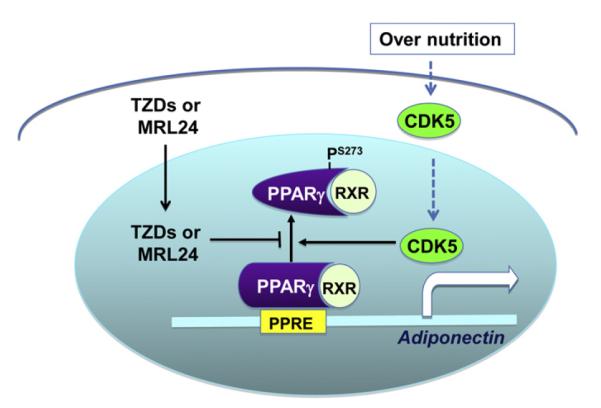
While it is well established that adiponectin is regulated by PPARγ, much less is known on the signal pathways modulating PPARγ activity involved in the regulation of adiponectin expression, multimerization, and secretion. PPARγ has recently been found to undergo obesity-induced and protein kinase cdk5-mediated phosphorylation at Ser273 [20] (Fig. 2). Interestingly, this phosphorylation neither activates nor suppresses the adipogenic capacity of PPARγ, but instead mediates obesity-induced down-regulation of a large number of genes including adiponectin in white adipose tissue [20]. The obesity-induced and cdk5-mediated PPARγ phosphorylation and adiponectin down-regulation in vivo is prevented by treating mice with TZDs or the PPARγ ligand MRL24, suggesting that inhibition of cdk5-meidated phosphorylation could be a mechanism by which TZDs enhance PPARγ action [20]. PPARγ has also been shown to be phosphorylated by ERK1/2 (extracellular signal–regulated kinases 1 and 2) at Ser82 [32] and Ser112 [33]. Phosphorylation at these sites has been shown to inhibit PPARγ transcriptional activity, but whether the phosphorylation has any effect on adiponectin gene expression remains to be clarified [32,34,35].
Fig. 2.
PPARγ transcriptional activity is regulated by phosphorylation. High fat diet induces CDK5 activation in mouse white fat tissue, leading to phosphorylation of PPARγ at Ser273. Phosphorylation at this site reduces PPARγ transcriptional activity towards specific genes such as adiponectin. TZDs or MRL24 protects obesity-induced adiponectin down-regulation by suppressing CDK5-meidated PPARγ phosphorylation.
In addition to being regulated by phosphorylation, PPARγ activity is also modulated by nuclear factors such as retinoid acid receptors (RARs) and silencing mediator of retinoid and thyroid hormone receptors (SMRT) (Fig. 3). Retinoid X receptor (RXR) and retinoid acid receptor (RAR) play opposite roles in term of regulating PPARγ activity. In response to PPARγ agonist stimulation, RXR dimerizes with PPARγ to form a PPARγ/RXR heterodimer, which directly binds to the PPARγ-responsive element (PPRE) (–273/–285) in the adiponectin promoter to facilitate adiponectin gene transcription [15]. On the other hand, the transcriptional activity of PPARγ is negatively regulated by RARs [36], which are members of the ligand-dependent nuclear receptor families that compete with PPARγ in binding to the same heterodimer partner RXR. The PPARγ/RXR-stimulated adiponectin gene expression and adipogenesis are suppressed in mice by in vivo administration of retinaldehyde, a retinoic acid precursor derived from vitamin A that activates RAR/RXR signaling [37]. Adiponectin gene expression is also regulated by silencing mediator of retinoid and thyroid hormone receptors (SMRT), a nuclear corepressor that interacts with a subset of nuclear receptors (NRs), including RAR and PPARγ. The interaction, which is mediated via the receptor interacting domain 1 or 2 (RID1/2) in SMRT, represses the transcriptional activity of RAR or PPARγ, respectively [38].Knock-in the RID1 mutated form of SMRT in mice led to reduced PPARγ activity, accompanied with decreased adiponectin levels and enhanced diet-induced obesity [38] [39]. These findings suggest that retinoid acid may regulate adiponectin expression modulating the PPARγ/RXR complex formation.
Fig. 3.
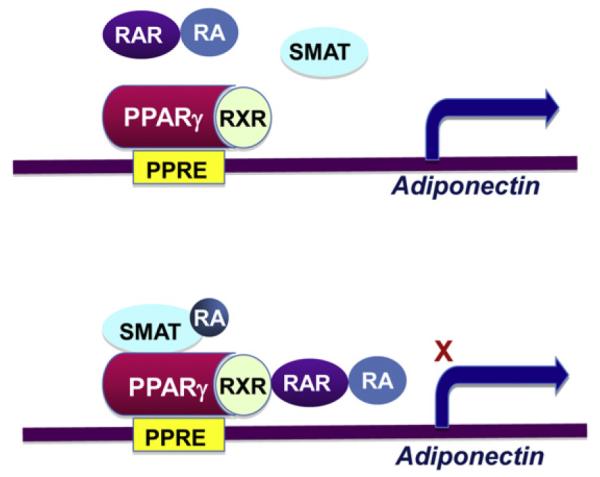
SMRT acts as a corepressor to regulate PPARγ activity and adiponectin transcription. The ligand-dependent nuclear corepressor SMRT interacts with RAR and PPARγ through receptor interacting domain 1 and 2 (RID1/2), respectively. The interaction of SMRT represses the transcriptional activity of RAR and PPARγ. Retinoid and thyroid hormone deficiency leads to dissociation of SMRT from PPARγ and subsequently activation of PPARγ and enhanced adiponectin transcription.
4. Regulation of PPARγ activity by small molecules
A number of small native or chemically synthesized compounds, including sulfonylurea (SU), a class of oral hypoglycemic agents used in treating type 2 diabetes [40], resveratrol, a stilbenoid [41], metformin, an oral anti-diabetic drug in the biguanide class [42], cilostazol, a potent phosphodiesterase type III inhibitor [43], and puerarin, a major isoflavone glycoside from Kudzu root [44], have been shown to enhance adiponectin production. Cilostazol and puerarin activate PPARγ transcription, which may provide a mechanism to up-regulate adiponectin production [43,44]. SU, resveratrol and metformin, on the other hand, appear to promote adiponectin expression and multimerization through PPARγ-independent mechanisms.
SU agents are the most widely used oral hypoglycemic drugs that stimulate insulin secretion primarily by binding to the SU receptor on the plasma membrane of pancreatic β-cells. However, some studies have suggested that SU may also improve insulin resistance and its related disorders by up-regulation of adiponectin [40,45,46]. Two SU agents, glimepiride and glibenclamide, have been found to exert partial PPARγ agonist activity and potentiate PPARγ-mediated increase in adiponectin production [47]. In addition to a direct binding to PPARγ, these two SUs also recruit the PPARγ co-activator DRIP205 and subsequently dissociate PPARγ co-repressors such as the nuclear receptor corepressor and SMRT. There is also data showing that SUs could enhance adiponectin expression and insulin sensitivity through inhibiting protein kinase A (PKA) via a PPARγ-independent mechanism [48]. Treating adipocytes with the PKA-selective activator forskolin significantly reduced adiponectin expression and secretion in adipocytes [48]. Additionally, Rp-cAMP, a diastereomer of cAMP that potently and selectively inhibits PKA activity by competitively binding to the regulatory subunit of PKA, further potentiated the stimulatory effect of rosiglitazone on adiponectin production [48]. One possible mechanism by which PKA inhibits adiponectin gene transcription is to activate CREB, a transcriptional repressor of adiponectin gene expression [49]. Consistent with this, adiponectin mRNA expression is also suppressed via β-adrenergic signaling-mediated activation of PKA [50], which could partly explain the role of β-adrenergic signaling in insulin resistance [51].
Resveratrol, a polyphenol originally found in different plant species such as grapes, has been shown to exert anti-diabetic activity in vitro and in vivo by improving mitochondria function and energy expenditure [23] [21]. Numerous studies have demonstrated that resveratrol enhances adiponectin levels, which could be one of the potential mechanisms by which resveratrol, improves insulin sensitivity. Resveratrol enhances adiponectin expression and improves insulin sensitivity in adipocytes and this effect is mediated by inhibition of inflammation [52] [53]. We have recently demonstrated that resveratrol enhances adiponectin cellular levels and multimerization by up-regulation of DsbA-L, which is mediated by the FOXO1 and AMPK signaling pathways [41]. Consistent with this finding, both FOXO1 and adiponectin mRNA expression are up-regulated by resveratrol treatment in human visceral adipocytes [54]. However, we found that while resveratrol treatment significantly enhanced the expression levels of DsbA-L, it had little effect on the mRNA levels of adiponectin [41] (Fig. 1). The exact reason for this discrepancy remains unknown but FOXO1 has been found to suppress PPARγ gene expression [41]. Since PPARγ positively regulates adiponectin gene expression and secretion, these findings suggest that the effects of FOXO1 on adiponectin biosynthesis may depend on cell content and upstream signal events.
Metformin is the first-line drug of choice for the treatment of 2 diabetes due to its potent anti-gluconeogenesis action and insulin sensitizing property. However, the mechanisms underlying metformin action remain to be established. Metformin activates AMP-dependent kinase (AMPK) and this property could play a key role in the inhibition of liver glucose production and promotion of glucose uptake and fatty acid oxidation in skeletal muscle [55,56]. Since AMPK plays an important role in resveratrol-stimulated adiponectin multimerization, it is conceivable that metformin may also promote adiponectin production by activation of AMPK. Consistent with this, six months of metformin therapy ameliorates insulin action in type 2 diabetes patients and leads to an increase of plasma adiponectin levels [42]. In addition, metformin has been found to up-regulate adiponectin expression and secretion from human subcutaneous adipose tissue (SAT) but not visceral adipose tissue (VAT), which provides evidence that metformin action on adiponectin production may be tissue specific [57]. However, there are studies showing that metformin has no effect on intracellular levels and secretion of adiponectin [58,59]. Further studies will be needed to address these controversies.
5. Summary
PPARγ is the major player regulating adiponectin expression, assembly and secretion. Two mechanisms, phosphorylation and interaction with other nuclear receptors such as SMRT and RARs, are shown to modulate PPARγ activity and its roles in regulating adiponectin gene activation. The cellular levels and multimerization of adiponectin could also be regulated by post-translational mechanisms via PPARγ-independent mechanisms, involving the FOXO1 and AMPK signaling pathways and DsbA-L. Understanding the mechanisms regulating adiponectin multimerization and cellular levels and identification of novel molecules enhancing adiponectin expression and multimerization could be an effective therapeutic approach for the treatment of insulin resistance and its associated metabolic and cardiovascular diseases.
References
- [1].Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- [2].Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- [3].Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem. Biophys. Res. Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- [4].Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J. Biol. Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- [5].Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C., Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc. Natl. Acad. Sci. U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Haluzik M, Parizkova J, Haluzik MM. Adiponectin and its role in the obesity-induced insulin resistance and related complications. Physiol. Res. 2004;53:123–129. [PubMed] [Google Scholar]
- [7].Gil-Campos M, Canete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin. Nutr. 2004;23:963–974. doi: 10.1016/j.clnu.2004.04.010. [DOI] [PubMed] [Google Scholar]
- [8].Hoffstedt J, Arvidsson E, Sjolin E, Wahlen K, Arner P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2004;89:1391–1396. doi: 10.1210/jc.2003-031458. [DOI] [PubMed] [Google Scholar]
- [9].Cascon A, Ruiz-Llorente S, Cebrian A, Telleria D, Rivero JC, Diez JJ, Lopez-Ibarra PJ, Jaunsolo MA, Benitez J, Robledo M. Identification of novel SDHD mutations in patients with phaeochromocytoma and/or paraganglioma. Eur. J. Hum. Genet. 2002;10:457–461. doi: 10.1038/sj.ejhg.5200829. [DOI] [PubMed] [Google Scholar]
- [10].Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J. Clin. Endocrinol. Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- [11].Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat. Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocytesecreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J. Biol. Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- [14].Fisher FF, Trujillo ME, Hanif W, Barnett AH, McTernan PG, Scherer PE, Kumar S. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48:1084–1087. doi: 10.1007/s00125-005-1758-7. [DOI] [PubMed] [Google Scholar]
- [15].Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I. Induction of adiponectin, a fat-derived antidiabetic and anti-atherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- [16].Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- [17].Kanatani Y, Usui I, Ishizuka K, Bukhari A, Fujisaka S, Urakaze M, Haruta T, Kishimoto T, Naka T, Kobayashi M. Effects of pioglitazone on suppressor of cytokine signaling 3 expression: potential mechanisms for its effects on insulin sensitivity and adiponectin expression. Diabetes. 2007;56:795–803. doi: 10.2337/db06-1039. [DOI] [PubMed] [Google Scholar]
- [18].Liu M, Zhou L, Xu A, Lam KS, Wetzel MD, Xiang R, Zhang J, Xin X, Dong LQ, Liu F. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc. Natl. Acad. Sci. U S A. 2008;105:18302–18307. doi: 10.1073/pnas.0806341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gray B, Swick J, Ronnenberg AG. Vitamin E and adiponectin: proposed mechanism for vitamin E-induced improvement in insulin sensitivity. Nutr. Rev. 2011;69:155–161. doi: 10.1111/j.1753-4887.2011.00377.x. [DOI] [PubMed] [Google Scholar]
- [20].Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, Griffin PR, Spiegelman BM. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- [24].Zulian A, Cancello R, Girola A, Gilardini L, Alberti L, Croci M, Micheletto G, Danelli P, Invitti C. In vitro and in vivo effects of metformin on human adipose tissue adiponectin. Obes. Facts. 2011;4:27–33. doi: 10.1159/000324582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding YY, Russell RG, Lindemann D, Hartley A, Baker GR, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer PE. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- [26].Phillips SA, Kung J, Ciaraldi TP, Choe C, Christiansen L, Mudaliar S, Henry RR. Selective regulation of cellular and secreted multimeric adiponectin by antidiabetic therapies in humans. Am. J. Physiol. Endocrinol. Metab. 2009;297:E767–E773. doi: 10.1152/ajpendo.00378.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Landrier JF, Gouranton E, El Yazidi C, Malezet C, Balaguer P, Borel P, Amiot MJ. Adiponectin expression is induced by vitamin E via a peroxisome proliferator-activated receptor gamma-dependent mechanism. Endocrinology. 2009;150:5318–5325. doi: 10.1210/en.2009-0506. [DOI] [PubMed] [Google Scholar]
- [28].Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur. J. Pharmacol. 2005;517:174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- [29].Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol. Cell Biol. 2007;27:4698–4707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Long Q, Lei T, Feng B, Yin C, Jin D, Wu Y, Zhu X, Chen X, Gan L, Yang Z. Peroxisome proliferator-activated receptor-gamma increases adiponectin secretion via transcriptional repression of endoplasmic reticulum chaperone protein ERp44. Endocrinology. 2010;151:3195–3203. doi: 10.1210/en.2009-1501. [DOI] [PubMed] [Google Scholar]
- [31].Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, Scherer PE. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol. Cell Biol. 2007;27:3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J. Biol. Chem. 1997;272:10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- [33].Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- [34].Hsi LC, Wilson L, Nixon J, Eling TE. 15-lipoxygenase-1 metabolites downregulate peroxisome proliferator-activated receptor gamma via the MAPK signaling pathway. J. Biol. Chem. 2001;276:34545–34552. doi: 10.1074/jbc.M100280200. [DOI] [PubMed] [Google Scholar]
- [35].Chan GK, Deckelbaum RA, Bolivar I, Goltzman D, Karaplis AC. PTHrP inhibits adipocyte differentiation by down-regulating PPAR gamma activity via a MAPK-dependent pathway. Endocrinology. 2001;142:4900–4909. doi: 10.1210/endo.142.11.8515. [DOI] [PubMed] [Google Scholar]
- [36].DiRenzo J, Soderstrom M, Kurokawa R, Ogliastro MH, Ricote M, Ingrey S, Horlein A, Rosenfeld MG, Glass CK. Peroxisome proliferator-activated receptors and retinoic acid receptors differentially control the interactions of retinoid X receptor heterodimers with ligands, coactivators, and corepressors. Mol. Cell Biol. 1997;17:2166–2176. doi: 10.1128/mcb.17.4.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, Duester G, Plutzky J. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat. Med. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fang S, Suh JM, Atkins AR, Hong SH, Leblanc M, Nofsinger RR, Yu RT, Downes M, Evans RM. Corepressor SMRT promotes oxidative phosphorylation in adipose tissue and protects against diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. U S A. 2011;108:3412–3417. doi: 10.1073/pnas.1017707108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Reilly SM, Bhargava P, Liu S, Gangl MR, Gorgun C, Nofsinger RR, Evans RM, Qi L, Hu FB, Lee CH. Nuclear receptor corepressor SMRT regulates mitochondrial oxidative metabolism and mediates aging-related metabolic deterioration. Cell Metab. 2010;12:643–653. doi: 10.1016/j.cmet.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Koshiba K, Nomura M, Nakaya Y, Ito S. Efficacy of glimepiride on insulin resistance, adipocytokines, and atherosclerosis. J. Med. Invest. 2006;53:87–94. doi: 10.2152/jmi.53.87. [DOI] [PubMed] [Google Scholar]
- [41].Wang A, Liu M, Liu X, Dong LQ, Glickman RD, Slaga TJ, Zhou Z, Liu F. Up-regulation of adiponectin by resveratrol: the essential roles of the Akt/FOXO1 and AMP-activated protein kinase signaling pathways and DsbA-L. J. Biol. Chem. 2011;286:60–66. doi: 10.1074/jbc.M110.188144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Adamia N, Virsaladze D, Charkviani N, Skhirtladze M, Khutsishvili M. Effect of metformin therapy on plasma adiponectin and leptin levels in obese and insulin resistant postmenopausal females with type 2 diabetes. Georgian Med. News. 2007:52–55. [PubMed] [Google Scholar]
- [43].Park SY, Shin HK, Lee JH, Kim CD, Lee WS, Rhim BY, Hong KW. Cilostazol ameliorates metabolic abnormalities with suppression of proinflammatory markers in a db/db mouse model of type 2 diabetes via activation of peroxisome proliferator-activated receptor gamma transcription. J. Pharmacol. Exp. Ther. 2009;329:571–579. doi: 10.1124/jpet.108.146456. [DOI] [PubMed] [Google Scholar]
- [44].Lee OH, Seo DH, Park CS, Kim YC. Puerarin enhances adipocyte differentiation, adiponectin expression, and antioxidant response in 3T3-L1 cells. Biofactors. 2010;36:459–467. doi: 10.1002/biof.119. [DOI] [PubMed] [Google Scholar]
- [45].Tsunekawa T, Hayashi T, Suzuki Y, Matsui-Hirai H, Kano H, Fukatsu A, Nomura N, Miyazaki A, Iguchi A. Plasma adiponectin plays an important role in improving insulin resistance with glimepiride in elderly type 2 diabetic subjects. Diabetes Care. 2003;26:285–289. doi: 10.2337/diacare.26.2.285. [DOI] [PubMed] [Google Scholar]
- [46].Araki T, Emoto M, Konishi T, Ikuno Y, Lee E, Teramura M, Motoyama K, Yokoyama H, Mori K, Koyama H, Shoji T, Nishizawa Y. Glimepiride increases high-density lipoprotein cholesterol via increasing adiponectin levels in type 2 diabetes mellitus. Metabolism. 2009;58:143–148. doi: 10.1016/j.metabol.2008.09.006. [DOI] [PubMed] [Google Scholar]
- [47].Fukuen S, Iwaki M, Yasui A, Makishima M, Matsuda M, Shimomura I. Sulfonylurea agents exhibit peroxisome proliferator-activated receptor gamma agonistic activity. J. Biol. Chem. 2005;280:23653–23659. doi: 10.1074/jbc.M412113200. [DOI] [PubMed] [Google Scholar]
- [48].Kanda Y, Matsuda M, Tawaramoto K, Kawasaki F, Hashiramoto M, Matsuki M, Kaku K. Effects of sulfonylurea drugs on adiponectin production from 3T3-L1 adipocytes: implication of different mechanism from pioglitazone. Diabetes Res. Clin. Pract. 2008;81:13–18. doi: 10.1016/j.diabres.2008.01.031. [DOI] [PubMed] [Google Scholar]
- [49].Qi L, Saberi M, Zmuda E, Wang Y, Altarejos J, Zhang X, Dentin R, Hedrick S, Bandyopadhyay G, Hai T, Olefsky J, Montminy M. Adipocyte CREB promotes insulin resistance in obesity. Cell Metab. 2009;9:277–286. doi: 10.1016/j.cmet.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Adiponectin gene expression is inhibited by beta-adrenergic stimulation via protein kinase A in 3T3-L1 adipocytes. FEBS Lett. 2001;507:142–146. doi: 10.1016/s0014-5793(01)02960-x. [DOI] [PubMed] [Google Scholar]
- [51].Kirsch DM, Baumgarten M, Deufel T, Rinninger F, Kemmler W, Haring HU. Catecholamine-induced insulin resistance of glucose transport in isolated rat adipocytes. Biochem. J. 1983;216:737–745. doi: 10.1042/bj2160737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ahn J, Lee H, Kim S, Ha T. Resveratrol inhibits TNF-alpha-induced changes of adipokines in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2007;364:972–977. doi: 10.1016/j.bbrc.2007.10.109. [DOI] [PubMed] [Google Scholar]
- [53].Kang L, Heng W, Yuan A, Baolin L, Fang H. Resveratrol modulates adipokine expression and improves insulin sensitivity in adipocytes: relative to inhibition of inflammatory responses. Biochimie. 2010;92:789–796. doi: 10.1016/j.biochi.2010.02.024. [DOI] [PubMed] [Google Scholar]
- [54].Costa Cdos S, Rohden F, Hammes TO, Margis R, Bortolotto JW, Padoin AV, Mottin CC, Guaragna RM. Resveratrol upregulated SIRT1, FOXO1, and adiponectin and downregulated PPARgamma1-3 mRNA expression in human visceral adipocytes. Obes. Surg. 2010;21:356–361. doi: 10.1007/s11695-010-0251-7. [DOI] [PubMed] [Google Scholar]
- [55].Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kim YD, Park KG, Lee YS, Park YY, Kim DK, Nedumaran B, Jang WG, Cho WJ, Ha J, Lee IK, Lee CH, Choi HS. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes. 2008;57:306–314. doi: 10.2337/db07-0381. [DOI] [PubMed] [Google Scholar]
- [57].Asensio-Lopez MC, Lax A, Pascual-Figal DA, Valdes M, Sanchez-Mas J. Metformin protects against doxorubicin-induced cardiotoxicity: involvement of the adiponectin cardiac system. Free Radic. Biol. Med. 2011;51:1861–1871. doi: 10.1016/j.freeradbiomed.2011.08.015. [DOI] [PubMed] [Google Scholar]
- [58].Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169–2176. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- [59].Fujita H, Fujishima H, Koshimura J, Hosoba M, Yoshioka N, Shimotomai T, Morii T, Narita T, Kakei M, Ito S. Effects of antidiabetic treatment with metformin and insulin on serum and adipose tissue adiponectin levels in db/db mice. Endocr. J. 2005;52:427–433. doi: 10.1507/endocrj.52.427. [DOI] [PubMed] [Google Scholar]