Abstract
Objective
Many studies suggest optimal sleep duration for survival is seven to eight hours/night. We report the gender-specific independent association of all-cause mortality with nighttime sleep and daytime nap duration in older adults who were followed for up to 19 years.
Methods
Between 1984 and 1987, 2,001 community-dwelling, mostly retired, adults (1,112 women), age 60–96 years, answered questions about health, mood, medications, life-style, daytime napping, and nighttime sleep duration. Vital status was confirmed for 96% through July 2001.
Results
At baseline, men reported significantly longer nighttime sleep and daytime napping than women. In both men and women, nighttime sleep <six hours was associated with depressed mood and sleep-related medication, and ≥nine hours was associated with more alcohol consumption. Napping ≥30 minutes was associated with prevalent depressed mood, coronary heart disease, and cancer. Of the group, 61% died over the next 19 yrs, at an average age of 85.6 years. Mortality risk was lowest among those sleeping 7–7.9 hours/night in both men and women. Multiple-adjusted analyses showed that increased mortality was associated with nighttime sleep ≥nine hours in women (HR 1.51: 95% CI = 1.05–2.18), and with daytime napping ≥30 minutes in men (HR 1.28: 95% CI, 1.00–1.64).
Conclusions
Mechanisms for these differences are unknown.
Keywords: elderly, mortality, nap duration, prospective study, sleep duration
INTRODUCTION
Epidemiologic studies have found that a usual self-reported nighttime sleep duration of approximately 7–8 hours is associated with a lower mortality than sleeping fewer or more hours [1–9]. The largest study of self-reported sleep and mortality, the Cancer Prevention Study II, followed 1.1 million men and women aged 30 to 102 for 6 years. The optimal nighttime sleep duration for survival was 7 hours with those sleeping >8 hours or <6 hours experiencing significantly increased mortality [6]. In the Nurses’ Health Study of 82,969 women aged 30 to 55 years, the 14-year mortality was lowest among women self-reporting sleeping 7 hours, and the highest mortality was in those sleeping <6 or >7 hours; the very large sample allowed authors to distinguish between narrow categories but it is unclear if hours of sleep included naps [1]. Three additional large studies of many thousands of older adults confirmed that both short sleep and long sleep are associated with greater mortality than sleeping about 7–8 hours [7–9]. Two recent meta-analyses confirmed that both short and long sleep durations (hours sleep/night) were associated with increased mortality in most populations [10,11]; neither of these studies considered daytime napping.
A few studies suggest that daytime napping may increase mortality [12–14]. Two studies reported that siestas were associated with a significantly increased mortality in older men and women [12,13]. One study examined gender differences and found that this association was only significant in older men [14]. On the other hand, in a seven-year prospective cohort study of 8101 women aged 69 years and older, women who reported napping daily were 44% more likely to die from any cause (95% CI, 1.23–1.67), 58% more likely to die from cardiovascular causes (95% CI, 1.25–2.00), and 59% more likely to die from noncardiovascular, noncancer causes (95% CI, 1.24–2.03) than women who did not nap daily [9].
The present study of community-dwelling, largely retired, older adults was designed to examine the gender-specific cross-sectional association between reported nighttime sleep and daytime nap durations with all-cause mortality 19-years later, before, and after adjusting for plausible covariates (age, lifestyle, common medical conditions, depressed mood, and sleep-related medications).
METHODS
Participants
Between 1972 and 1974, 82.1% (5,052) of the target population of all community-dwelling residents aged 30 to 79 years (median age 61) from the southern California community of Rancho Bernardo were enrolled in a heart disease risk factor survey. Participants were largely Caucasians of European origin, middle to upper-middle social class, and relatively healthy; all were ambulatory. Between 1984 and 1987, 2,711 of these older adults (nearly 80% of surviving community-dwelling men and women) participated in a follow-up research clinic evaluation. The present analyses included 889 men and 1,112 women aged >60 years who attended this clinic visit and completed a questionnaire about their usual duration of nighttime sleep and daytime sleep (napping).
All participants provided written informed consent. The study protocol was approved by the Human Research Protection Program at the University of California, San Diego.
Clinic Visit Data Collected
Sleep duration was based on two questions: “How many hours do you sleep each night?” and “How many hours do you nap each day?” Based on existing literature [15,16], nighttime sleep responses were grouped into five categories: < 6.0, 6.0–6.9, 7.0–7.9, 8.0–8.9, and ≥ 9.0 hours. Daytime naps were grouped into three categories: zero, < 30, and ≥ 30 minutes.
Covariates were assessed at the same visit using standard questions about medical history, cigarette smoking (never, ever), alcohol consumption (amount [ml] during previous week), regular strenuous exercise (≥ 3 times/week), and current medication use for diabetes, hypertension, coronary heart disease, stroke, cancer, sleep-related medications (sedating antidepressants, antianxiety drugs, and sedative hypnotics), and postmenopausal estrogen (women). Current medication use was validated by examination of pills and prescriptions brought to the clinic for that purpose. Depressed mood was assessed with the Beck Depression Inventory (BDI) [17,18].
Height and weight were measured in participants wearing light clothing and no shoes. BMI was calculated as weight (kg)/height (m2). Systolic and diastolic blood pressures were measured twice in resting seated participants. Morning fasting plasma glucose was measured after a requested 12-hour fast.
Follow-up
Annual mail or telephone follow-up was continued for 19 years through July 2001; vital status was ascertained for 96% of the sample. Date of death was based on death certificates (90%), or notice from a family member or published obituary (10%). Cause of death in the elderly is inaccurate even when it occurs in the hospital. For this reason, and because cutting the pie into smaller pieces would have reduced statistical power, we did not attempt to specify cause of death but instead reported all-cause mortality.
Statistical analysis
Independent sample t-tests were used to compare mean levels of covariates between men and women. Analyses of covariance (ANCOVA) were used to compare mean values of age-adjusted covariates according to night sleep duration using 7–7.9 hours of nighttime sleep as the reference category for mortality analyses, based on published literature showing 7–7.9 hours sleep per night being associated with the lowest mortality rates [1–4]. For nap duration, <30 minutes was used as the reference category, based on reports suggesting that <30-minute napping has beneficial effects [15]. Time-to-event Cox proportional hazards survival models were used to evaluate the sex-specific hazard ratio (HR) for death by each nighttime sleep and daytime nap duration category relative to the reference group, before and after adjustment for covariates. To increase statistical power, we report all-cause mortality.
Three gender-specific models were used. The first model adjusted only for age. The second adjusted for age and each of the covariates that differed by nighttime sleep or daytime napping duration (P < 0.10), including BMI, alcohol consumption, daytime nap duration (when comparing nighttime sleep duration with mortality), nighttime sleep duration (when comparing daytime nap duration with mortality), BDI, physical exercise, education, and smoking history. Additional analyses adjusted for diabetes, hypertension, coronary heart disease, stroke, and cancer. A third model adjusted for all covariates that were significant in the second model plus use of sleep-related medications and use of postmenopausal estrogen.
Data were analyzed using SPSS (version 12.0, SPSS, Inc., Chicago, IL). All P values were based on two-tailed tests of statistical significance (P < 0.05) for hazard ratios; 95% confidence intervals (CIs) are shown.
RESULTS
Baseline characteristics
There were significant differences between men and women for nearly all covariates at baseline, as shown in Table 1. Men were slightly but significantly older (mean age 74.1±7.3 years; range 60–92 years) than women (mean age 73.3±7.1 years; range 60–96 years), were better educated, more likely to be past-smokers, and more likely to have lower depression symptom scores on the BDI; they also consumed more alcohol and reported more physical exercise. Men reported a higher prevalence of diabetes, coronary heart disease, stroke, and cancer and used sleep-related medications less often than women. Mean reported nighttime sleep duration and daytime nap duration were significantly longer in men than women. Napping was reported by 45% (n=400) of men and 36% (n=399) of women, with the same wide range of nap duration (0–240 minutes) for both.
Table 1.
Characteristics of cohort, 1984–1987
| Men (n=889) | Women (n=1112) | P-value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) † | 74.1 (7.3) | 73.3 (7.1) | 0.006 |
| BMI (kg/m2) † | 25.5 (3.3) | 24.1 (3.7) | < 0.001 |
| Alcohol consumption (ml/week) † | 131.7 (149.3) | 81.5 (109.3) | < 0.001 |
| Night-time sleep duration (hours) † | 7.4 (1.2) | 7.1 (1.3) | <0 .001 |
| Nap duration (minutes) † | 18 (30) | 12 (18) | 0.003 |
| Beck Depression Inventory† | 5.8 (4.3) | 6.8 (4.8) | <0 .001 |
| % | % | ||
| Exercise (≥ 3 times/week) § | 84.9 | 78.9 | 0.001 |
| At least some college § | 77.3 | 62.0 | <0 .001 |
| Smoking history§ | 69.7 | 51.5 | <0 .001 |
| Diabetes§ | 13.7 | 8.1 | <0 .001 |
| Hypertension§ | 38.1 | 40.9 | 0.21 |
| Coronary heart disease§ | 13.9 | 6.9 | <0 .001 |
| Stroke§ | 8.6 | 5.0 | 0.004 |
| Cancer§ | 32.5 | 26.2 | 0.002 |
| Medication Use§ | |||
| All sleep-related drugs§ | 9.3 | 12.7 | 0.03 |
| Sedating antidepressants § | 1.6 | 3.1 | 0.03 |
| Antianxiety drugs§ | 6.5 | 8.4 | 0.12 |
| Hypnotics§ | 1.1 | 1.1 | 0.96 |
| All estrogen use§ | - | 30.3 | - |
| Estrogen with progesterone§ | - | 11.3 | - |
| Estrogen without progesterone§ | - | 19.0 | - |
Independent samples t-test
Chi-square test
Table 2a shows the men’s baseline characteristics by nighttime sleep duration; men sleeping <six hours reported the longest daytime nap duration, men sleeping 6–6.9 hours had the highest prevalence of a cancer history and of reported physical exercise, men sleeping 7–7.9 hours and men sleeping 8–8.9 hours or sleeping >nine hours were the most educated. Table 2b shows the data for women: women sleeping ≥nine hours reported the highest prevalence of smoking. Both men and women sleeping <six hours had the highest depression (BDI) score and the highest prevalence of sleep-related medication use; both men and women sleeping ≥nine hours reported the highest alcohol consumption.
Table 2.
| a. Age and age-adjusted mean values of covariates by reported nighttime sleep duration in men | ||||||
|---|---|---|---|---|---|---|
| Night-time sleep duration/day (hours) | ||||||
| <6 hoursa (n=55) |
6–6.9 hoursb (n=137) |
7–7.9 hoursc (n=248) |
8–8.9 hoursd (n=330) |
≥9 hourse (n=119) |
p-value | |
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | ||
| Age (years) † | 75.7 (1.0) | 74.0 (0.6) | 73.4 (0.5) | 73.9 (0.4) | 75.7 (0.7) | 0.03 |
| BMI (kg/m2) ‡ | 26.5 (0.4) | 25.1 (0.3) | 25.3 (0.2) | 25.6 (0.2) | 25.6 (0.3) | 0.10 |
| Alcohol consumption (ml/week) ‡ | 126.3 (19.8) | 103.9 (12.5) | 123.5 (9.3) | 137.7 (8.1) | 166.0 (13.5) | 0.02 |
| Nap duration (minutes) ‡ | 30.6 (4.2) | 24.0 (2.4) | 14.4 (1.8) | 16.8 (1.8) | 18.6 (3.0) | <0.001 |
| Beck Depression Inventory‡ | 8.7 (0.6) | 7.2 (0.4) | 5.4 (0.3) | 5.0 (0.2) | 5.8 (0.4) | <0.001 |
| % | % | % | % | % | ||
| Exercise (≥3 times/week) § | 84.2 | 89.0 | 89.2 | 83.8 | 74.6 | 0.004 |
| Some college§ | 65.9 | 68.6 | 76.3 | 80.7 | 80.4 | 0.01 |
| Smoking history§ | 64.0 | 63.9 | 68.8 | 72.4 | 73.5 | 0.29 |
| Diabetes§ | 19.6 | 13.9 | 12.3 | 14.9 | 10.5 | 0.48 |
| Hypertension§ | 46.9 | 33.1 | 41.5 | 36.2 | 37.7 | 0.30 |
| Coronary heart disease§ | 14.4 | 18.3 | 10.5 | 14.0 | 15.8 | 0.30 |
| Stroke§ | 8.9 | 8.9 | 8.4 | 6.6 | 14.4 | 0.17 |
| Cancer§ | 24.4 | 43.9 | 33.6 | 27.4 | 34.7 | 0.006 |
| Sleep-related medication use§* | 15.0 | 10.5 | 10.0 | 4.2 | 12.2 | 0.009 |
Sleep-related medications: sedating antidepressants, antianxiety drugs, and hypnotics
ANOVA
ANCOVA adjusted for age
Chi-square test
P (for multiple comparison)
in age, a–c: p=0.04, b–e: p=0.049, c–e: p=0.004, d–e: p=0.02
in alcohol consumption, b–d: p=0.02, b–e: p=0.001, c–e: p=0.01
in nap duration, a–c: p<0.001, a–d: p=0.001, a–e: p=0.01, b–c: p=0.002, b–d: p=0.02
in Beck Depression Inventory, a–b: p=0.02, a–c, a–d, a–e, b–c, and b–d: p<0.001, b–e: p=0.01
Table 2.
| b. Age and age-adjusted mean values of covariates by reported nighttime sleep duration in women | ||||||
|---|---|---|---|---|---|---|
| Night-time sleep duration/day (hours) | ||||||
| <6 hoursa (n=115) |
6–6.9 hoursb (n=225) |
7–7.9 hoursc (n=282) |
8–8.9 hoursd (n=382) |
≥9 hourse (n=108) |
p-value | |
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | ||
| Age (years) † | 74.6 (0.7) | 73.6 (0.5) | 72.4 (0.4) | 72.9 (0.4) | 75.6 (0.7) | 0.008 |
| BMI (kg/m2) ‡ | 24.2 (0.3) | 24.5 (0.2) | 24.6 (0.2) | 24.3 (0.2) | 23.6 (0.4) | 0.13 |
| Alcohol consumption (ml/week) ‡ | 67.6 (10.1) | 70.3 (7.2) | 78.4 (6.4) | 88.0 (5.5) | 104.3 (10.4) | 0.03 |
| Nap duration (minutes) ‡ | 18.6 (2.4) | 13.8 (1.8) | 12.0 (1.8) | 14.4 (1.2) | 18.0 (2.4) | 0.14 |
| Beck Depression Inventory‡ | 10.0 (0.4) | 7.4 (0.3) | 6.3 (0.3) | 5.9 (0.2) | 7.0 (0.5) | <0.001 |
| % | % | % | % | % | ||
| Exercise (≥3 times/week) § | 75.6 | 76.7 | 83.1 | 79.6 | 73.0 | 0.15 |
| Some college§ | 64.2 | 62.4 | 69.0 | 65.5 | 67.4 | 0.60 |
| Smoking history§ | 50.1 | 47.1 | 46.2 | 52.2 | 63.8 | 0.02 |
| Diabetes§ | 8.8 | 9.4 | 7.0 | 8.1 | 7.5 | 0.91 |
| Hypertension§ | 38.9 | 41.5 | 42.3 | 41.3 | 36.2 | 0.84 |
| Coronary heart disease§ | 9.9 | 5.6 | 5.7 | 6.4 | 11.5 | 0.17 |
| Stroke§ | 6.6 | 5.7 | 3.9 | 4.1 | 8.1 | 0.35 |
| Cancer§ | 32.5 | 26.6 | 25.4 | 25.3 | 23.8 | 0.57 |
| Sleep-related medication use§* | 22.1 | 14.7 | 9.2 | 9.8 | 6.8 | 0.001 |
| Postmenopausal estrogen use§ | 30.8 | 27.1 | 27.3 | 29.6 | 26.2 | 0.91 |
Sleep-related medications: sedating antidepressants, antianxiety drugs, and hypnotics
ANOVA
ANCOVA adjusted for age
Chi-square test
P for multiple comparison
in age, a–c: p=0.004, a–d: p=0.02, c–e: p=0.006, d–e: p=0.03
in alcohol consumption, a–e: p=0.01, b–e: p=0.007, c–e: p=0.04
in Beck Depression Inventory, a–b, a–c, a–d, and a–e: p<0.001, b–c: p<0.006, b–d: p<0.001, d–e: p=0.04
Tables 3a and 3b show gender-specific baseline characteristics according to reported napping. In men only, those not napping reported the longest nighttime sleep duration. Those napping ≥30 minutes had the highest prevalence of diabetes. In both sexes, napping ≥30 minutes had the highest BDI score, and the highest prevalence of coronary heart disease and stroke history.
Table 3.
| a. Age and age-adjusted mean values of covariates by reported daytime napping duration in men | ||||
|---|---|---|---|---|
| Nap duration/day (minutes) | ||||
| 0a (n=489) |
<30b (n=236) |
≥30c (n=164) |
p-value | |
| Mean (SE) | Mean (SE) | Mean (SE) | ||
| Age (years) † | 72.6 (0.3) | 75.4 (0.5) | 76.8 (0.6) | <0.001 |
| BMI (kg/m2) ‡ | 25.4 (0.1)) | 25.7 (0.2) | 25.3 (0.3) | 0.33 |
| Alcohol consumption (ml/week) ‡ | 139.9 (6.7) | 118.1 (9.6) | 126.5 (11.7) | 0.17 |
| Night-time sleep duration/day (hours) ‡ | 7.51 (0.55) | 7.28 (0.79) | 7.21 (0.96) | 0.008 |
| Beck Depression Inventory‡ | 5.40 (0.20) | 5.98 (0.28) | 6.91 (0.35) | <0.001 |
| % | % | % | ||
| Exercise (≥3 times/week) § | 85.9 | 86.5 | 79.6 | 0.12 |
| Some college§ | 78.0 | 75.8 | 73.9 | 0.55 |
| Smoking history§ | 70.0 | 68.9 | 70.0 | 0.95 |
| Diabetes§ | 11.3 | 12.1 | 23.4 | <0.001 |
| Hypertension§ | 36.9 | 39.7 | 39.2 | 0.74 |
| Coronary heart disease§ | 11.2 | 14.4 | 21.4 | 0.006 |
| Stroke§ | 5.6 | 9.5 | 16.5 | <0.001 |
| Cancer§ | 32.9 | 30.8 | 33.5 | 0.80 |
| Sleep-related medication use§* | 7.6 | 9.4 | 9.8 | 0.62 |
Sleep-related medications: sedating antidepressants, antianxiety drugs, and hypnotics
ANOVA
ANCOVA adjusted for age
Chi-square test
P for multiple comparison
in age, a–b and a–c: p<0.001, b–c: p=0.04
in night-time duration, a–b: p=0.02, a–c: p=0.007
in Beck Depression Inventory, a–c: p<0.001, b–c: p=0.04
Table 3.
| b. Age and age-adjusted mean values of covariates by reported daytime napping duration in women | ||||
|---|---|---|---|---|
| Nap duration/day (minutes) | ||||
| 0a (n=713) |
<30 minutesb (n=252) |
≥30 minutesc (n=147) |
p-value | |
| Mean (SE) | Mean (SE) | Mean (SE) | ||
| Age (years) † | 72.1 (0.3) | 74.5 (0.4) | 76.5 (0.6) | <0.001 |
| BMI (kg/m2) ‡ | 24.0 (0.1) | 24.2 (0.2) | 24.5 (0.3) | 0.37 |
| Alcohol consumption (ml/week) ‡ | 88.2 (4.1) | 70.9 (6.8) | 66.9 (9.0) | 0.02 |
| Night-time sleep duration/day (hours) ‡ | 7.13 (0.05) | 7.14 (0.08) | 7.15 (1.11) | 0.99 |
| Beck Depression Inventory‡ | 6.6 (0.2) | 6.6 (0.3) | 8.2 (0.4) | 0.003 |
| % | % | % | ||
| Exercise (≥3 times/week) § | 81.4 | 75.7 | 71.8 | 0.02 |
| Some college§ | 65.4 | 67.5 | 65.1 | 0.81 |
| Smoking history§ | 51.8 | 49.2 | 46.9 | 0.50 |
| Diabetes§ | 7.5 | 8.0 | 11.1 | 0.35 |
| Hypertension§ | 39.9 | 39.2 | 48.5 | 0.13 |
| Coronary heart disease§ | 6.2 | 5.9 | 12.4 | 0.02 |
| Stroke§ | 5.2 | 2.0 | 9.3 | 0.005 |
| Cancer§ | 25.1 | 27.7 | 28.9 | 0.54 |
| Sleep-related medication use§* | 11.4 | 10.1 | 15.3 | 0.32 |
| Postmenopausal estrogen use§ | 28.5 | 26.9 | 28.9 | 0.89 |
Sleep-related medications: sedating antidepressants, antianxiety drugs, and hypnotics
ANOVA
ANCOVA adjusted for age
Chi-square test
P for multiple comparison
in age, a–b and a–c: p<0.001, b–c: p=0.006
in alcohol consumption, a–b and a–c: p=0.03
in Beck Depression Inventory, a–c: p=0.001, b–c: p=0.003
Prospective Associations of sleep duration with all-cause mortality
At the 19-year follow up, 1224 participants had died (632 men and 592 women) at an average age of 85.0 years (SD=6.5 years) for men and 86.1 years (SD=7.0 years) for women. Table 4 shows the association of nighttime sleep duration with all-cause mortality in three models. Age at death showed a U-shaped association with nighttime sleep duration in both men and women (not shown). Reported nighttime sleep duration was not significantly associated with mortality in men in any of the models. In contrast, women sleeping ≥nine hours per night had a significantly higher risk of death than those sleeping 7–7.9 hours with an age-adjusted HR for all-cause mortality of 1.50 (95% CI, 1.12–2.00) (Model 1; Figure 1). This association remained statistically significant in Model 2 (after additional adjustment for nap duration, smoking, alcohol consumption, BDI, and history of hypertension, diabetes, coronary heart disease, stroke, and cancer) with a slight attenuation of this association [HR, 1.40; 95% CI, 1.02–1.94]. This association also remained statistically significant in Model 3 (after additional adjustment for use of sleep-related medications and postmenopausal estrogen use; HR, 1.51; 95% CI, 1.05–2.18). There was no significant difference in the HR for all-cause mortality in women sleeping <six, six to seven, or 8–8.9 hours per night, compared to those sleeping 7–7.9 hours per night, in any of the three models.
Table 4.
Associations of night-time sleep duration with all-cause mortality in men and women
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Night-time sleep duration (hours) |
No. of deaths | HR (95% CI) |
p-value | HR (95% CI) |
p-value | HR (95% CI) |
p-value |
| Men | |||||||
| <6 (n=55) | 42 | 1.10 (0.79–1.55) |
0.57 | 0.96 (0.67–1.38) |
0.83 | 0.98 (0.673–1.43) |
0.92 |
| 6.0 – 6.9 (n=137) | 101 | 1.14 (0.89–1.46) |
0.30 | 1.12 (0.85–1.46) |
0.43 | 1.12 (0.85–1.48) |
0.41 |
| 7.0 – 7.9 (n=248) | 164 | 1.0 (reference) |
- | 1.0 (reference) |
- | 1.0 (reference) |
- |
| 8.0 – 8.9 (n=330) | 229 | 1.02 (0.83–1.24) |
0.89 | 1.02 (0.82–1.26) |
0.86 | 0.98 (0.79–1.22) |
0.85 |
| ≥9.0 (n=119) | 96 | 1.18 (0.92–1.52) |
0.19 | 1.07 (0.81–1.41) |
0.64 | 1.09 (0.82–1.45) |
0.55 |
| Women | |||||||
| <6 (n=115) | 65 | 1.07 (0.79–1.44) |
0.67 | 1.06 (0.76–1.46) |
0.75 | 1.11 (0.77–1.60) |
0.59 |
| 6.0 – 6.9 (n=225) | 124 | 1.19 (0.93–1.52) |
0.17 | 1.03 (0.79–1.36) |
0.82 | 1.17 (0.85–1.61) |
0.33 |
| 7.0 – 7.9 (n=282) | 128 | 1.0 (reference) |
- | 1.0 (reference) |
- | 1.0 (reference) |
- |
| 8.0 – 8.9 (n=382) | 202 | 1.19 (0.95–1.49) |
0.12 | 1.21 (0.95–1.59) |
0.12 | 1.19 (0.90–1.57) |
0.23 |
| ≥9.0 (n=108) | 73 | 1.50 (1.12–2.00) |
0.006 | 1.40 (1.02–1.94) |
0.04 | 1.51 (1.05–2.18) |
0.03 |
Time-to-event Cox proportional hazards survival models
HR (95% CI): Hazard ratios (95% confidence interval)
Model 1: adjusted for age
Model 2: adjusted for age, nap duration, Beck Depression Inventory (only in men), education (only in men), exercise (only in men), smoking (only in women), alcohol consumption, and medical history of hypertension, diabetes, coronary heart disease, stroke, and cancer
Model 3: adjusted for Model 2 plus use of sleep-related medications (sedating antidepressants, antianxiety drugs, and hypnotics) and postmenopausal estrogen (only in women)
Figure 1.
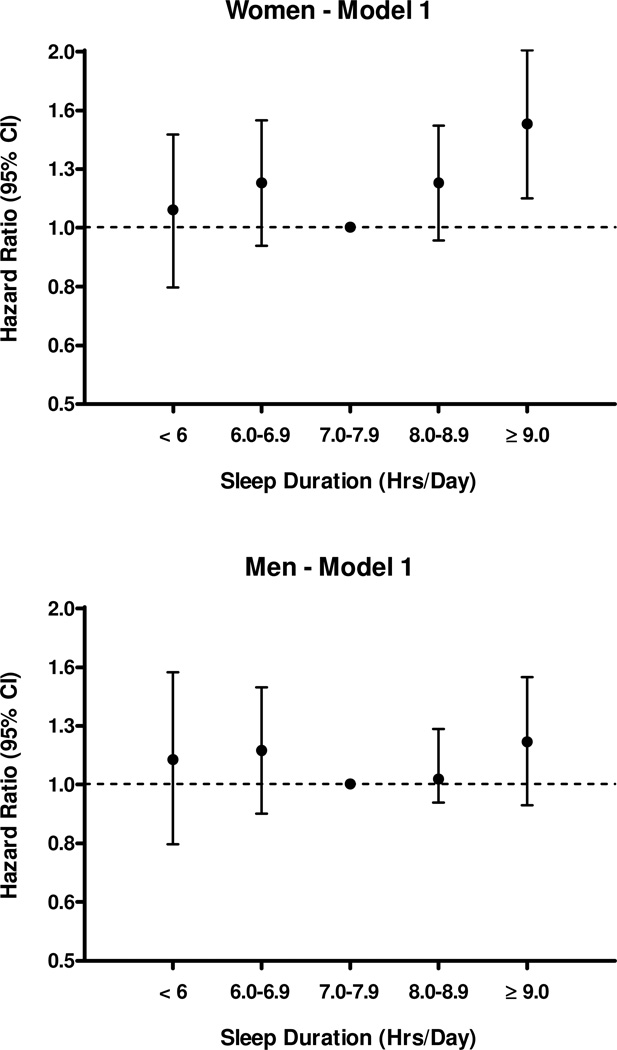
Association of nighttime sleep duration with all-cause mortality in men and women. Only data for Model 1 are shown, adjusted for age (Hazard ratios (95% confidence interval). There were no significant associations with mortality in men and only sleeping >9 hours was significantly associated with increased mortality in women (p=0.006). Results were similar for models 2 and 3.
Table 5 shows that men reporting napping ≥30 minutes per day had a significantly increased risk of mortality compared to men napping ≤30 minutes (HR, 1.37; 95% CI, 1.10–1.72) (Model 1). This association remained significant after additional adjustment in Model 2 for nighttime sleep duration, BDI, and history of hypertension, diabetes, coronary heart disease, stroke, and cancer (HR, 1.31; 95% CI, 1.03–1.66) and in Model 3 after additional adjustment for use of sleep-related medications (HR, 1.28; 95% CI, 1.00–1.64). There were no significant differences in the HR for all-cause mortality in men not napping, compared to those napping <30 minutes per day, in any of the three models. Similar analyses in women (Table 5) showed no significant difference in the HR for all-cause mortality by nap duration in any of the three models.
Table 5.
Associations of nap duration with all-cause mortality in men and women
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Daytime napping duration (minutes) |
No. of deaths | HR (95% CI) |
p-value | HR (95% CI) |
p-value | HR (95% CI) |
p-value |
| Men | |||||||
| 0 (n=489) |
318 | 1.01 (0.84–1.22) |
0.90 | 1.06 (0.87–1.29) |
0.60 | 1.03 (0.84–1.26) |
0.79 |
| ≤ 30 (n=236) |
172 | 1.00 (reference) |
- | 1.00 (reference) |
- | 1.00 (reference) |
- |
| ≥ 30 (n=164) |
142 | 1.37 (1.10–1.72) |
0.006 | 1.31 (1.03–1.66) |
0.03 | 1.28 (1.00–1.64) |
0.049 |
| Women | |||||||
| 0 (n=713) |
342 | 0.88 (0.72–1.07) |
0.19 | 0.90 (0.73–1.11) |
0.33 | 0.88 (0.69–1.11) |
0.28 |
| ≤ 30 (n=252) |
150 | 1.00 (reference) |
- | 1.00 (reference) |
- | 1.00 (reference) |
- |
| ≥ 30 (n=147) |
100 | 1.15 (0.89–1.48) |
0.27 | 1.11 (0.84–1.47) |
0.46 | 1.08 (0.79–1.49) |
0.63 |
Time-to-event Cox proportional hazards survival models
HR (95% CI): Hazard ratios (95% confidence interval)
Model 1: adjusted for age
Model 2: adjusted for age, night-time sleep duration, exercise (only in women), alcohol consumption (only in women), Beck Depression
Inventory, and medical history of hypertension, diabetes, coronary heart disease, stroke, and cancer
Model 3: adjusted for Model 2 plus use of sleep-related medications (sedating antidepressants, antianxiety drugs, and hypnotics) and postmenopausal estrogen (only in women)
DISCUSSION
The results of this study suggest that there is a differential relationship between reported sleep duration and mortality in men and women. In men, mortality was associated with napping more than 30 minutes each day while in women mortality was associated with sleeping more than 9 hours a night. These results were significant after adjusting for multiple co-variates including age, education, exercise, depression, smoking, alcohol consumption, medical history of hypertension, diabetes, coronary heart disease, stroke, cancer, and use of sleep medications, and, in women, estrogen. Previous studies of differences between men and women in the association of sleep duration and mortality have been inconsistent [7,8,19]. While our study adjusted for many covariates, differences in results in other studies could reflect differences in age, health, or other unmeasured attributes, failure to control for common covariates, or cultural characteristics (many of the epidemiological studies were conducted in different countries).
Our study also showed different sleep patterns in men vs. women. Men sleeping <six hours at night napped longer during the day; this pattern was not seen in the women. It is possible that, for the men, the short sleep at night may have resulted in daytime sleepiness which led to more napping. Presumably, older men who slept more at night did not need to nap during the day. It is also possible that the shorter sleep at night was secondary to nocturia, which is more common in men than women [20]. In one report, 66% of men aged 50 to 59 years and 91% of men older than 80 years reported nocturia [21], which both shortens and fragments sleep [22]. In interviews of 1506 older adults, the National Sleep Foundation showed that nocturia was associated with excessive daytime sleepiness [23,24]. Unfortunately, our study did not collect information about nocturia.
A second explanation for fragmented sleep and daytime napping might be sleep apnea, which was not evaluated in our present study. The prevalence of sleep apnea increases with age, causes sleep fragmentation and frequent awakenings, is associated with increased nocturia, and is more common in men than women [25,26]. However, sleep apnea seems a less likely explanation given the weak or absent association of reported sleep duration with obesity (BMI). However, not all older adults with sleep apnea are obese, so sleep apnea cannot be totally ruled out. We can only infer that covariates are partially controlled for by adjusting for daytime nap duration in our nighttime sleep analyses, on the assumption that sleep apnea contributed to longer daytime nap duration.
In contrast to the present study, several groups have published studies showing an association between short sleep duration and all-cause mortality [1,3,7]. In short-term clinical trials, experimental partial sleep deprivation impairs glucose tolerance, raises evening cortisol levels, increases sympathetic nervous system activity, and reduces leptin levels [27,28]. These results could imply that prolonged sleep deprivation could cause diabetes and hypertension and therefore increase mortality. However, sleep deprivation is not the same as short sleep duration. In the present study, short sleep duration was not associated with mortality 19 years later in men or women. Since participants were not asked about their sleep at follow-up, it is also unknown if those reporting short sleep continued to be short sleepers.
Other possible explanations why long sleep duration predicts increased mortality have been suggested [29]; a long time awake in bed is associated with increased sleep fragmentation, which may cause poor health [29]. In our study, respondents may not have discriminated between long sleep and time awake in bed; if this was an important misclassification, a stronger effect of adjusting for sleep medications might have been expected, but was not observed. Also, feelings of lethargy may be more common in long sleepers, with untoward consequences. One study suggested that the differences in sleep need may be a response to the differences in life-style and personality, and that long sleepers were depressed or anxious “worriers” [30]. Contrary to that hypothesis, in our study both male and female short nighttime sleepers (<six hr) showed more depressed mood scores and also used more sleep-related medications, which included antidepressants. Alternatively, long night sleep may be physiologically similar to spending a long time in the dark (the photoperiod); differences in photoperiod have been associated with mortality in several species [31].
In the present study, a daytime nap duration >30 minutes was significantly and independently associated with all-cause mortality in men but not in women. Previous studies suggested that habitual daytime naps would be a risk factor for mortality in older adults. One study reported that older women napping daily were at significantly greater risk of death from all causes other than cancer [9]. Another study reported the siesta as an independent mortality predictor in old age [12]. Additionally, Stang et al. reported siestas >1 hour had positive associations with prevalence of several cardiovascular risk factors and measures of subclinical atherosclerosis [32]. From a hemodynamic viewpoint, the afternoon awakening, like the morning one, may also be associated with increased mortality. A rise in blood pressure and heart rate upon awakening is considered one of the causes of clustering of cardiovascular events in the morning and afternoon [33–36]. Several previous studies also suggested that diabetes mellitus was associated with daytime nap [32, 37–39].
In contrast, others suggested that proper daytime nap duration is beneficial. Several studies suggested that a short nap promoted wakefulness and enhanced performance and learning ability. One previous study reported that siestas are inversely associated with coronary mortality, and the association was particularly evident among working men [40]. In our study, daytime naps were divided into three categories: zero, < 30, and ≥ 30 minutes based on reports suggesting that <30-minute napping has beneficial effects [15,41], and because naps >30 minutes can result in sleep inertia and are not recommended clinically [16]. The present study did not show any beneficial effect of daytime napping on mortality.
Some have reported that hypnotics increase mortality [5,42–44], although another study reported otherwise [45]. In the present study, both men and women sleeping <six hours were more likely to use sleep-related medications than other participants, but use of these medications showed no significant influence on the association between sleep duration and all-cause mortality in either group. Although most sleep medication users likely used them for relief of insomnia, indication for sleep medications was not asked specifically.
The present study had several limitations. Participants were mostly retired, Caucasian, and middle to upper-middle class, which limits generalizability to other age, ethnic, and socioeconomic groups, but reduces the confounding of age, work hours, and socioeconomic stressors. Information about sleep was self-reported and is imprecise. We could not evaluate sleep qualities such as impaired sleep architecture or sleep apnea, which are likely to influence mortality. It is plausible that long sleep duration is a marker for undiagnosed disease(s) with onset after baseline in an elderly cohort, but this bias would have been stronger in a cohort study with a shorter follow up. Reported nighttime sleep duration may include time awake in bed, not physiologic sleep. We did not control for employment, as nearly all participants were retired. Finally our healthy cohort with remarkable longevity may have obscured or unveiled associations.
The strengths of this study are the well-characterized cohort with a large number of potential covariates including validated medication use, the 19-year duration of >90% follow-up for vital status, the data allowing separate analyses of nighttime sleep and daytime napping, and the study of potential differences between men and women. Because the weak association observed in women did not emerge until after five years of follow up, and increased thereafter (over the average 19-year follow up), this pattern of delayed onset of increased mortality does not suggest that longer sleep duration was merely a marker for an underlying, undiagnosed disease. The very long survival of this cohort into their 80s permits observations about late life mortality that could not be made in sleep studies in younger adults and would be difficult to replicate in a clinical trial.
In conclusion, women in this cohort who reported nighttime sleep longer than nine hours and men who reported daytime naps longer than 30 minutes were at significantly increased risk of earlier mortality over the next 19 years in time-to-event analyses. These gender-specific results were independent of multiple covariates assessed at baseline, including comorbidity and sleep-related medications. Additional long-term gender-specific studies in the elderly are needed to confirm and understand the mechanisms of these sex-specific delayed sleep-mortality associations.
Acknowledgments
Supported by: The Rancho Bernardo Study was funded by the National Institute on Aging, grants AG07181 and AG02857, and the National Institute of Diabetes and Digestive and Kidney Diseases, grant DK31801, and Dr. Ancoli-Israel was supported by National Institute on Aging grant AG08415.
Abbreviations
- BDI
Beck Depression Inventory
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest.
REFERENCES
- 1.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27(3):440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 2.Wingard DL, Berkman LF, Brand RJ. A multivariate analysis of health-related practices: a nine-year mortality follow-up of the Alameda County Study. American Journal of Epidemiology. 1982;116(5):765–775. doi: 10.1093/oxfordjournals.aje.a113466. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan GA, Seeman TE, Cohen RD, Knudsen LP, Guralnik J. Mortality among the elderly in the Alameda County Study: behavioral and demographic risk factors. American journal of public health. 1987;77(3):307–312. doi: 10.2105/ajph.77.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Archives of general psychiatry. 1979;36(1):103–116. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 5.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30(10):1245–1253. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Archives of general psychiatry. 2002;59(2):131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 7.Heslop P, Smith GD, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: The effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep medicine. 2002;3(4):305–314. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 8.Burazeri G, Gofin J, Kark JD. Over 8 hours of sleep--marker of increased mortality in Mediterranean population: follow-up population study. Croatian medical journal. 2003;44(2):193–198. [PubMed] [Google Scholar]
- 9.Stone KL, Ewing SK, Ancoli-Israel S, et al. Self-reported sleep and nap habits and risk of mortality in a large cohort of older women. J Am Geriatr Soc. 2009;57(4):604–611. doi: 10.1111/j.1532-5415.2008.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18(2):148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 12.Bursztyn M, Stessman J. The siesta and mortality: twelve years of prospective observations in 70-year-olds. Sleep. 2005;28(3):345–347. [PubMed] [Google Scholar]
- 13.Hays JC, Blazer DG, Foley DJ. Risk of napping: excessive daytime sleepiness and mortality in an older community population. Journal of the American Geriatrics Society. 1996;44(6):693–698. doi: 10.1111/j.1532-5415.1996.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 14.Burazeri G, Gofin J, Kark JD. Siesta and mortality in a Mediterranean population: a community study in Jerusalem. Sleep. 2003;26(5):578–584. doi: 10.1093/sleep/26.5.578. [DOI] [PubMed] [Google Scholar]
- 15.Tamaki M, Shirota A, Hayashi M, Hori T. Restorative effects of a short afternoon nap (<30 min) in the elderly on subjective mood, performance and eeg activity. Sleep Res Online. 2000;3(3):131–139. [PubMed] [Google Scholar]
- 16.Tassi P, Muzet A. Sleep inertia. Sleep Med Rev. 2000;4(4):341–353. doi: 10.1053/smrv.2000.0098. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher D, Nies G, Thompson LW. Reliability of the Beck Depression Inventory with older adults. Journal of consulting and clinical psychology. 1982;50(1):152–153. doi: 10.1037//0022-006x.50.1.152. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of general psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 19.Amagai Y, Ishikawa S, Gotoh T, et al. Sleep duration and mortality in Japan: the Jichi Medical School Cohort Study. Journal of epidemiology. 2004;14(4):124–128. doi: 10.2188/jea.14.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rembratt A, Norgaard JP, Andersson KE. Nocturia and associated morbidity in a community-dwelling elderly population. BJU international. 2003;92(7):726–730. doi: 10.1046/j.1464-410x.2003.04467.x. [DOI] [PubMed] [Google Scholar]
- 21.Sommer P, Nielsen KK, Bauer T, et al. Voiding patterns in men evaluated by a questionnaire survey. British journal of urology. 1990;65(2):155–160. doi: 10.1111/j.1464-410x.1990.tb14688.x. [DOI] [PubMed] [Google Scholar]
- 22.Ancoli-Israel S, Bliwise DL, Norgaard JP. The effect of nocturia on sleep. Sleep Med Rev. 15(2):91–97. doi: 10.1016/j.smrv.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation '2003 Sleep in America' Poll. Am J Geriatr Psychiatry. 2007;15(4):344–350. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 24.Bliwise DL, Foley DJ, Vitiello MV, Ansari FP, Ancoli-Israel S, Walsh JK. Nocturia and disturbed sleep in the elderly. Sleep Med. 2009;10(5):540–548. doi: 10.1016/j.sleep.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoch CC, Reynolds CF, Monk TH, et al. Comparison of sleep-disordered breathing among healthy elderly in the seventh, eighth, and ninth decades of life. Sleep. 1990;13(6):502–511. doi: 10.1093/sleep/13.6.502. [DOI] [PubMed] [Google Scholar]
- 27.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. The lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 28.Spiegel K, Leproult R, L'Hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. The Journal of clinical endocrinology & metabolism. 2004;89(11):5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 29.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;8(3):159–174. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Hartmann E, Baekeland F, Zwilling GR. Psychological differences between long and short sleepers. Arch Gen Psychiatry. 1972;26(5):463–468. doi: 10.1001/archpsyc.1972.01750230073014. [DOI] [PubMed] [Google Scholar]
- 31.Gordon SH, Tucker SA. Effect of photoperiod and daily food-access time on mortality and performance of male broilers. Br Poult Sci. 1998;39(Suppl):S11–S12. doi: 10.1080/00071669888070. [DOI] [PubMed] [Google Scholar]
- 32.Stang A, Dragano N, Poole C, et al. Daily siesta, cardiovascular risk factors, and measures of subclinical atherosclerosis: results of the Heinz Nixdorf Recall Study. Sleep. 2007;30(9):1111–1119. doi: 10.1093/sleep/30.9.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulcahy D, Wright C, Sparrow J, et al. Heart rate and blood pressure consequences of an afternoon SIESTA (Snooze-Induced Excitation of Sympathetic Triggered Activity) The American journal of cardiology. 1993;71(7):611–614. doi: 10.1016/0002-9149(93)90524-g. [DOI] [PubMed] [Google Scholar]
- 34.Bursztyn M, Mekler J, Ben Ishay D. The siesta and ambulatory blood pressure: is waking up the same in the morning and afternoon? Journal of human hypertension. 1996;10(5):287–292. [PubMed] [Google Scholar]
- 35.Stergiou GS, Vemmos KN, Pliarchopoulou KM, Synetos AG, Roussias LG, Mountokalakis TD. Parallel morning and evening surge in stroke onset, blood pressure, and physical activity. Stroke. 2002;33(6):1480–1486. doi: 10.1161/01.str.0000016971.48972.14. [DOI] [PubMed] [Google Scholar]
- 36.Stergiou GS, Mastorantonakis SE, Roussias LG. Intraindividual reproducibility of blood pressure surge upon rising after nighttime sleep and siesta. Hypertens Res. 2008;31(10):1859–1864. doi: 10.1291/hypres.31.1859. [DOI] [PubMed] [Google Scholar]
- 37.Lam KB, Jiang CQ, Thomas GN, et al. Napping is associated with increased risk of type 2 diabetes: the Guangzhou Biobank Cohort Study. Sleep. 2010;33(3):402–407. doi: 10.1093/sleep/33.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Q, Song Y, Hollenbeck A, Blair A, Schatzkin A, Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33(1):78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picarsic JL, Glynn NW, Taylor CA, et al. Self-reported napping and duration and quality of sleep in the lifestyle interventions and independence for elders pilot study. J Am Geriatr Soc. 2008;56(9):1674–1680. doi: 10.1111/j.1532-5415.2008.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naska A, Oikonomou E, Trichopoulou A, Psaltopoulou T, Trichopoulos D. Siesta in healthy adults and coronary mortality in the general population. Archives of internal medicine. 2007;167(3):296–301. doi: 10.1001/archinte.167.3.296. [DOI] [PubMed] [Google Scholar]
- 41.Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res. 2009;18(2):272–281. doi: 10.1111/j.1365-2869.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 42.Rumble R, Morgan K. Hypnotics, sleep, and mortality in elderly people. Journal of the American Geriatrics Society. 1992;40(8):787–791. doi: 10.1111/j.1532-5415.1992.tb01850.x. [DOI] [PubMed] [Google Scholar]
- 43.Kripke DF, Klauber MR, Wingard DL, Fell RL, Assmus JD, Garfinkel L. Mortality hazard associated with prescription hypnotics. Biological Psychiatry. 1998;43(9):687–693. doi: 10.1016/s0006-3223(97)00292-8. [DOI] [PubMed] [Google Scholar]
- 44.Kripke DF, Langer RD, Kline LE. Hypnotics' association with mortality or cancer: a matched cohort study. BMJ Open. 2012;vol 2 doi: 10.1136/bmjopen-2012-000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips B, Mannino DM. Does insomnia kill? Sleep. 2005;28(8):965–971. doi: 10.1093/sleep/28.8.965. [DOI] [PubMed] [Google Scholar]


