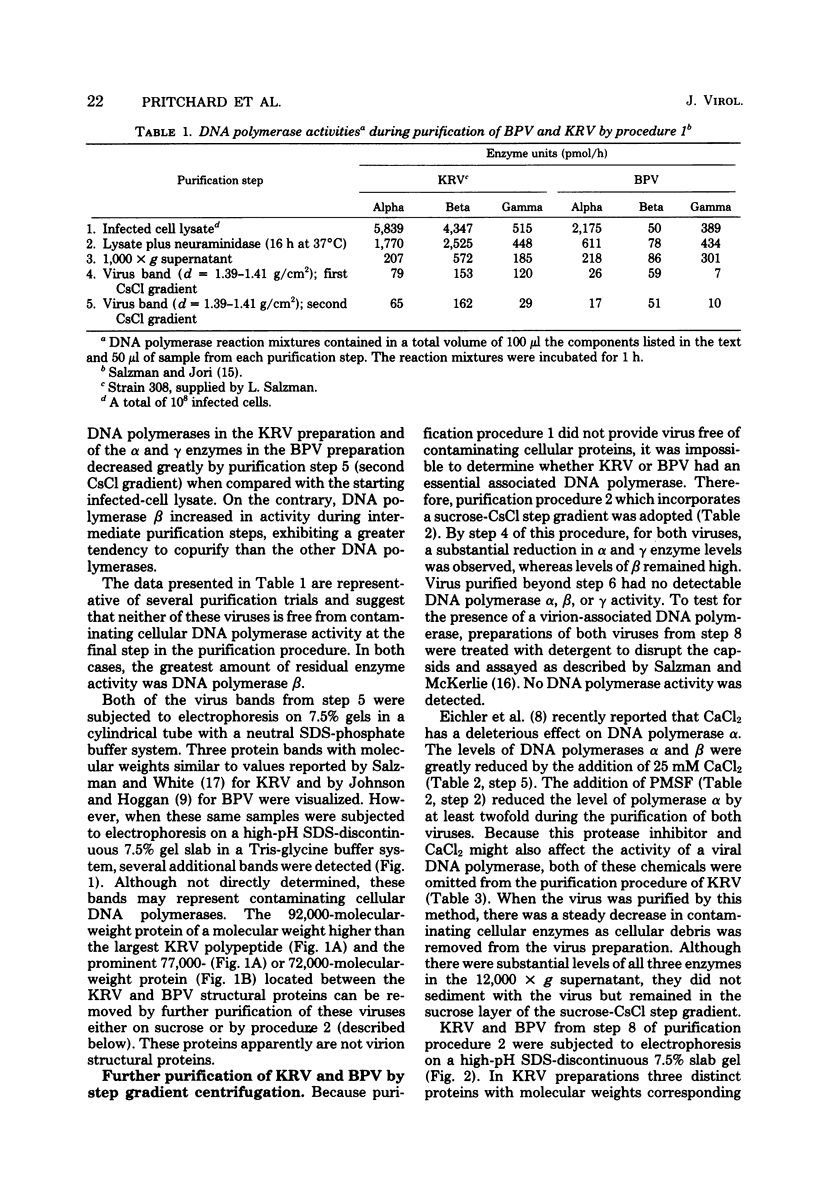
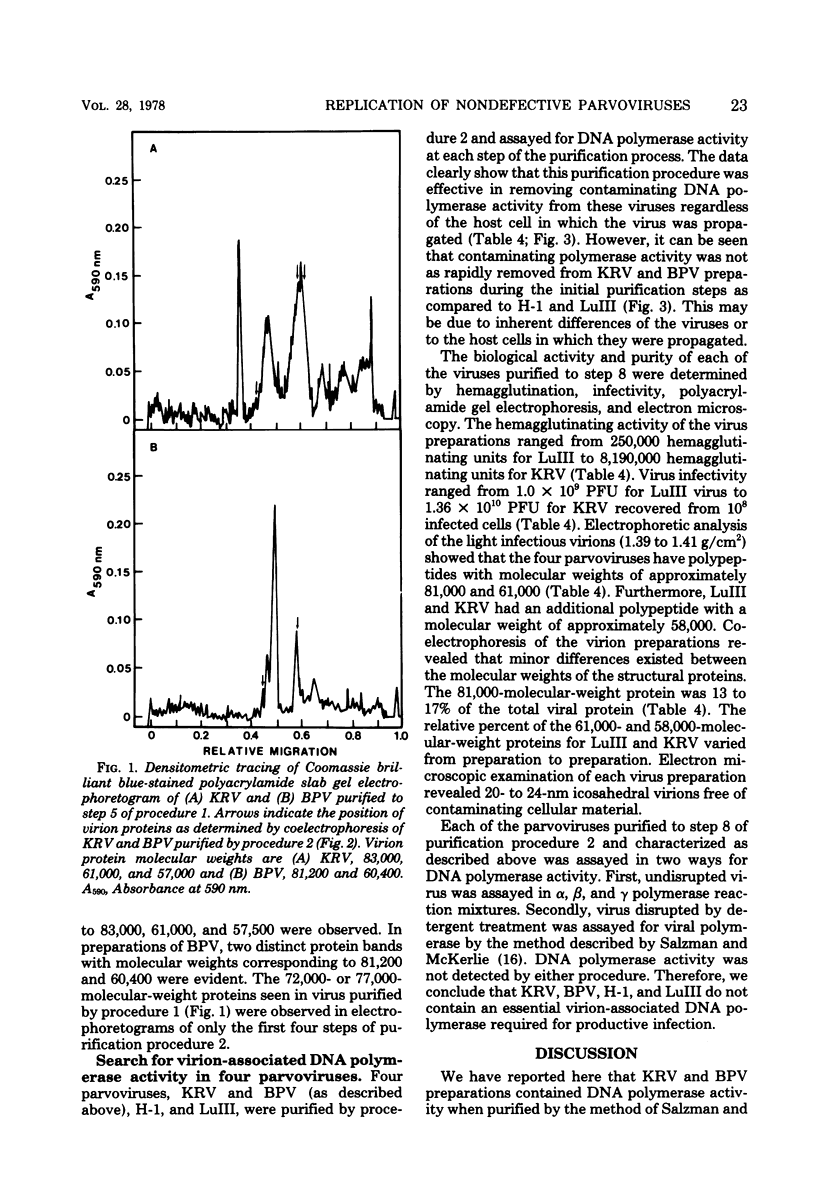
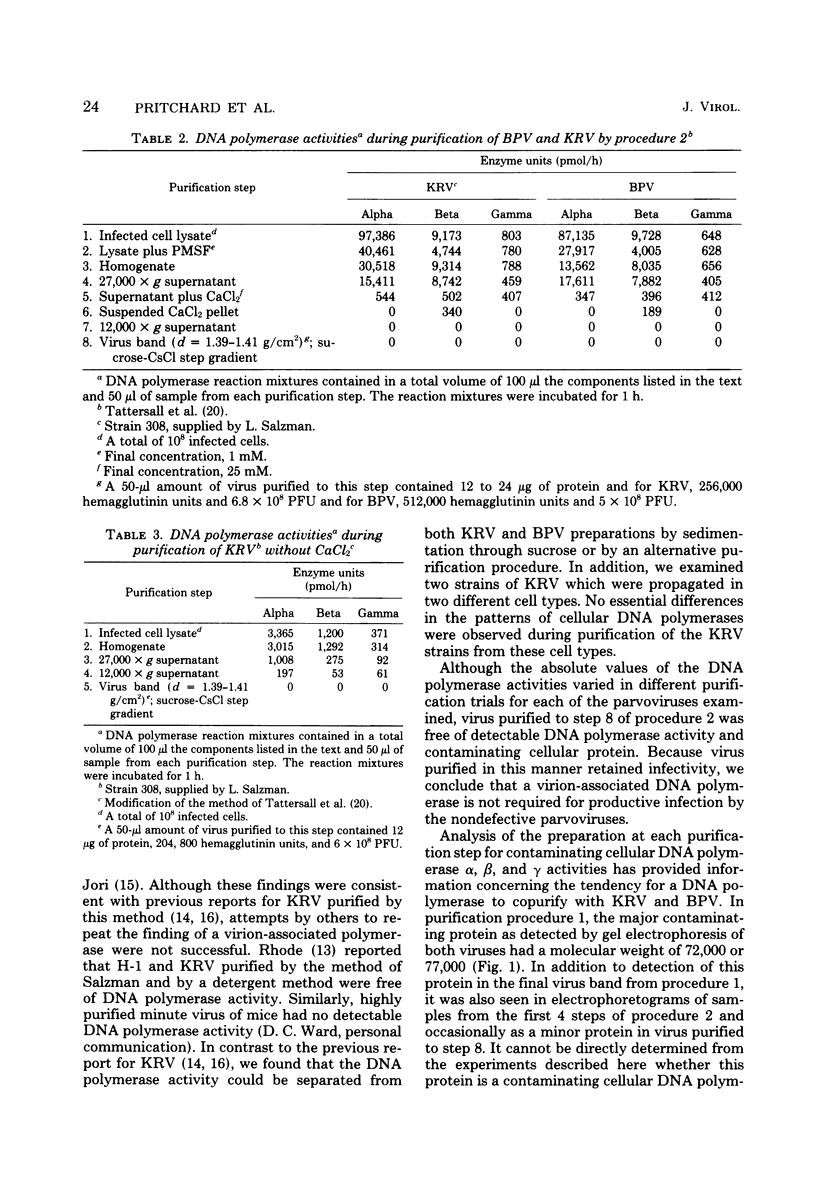
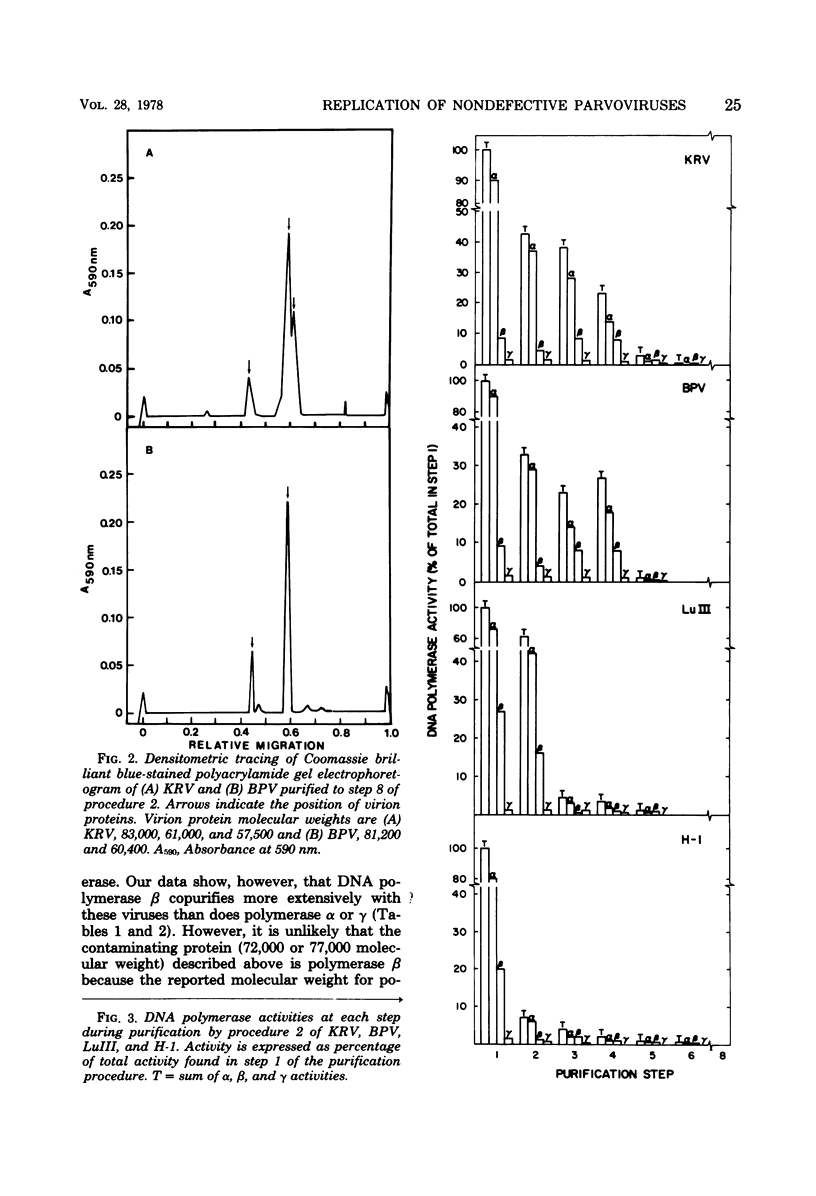
Abstract
We have examined four of the nondefective parvoviruses for an associated DNA polymerase. Virions were purified from neuraminidase-treated infected-cell lysates by isopycnic centrifugation in CsCl or from infected cell material by CaCl2 precipitation and centrifugation through sucrose into CsCl. Preparations of bovine parvovirus or Kilham rat virus obtained by the former procedure contained DNA polymerase activity but were not free of contaminating cellular proteins. The latter method produced viral preparations free of contaminating cellular proteins, and no DNA polymerase activity was detected in light infectious particles of H-1, LuIII, bovine parvovirus, or Kilham rat virus. Examination of levels of each cellular DNA polymerase in these preparations from each step of both purification procedures revealed that DNA polymerase β had a greater tendency to copurify with bovine parvovirus and Kilham rat virus than did DNA polymerases α or γ. Disruption of infectious virions obtained by the second purification method with detergents and sonic treatment did not result in the detection of a DNA polymerase activity. The biological activity and purity of each of the four different viruses obtained by the latter procedure were determined by hemagglutination and infectivity assays, polyacrylamide gel electrophoresis, and electron microscopy. In each case, the virions banding at a density of 1.39 to 1.41 g/cm2 in CsCl were infectious and contained only the virion structural proteins. DNA polymerase activity was not detected in any of these preparations, and we have concluded that a virion-associated DNA polymerase is not required for productive infection with the nondefective parvoviruses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates R. C., Storz J., Reed D. E. Isolation and comparison of bovine parvoviruses. J Infect Dis. 1972 Nov;126(5):531–536. doi: 10.1093/infdis/126.5.531. [DOI] [PubMed] [Google Scholar]
- Bollum F. J. Mammalian DNA polymerases. Prog Nucleic Acid Res Mol Biol. 1975;15(0):109–144. doi: 10.1016/s0079-6603(08)60118-x. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Rodbard D. Polyacrylamide gel electrophoresis. Science. 1971 Apr 30;172(3982):440–451. doi: 10.1126/science.172.3982.440. [DOI] [PubMed] [Google Scholar]
- Daugharty H., Warfield D. T., Nemingway N. D., Casey H. L. Mumps class-specific immunoglobulins in radioimmunoassay and conventional serology. Infect Immun. 1973 Mar;7(3):398–402. doi: 10.1128/iai.7.3.398-402.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler D. C., Fisher P. A., Korn D. Effect of calcium on the recovery and distribution of DNA polymerase alpha from cultured human cells. J Biol Chem. 1977 Jun 10;252(11):4011–4014. [PubMed] [Google Scholar]
- Johnson F. B., Hoggan M. D. Structural proteins of HADEN virus. Virology. 1973 Jan;51(1):129–137. doi: 10.1016/0042-6822(73)90373-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Parris D. S., Bates R. C. Effect of bovine parvovirus replication on DNA, RNA, and protein synthesis in S phase cells. Virology. 1976 Aug;73(1):72–78. doi: 10.1016/0042-6822(76)90061-1. [DOI] [PubMed] [Google Scholar]
- Pritchard C., Bates R. C., Stout E. R. Levels of cellular DNA polymerases in synchronized bovine paravovirus-infected cells. J Virol. 1978 Jul;27(1):258–261. [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973 Jun;11(6):856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A. DNA polymerase activity associated with purified Kilham rat virus. Nat New Biol. 1971 Jun 9;231(23):174–176. doi: 10.1038/newbio231174a0. [DOI] [PubMed] [Google Scholar]
- Salzman L. A., Jori L. A. Characterization of the Kilham rat virus. J Virol. 1970 Feb;5(2):114–122. doi: 10.1128/jvi.5.2.114-122.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., McKerle L. Characterization of the deoxyribonucleic acid polymerase associated with Kilham rat virus. J Biol Chem. 1975 Jul 25;250(14):5583–5588. [PubMed] [Google Scholar]
- Salzman L. A., White W. L. Structural proteins of Kilham rat virus. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1551–1556. doi: 10.1016/0006-291x(70)90564-4. [DOI] [PubMed] [Google Scholar]
- Schlabach A., Fridlender B., Bolden A., Weissbach A. DNA-dependent DNA polymerases from HeLa cell nuclei. II. Template and substrate utilization. Biochem Biophys Res Commun. 1971 Aug 20;44(4):879–885. doi: 10.1016/0006-291x(71)90793-5. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Layman K. R., Hand R. E. Effect of cell physiological state on infection by rat virus. J Virol. 1969 Dec;4(6):872–878. doi: 10.1128/jvi.4.6.872-878.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. S., Sedwick W. D., Korn D. Nuclear deoxyribonucleic acid polymerase. Further observations on the structure and properties of the enzyme from human KB cells. J Biol Chem. 1975 Sep 10;250(17):7040–7044. [PubMed] [Google Scholar]
- Weissbach A., Baltimore D., Bollum F., Gallo R., Korn D. Nomenclature of eukaryotic DNA polymerases. Eur J Biochem. 1975 Nov 1;59(1):1–2. doi: 10.1111/j.1432-1033.1975.tb02416.x. [DOI] [PubMed] [Google Scholar]


