Abstract
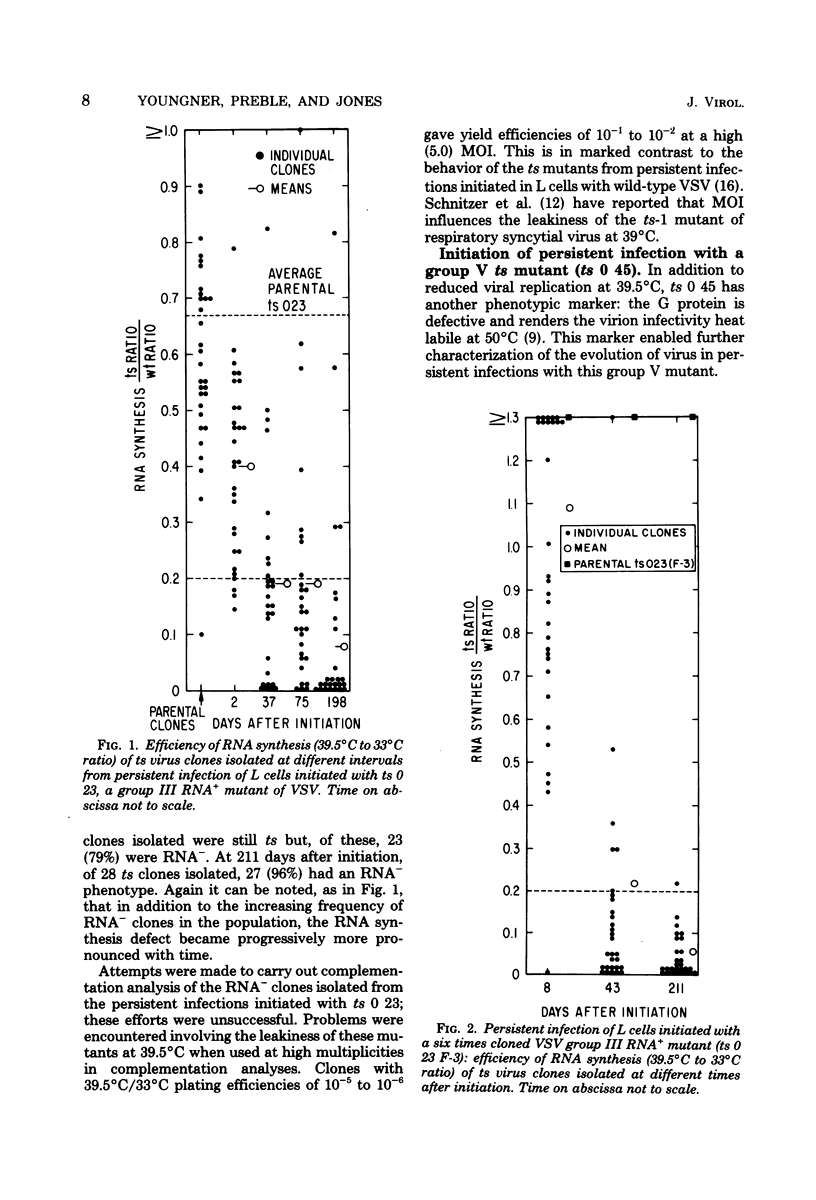
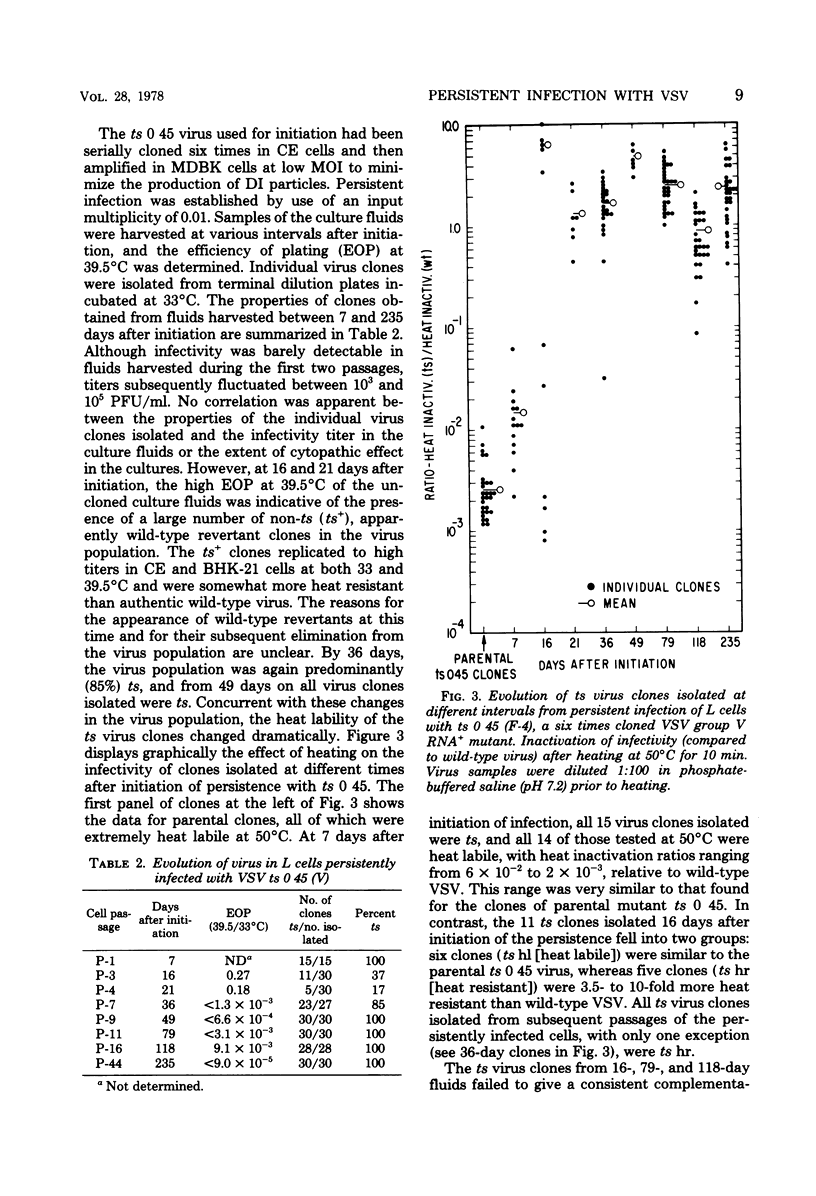
A previous report (Youngner et al., J. Virol. 19:90-101, 1976) documented that noncytocidal persistent infection can be established with wild-type vesicular stomatitis virus (VSV) in mouse L cells at 37°C and that a rapid selection of RNA−, group I temperature-sensitive (ts) mutants consistently occurs in this system. To assess the selective advantage of the RNA−ts phenotype, evolution of the virus population was studied in persistent infections initiated in L cells by use of VSV ts 0 23 and ts 0 45, RNA+ mutants belonging to complementation groups III and V. In L cells persistently infected with ts 0 23, the ts RNA+ virus population was replaced gradually by viruses which had a ts RNA− phenotype. VSV ts 0 45 (V) has another marker in addition to reduced virus yield at 39.5°C: a defective protein (G) which renders virion infectivity heat labile at 50°C. Persistent infections initiated with this virus (ts, heat labile, RNA+) evolved into a virus population which was ts, heat resistant, and RNA−. These findings suggest that the ts phenotype itself is not sufficient to stabilize the VSV population in persistently infected L cells and also indicate that the ts RNA− phenotype may have a unique selective advantage in this system. In addition to the selection of ts RNA− mutants, other mechanisms which also might operate in the maintenance of persistent VSV infections of L cells were explored. Whereas defective-interfering particles did not seem to mediate the carrier state, evidence was obtained that interferon may play a role in the regulation of persistent infections of L cells with VSV.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLETT A. J., COOPER P. D. Some properties of the transmissible interfering component of vesicular stomatitis virus preparations. J Gen Microbiol. 1959 Dec;21:498–509. doi: 10.1099/00221287-21-3-498. [DOI] [PubMed] [Google Scholar]
- Flamand A. Etude génétique du virus de la stomatite vésiculaire: classement de mutants thermosensibles spontanés en groupes de complémentation. J Gen Virol. 1970 Sep;8(3):187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Breindl M. Factors involved in the generation and replication of rhabdovirus defective T particles. J Virol. 1976 Mar;17(3):805–815. doi: 10.1128/jvi.17.3.805-815.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Purification of defective interfering T particles of vesicular stomatitis and rabies viruses generated in vivo in brains of newborn mice. Virology. 1975 Oct;67(2):438–449. doi: 10.1016/0042-6822(75)90445-6. [DOI] [PubMed] [Google Scholar]
- Inglot A. D., Albin M., Chudzio T. Persistent infection of mouse cells with Sindbis virus: role of virulence of strains, auto-interfering particles and interferon. J Gen Virol. 1973 Jul;20(1):105–110. doi: 10.1099/0022-1317-20-1-105. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y. Studies of L cells persistently infected with VSV: factors involved in the regulation of persistent infection. J Gen Virol. 1977 May;35(2):265–279. doi: 10.1099/0022-1317-35-2-265. [DOI] [PubMed] [Google Scholar]
- Potter K. N., Stewart R. B. Comparison of vesicular stomatitis virus defective interfering particle synthesis in chick embryo and L cells. Can J Microbiol. 1976 Oct;22(10):1458–1463. doi: 10.1139/m76-216. [DOI] [PubMed] [Google Scholar]
- Ramseur J. M., Friedman R. M. Prolonged infection of L cells with vesicular stomatitis virus. Defective interfering forms and temperature-sensitive mutants as factors in the infection. Virology. 1978 Mar;85(1):253–261. doi: 10.1016/0042-6822(78)90429-4. [DOI] [PubMed] [Google Scholar]
- Schnitzer T. J., Richardson L. S., Chanock R. M. Growth and genetic stability of the ts-1 mutant of respiratory syncytial virus at restrictive temperatures. J Virol. 1976 Feb;17(2):431–438. doi: 10.1128/jvi.17.2.431-438.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekellick M. J., Marcus P. I. Persistent infection. I Interferon-inducing defective-interfering particles as mediators of cell sparing: possible role in persistent infection by vesicular stomatitis virus. Virology. 1978 Mar;85(1):175–186. doi: 10.1016/0042-6822(78)90422-1. [DOI] [PubMed] [Google Scholar]
- Stanwick T. L., Hallum J. V. Role of interferon in six cell lines persistently infected with rubella virus. Infect Immun. 1974 Oct;10(4):810–815. doi: 10.1128/iai.10.4.810-815.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Dubovi E. J., Quagliana D. O., Kelly M., Preble O. T. Role of temperature-sensitive mutants in persistent infections initiated with vesicular stomatitis virus. J Virol. 1976 Jul;19(1):90–101. doi: 10.1128/jvi.19.1.90-101.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Quagliana D. O. Temperature-sensitive mutants isolated from hamster and canine cell lines persistently infected with Newcastle disease virus. J Virol. 1975 Nov;16(5):1332–1336. doi: 10.1128/jvi.16.5.1332-1336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Scott A. W., Hallum J. V., Stinebring W. R. Interferon production by inactivated Newcastle disease virus in cell cultures and in mice. J Bacteriol. 1966 Oct;92(4):862–868. doi: 10.1128/jb.92.4.862-868.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Meulen V., Martin S. J. Genesis and maintenance of a persistent infection by canine distemper virus. J Gen Virol. 1976 Sep;32(3):431–440. doi: 10.1099/0022-1317-32-3-431. [DOI] [PubMed] [Google Scholar]


