Abstract
Aged garlic extract (AGE) is known to have a protective effect against immune system, endothelial function, oxidative stress and inflammation. We examined the effects of exercise with and without aged garlic extract administration on body weight, lipid profiles, inflammatory cytokines, and oxidative stress marker in high-fat diet (HFD)-induced obese rats. Forty-five Sprague-Dawley rats were fed either a HFD (HFD, n = 40) or a normal diet (ND, n = 5) for 6 weeks and thereafter randomized into ND (n = 5), HFD (n = 10), HFD with AGE (n = 10), HFD with Exercise (n = 10), or HFD with Exercise+AGE (n = 10) for 4 weeks. AGE groups were administered at a dose of 2.86 g/kg·body weight, orally. Exercise consisted of running 15-60 min 5 days/week with gradually increasing intensity. AGE (P < 0.01), Exercise, and Exercise+AGE (P < 0.001) attenuated body weight gain and food efficiency ratio compared to HFD. Visceral fat and liver weight gain were attenuated (P < 0.05) with all three interventions with a greater effect on visceral fat in the Exercise+AGE than AGE (P < 0.001). In reducing visceral fat (P < 0.001), epididymal fat (P < 0.01) and liver weight (P < 0.001), Exercise+AGE was effective, but exercise showed a stronger suppressive effect than AGE. Exercise+AGE showed further additive effects on reducing visceral fat and liver weight (P < 0.001). AGE significantly attenuated the increase in total cholesterol and low-density lipoprotein-cholesterol compared with HFD (P < 0.05). Exercise+AGE attenuated the increase in triglycerides compared with HFD (P < 0.05). Exercise group significantly decrease in C-reactive protein (P < 0.001). These results suggest that AGE supplementation and exercise alone have anti-obesity, cholesterol lowering, and anti-inflammatory effects, but the combined intervention is more effective in reducing weight gain and triglycerides levels than either intervention alone.
Keywords: Aged garlic extract, exercise, high fat diet, obesity, metabolic parameters
Introduction
The prevalence of obesity has been known to indicate serious public health problems in the world. It is strongly associated with metabolic syndrome and many chronic diseases that result from an imbalance between energy intake and physical activity [1-3]. Excess energy intake and reduced energy expenditure promotes metabolic dysfunction [4-7], oxidative stress and inflammatory pathologic factors that increase the secretion of interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP) [1,2,4,5,8].
Increased adipose tissue plays an important role in the development of low-grade inflammation, which is characterized by cytokine production and stimulation of inflammatory cytokine signaling pathways [9]. Pro-inflammatory cytokines are associated with dyslipidemia and atherosclerosis [10]. Potential therapeutic regimens for severe obesity are non-conservative dietary interventions, drug therapy, and bariatric surgery [11]. Other interventions performed either alone or in combination include improving nutritional habits, increasing physical activity levels, and undergoing psychological treatment [12].
Previous studies have documented that nutritional application of various metabolic dysfunction have been conducted with the aim of regulating obesity [13-16]. There is a developing interest in the use of phytochemical compounds as medicinal alternative due to their lack of toxicity and the relative ease and cost of production. Garlic (Allium sativum) has several biological benefits including anti-oxidative, anti-inflammatory, and anti-dyslipidemic effects [17]. Aged garlic extract (AGE) differs from other garlic varieties; it has less stimulating and pungent properties than bulbs and contains more newly converted sulfur-containing compounds (gamma-glutamyl cysteine, S-allyl cysteine, S-allylmercaptocysteine, and S-methyl cysteine) than those found in cooked or raw garlic [18]. AGE and its individual components have been shown to improve plasma lipid concentrations and oxidative stress, and inflammatory cytokines [19-21]. However, the effect of AGE on reduced body weight is unknown [4,6,17-19].
Physical exercise training has been widely demonstrated to be beneficial for improving metabolic function in high-fat diet (HFD)-induced obese rats because it improves lipid profiles and reduces fat mass, inflammation, and oxidative stress [22,23]. However, the effect of AGE supplementation on exercise-mediated improvement of metabolic parameters in HFD-induced obese rats is unknown.
We hypothesized that AGE supplementation combined with exercise may improve metabolic parameters in HFD-induced obese rats. Therefore, we evaluated the additive effect of AGE on exercise-mediated changes in body weight, lipid profiles, inflammatory cytokines and oxidative stress markers in rats with HFD-induced obesity.
Materials and Methods
Animals and experimental design
Forty-five male Sprague Dawley rats (3 weeks old) were purchased from Dae Han Biolink (Chung Cheong do, Korea). The rats were housed two per cage on a 12:12-h- light/dark cycle in a temperature-maintained room at 25℃ with 45 ± 5% humidity in compliance with the animal care standards of the American College of Sports Medicine. All experiments were approved by the Animal Care and Use Committee at Pusan National University.
All rats were given 7 days to adjust to their new environment. At 4 weeks of age, the animals were fed either a normal diet (ND; n = 5) or high-fat diet (HFD; n = 40) for 6 weeks (Table 1 shows the composition of diet). After 6 weeks, animals in the HFD group were randomized into the following groups (Table 2): HFD (HFD, n = 10), HFD with AGE (AGE, n = 10), HFD with exercise (Exercise, n = 10), and HFD with exercise and AGE (Exercise+AGE, n = 10) for 4 weeks. The food efficiency ratios (g body weight/kcal) were calculated as the ratio of body weight gain (g) from 1 day to 16 days and the total amount of food (kcal) ingested over that period. The animals were sacrificed 48 h after last exercise bout and/or AGE ingestion.
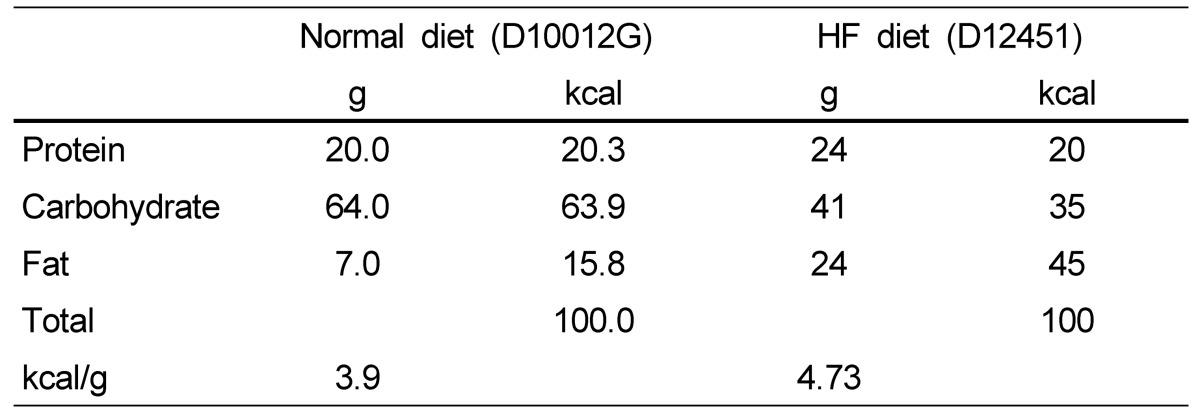
Table 1.
Composition of experimental diets1)
1)All dietary components were prepared by Research Diets.
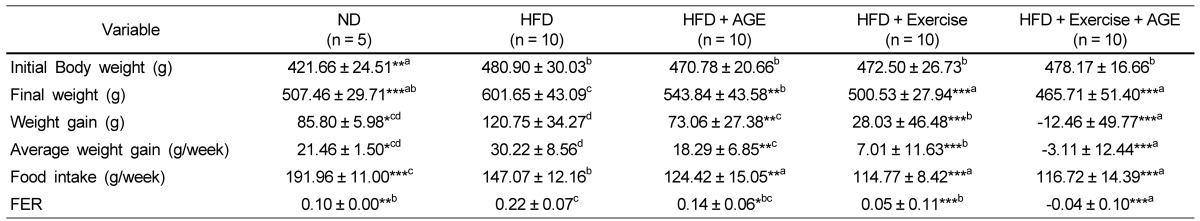
Table 2.
Effect of AGE and exercise on body weight changes and food intake in high fat diet-induces obese rats1)
1)Valuses are means ± SD.
HFD, high-fat diet; AGE, aged garlic extract; FER, food efficiency ratio.
Experimental values were compared with those for the high fat diet group using Duncan's multiple comparison test after one way ANOVA.
*P < 0.05 vs HFD, **P < 0.01 vs HFD, ***P < 0.001 vs HFD. a,b,c,ddenoting a significant post hoc difference from the other groups (P < 0.05).
Experimental dietand exercise training
The HFD contained 20% protein, 35% carbohydrate, and 45% fat as previously described [24]. The ND (fat; 15.8% of kilocalories consumed, D10012G) and HFD (fat; 45% of kilocalories consumed, D12541) formulations were purchased from Research Diets (New Brunswick, NJ), and their nutritional composition is shown in Table 1 [25]. AGE made from organically grown garlic and contacting sulfide, disulfide, S-ally-cysteine, and protein was obtained from Uiseong Black-Garlic Farming Association of Korea Co., Ltd. The AGE used contained 28.6% (w/v) solid material. S-Allyl-L-cysteine (SAC) was also present as 0.1% of the total solids, calculated on a dry weight basis [25]. AGE was orally provided at a dose of 2.86 g/kg to both the AGE and Exercise+AGE groups. In the combined group, AGE was provided 30 min before exercise five times per week for 4 weeks [26]. Other groups were treated similarly with distilled water instead of AGE. Animals were trained on a motorized treadmill (Dual treadmill, DJ344, Dae Jong, Seoul, Korea) five times per week for 4 weeks.
Exercise intensity and duration were as follows: first and second weeks: 15 m/min for 45 and 60 min, respectively; third and fourth weeks: 20 m/min for 30 and 45 min, respectively. [27]. Because Boor et al. [27] used a similar adaptation protocol (15 m/min for 30-60 min) to prepare the rats for a period of training at higher exercise intensity. In order to produce beneficial adaptations and avoid overtraining and injuries, the velocity of the treadmill was increased while the duration was maintained. If a longer training intervention is used, the speed will be kept at 20 m/min and the duration would increase from 45 to 60 min in third period. Due to the short duration of the current study, we decided to increase the velocity and maintain the duration up to 45 min in the 4th week. We used the needle plate only for a week to motivate the animals during the exercise training to accustom them into running. Additionally, although we called it needle plate, the edge is dull and did not cause any abrasion to the rats. It gave more merit than using electrical shock device the latter which heightens the rodents' stress level.
Body weight, tissue mass and blood chemistry assay
To avoid immediate effects of exercise and supplementation, rats were euthanized 48 h after the last exercise bout and AGE intake. The rats were anesthetized at theabdomen using a heparinized syringe. Visceral fat, epididymal fat, liver, gastrocnemius and soleus samples were removed and weighed after sacrificing the animals.
Blood samples were collected from the abdominal aorta into heparinized or EDTA-coated tubes. The plasma was immediately separated by centrifugation at 3,000 × g for 10 min and kept frozen at -80℃ until it was assayed. T-C, HDL-C, LDL-C, and TG concentrations were measured in an automatic blood analyzer (Hitachi 7600-210 and 7180, Tokyo, Japan) with enzymatic techniques based on a colorimetric assay.Plasma levels of interleukin-6 (IL-6; R&D Systems, Minneapolis, MN), tumor necrosis factor-α (TNF-α; R&D Systems, Minneapolis, MN), C-reactive protein (CRP, BD Biosciences, USA) were measured by enzyme-linked immunosorbent assay (ELISA) method. Malondialdehyde (MDA) was measured by spectrophotometry using a commercial thiobarbituric acid-reacting substances assay kit (Zeptometrix, USA). All assays were performed according to the manufacturers' instructions.
Statistical analysis
Data are expressed as mean ± standard deviation. Differences among the groups were evaluated using one-way analysis of variance (ANOVA) at baseline. If ANOVA indicated significance, a Duncan post hoc test was performed. The alpha level of significance was set at P < 0.05. All analyses were performed with SPSS version 19.0 (SPSS Inc., Chicago, IL).
Results
Effect of AGE supplementation and exercise on body weight, food intake, and food efficiency ratio
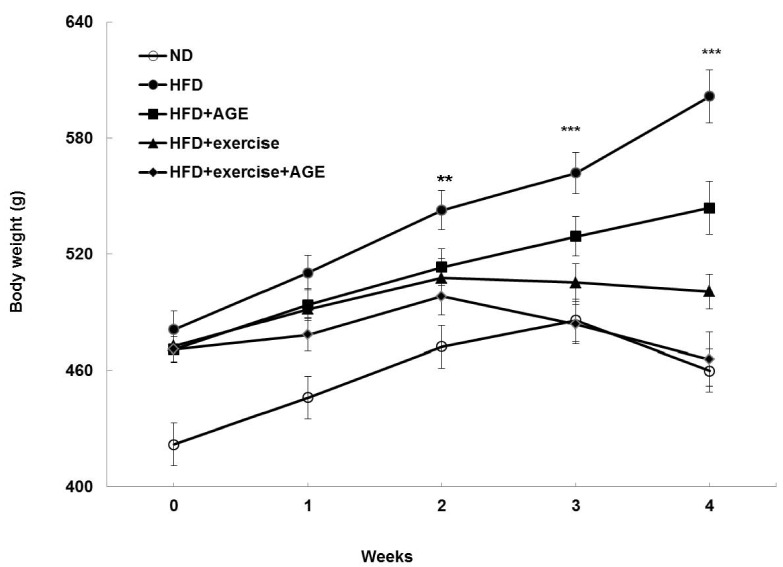
Body weight, food intake, and food efficiency ratio (FER) are shown in Table 2. ND-fed rats had a lower average body weight than HFD rats at baseline. Before AGE and exercise interventions, there were no significant differences in body weight among the HFD groups. However, the HFD rats had significantly increased body weight compared to the other four groups after 2, 3, and 4 weeks (Fig. 1). In addition, the final weight, weight gain, average weight gain, and FER were greater in the HFD group compared to the other four groups (Table 2). Exercise decreased body weight gain more strongly than AGE. Exercise+ AGE supplementation caused a significant weight loss compared to all the other groups. In addition, Exercise+AGE group was markedly reduced in contrast to HFD and HFD+AGE groups. On the other hand, there was no significant difference between the ND and Exercise+AGE group implying a beneficial effect. Relative food intake was reduced by AGE, Exercise, and Exercise+AGE compared with HFD, but the combined treatment did not have an additive effect on food intake.
Fig. 1.
Effect of AGE supplementation on body weight in HFD-induced obese rats with or without AGE supplementation for 4 weeks. AGE was orally administered at a dose of 2.86 g/kg 30 min before exercise. Statistical analysis was performed using one-way ANOVA. Values are means ± SD. **P < 0.01 and ***P < 0.001 vs. HFD.
Effect of AGE supplementation and exercise on fat, liver, and muscle weights
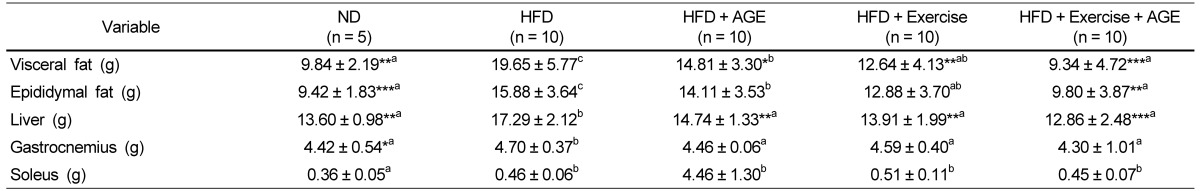
Visceral fat, epididymal fat, and liver weights were significantly higher in the HFD group compared to the other groups (Table 3). Exercise, AGE, and Exercise+AGE were effective in attenuating increased visceral fat and liver weight, but Exercise and Exercise+AGE had a stronger suppressive effect on visceral fat than AGE administration alone. Epididymal fat gain was not attenuated by AGE or Exercise. However, the combined of Exercise+AGE significantly inhibited epididymal fat accumulation compared to the other groups. HFD feeding slightly increased gastrocnemius weight but there was no significant difference following exercise.
Table 3.
Effect of AGE and exercise on obesity state in high fat diet-induced obese rats1)
1)Valuses are means ± SD.
HFD, high-fat diet; AGE, aged garlic extract.
Experimental values were compared with those for the high fat diet group using Duncan's multiple comparison test after one way ANOVA.
*P < 0.05 vs HFD, **P < 0.01 vs HFD, ***P < 0.001 vs HFD. a,b,cdenoting a significant post hoc difference from the other groups (P < 0.05).
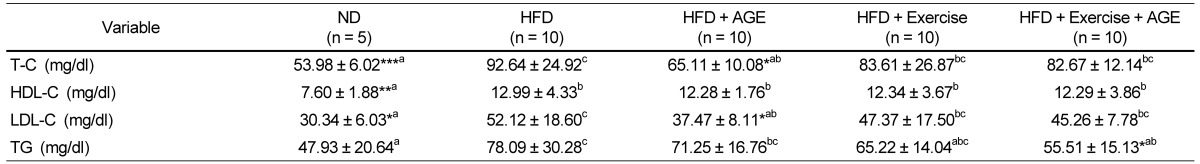
Effect of AGE supplementation and exercise on blood lipid profile
Serum T-C and LDL-C concentrations were higher in the HFD group compared with the ND and AGE groups (Table 4). TG levels were higher in the HFD group compared with the ND and Exercise+AGE groups. Individual AGE and exercise regimens were not effective in reducing TG, but Exercise+AGE did cause a decrease in TG levels.
Table 4.
Effect of AGE and exercise on serum parameters in high fat diet-induced obese rats1)
1)Valuses are means ± SD.
HFD, high-fat diet; AGE, aged garlic extract.
Experimental values were compared with those for the high fat diet group using Duncan's multiple comparison test after one way ANOVA.
*P < 0.05 vs HFD, **P < 0.01 vs HFD, ***P < 0.001 vs HFD. a,b,cdenoting a significant post hoc difference from the other groups (P < 0.05).
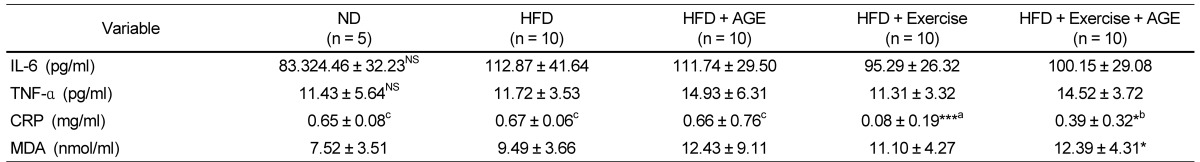
Effect of AGE supplementation and exercise on inflammatory markers
IL-6, TNF-α, and CRP were assessed to determine the effect of AGE and exercise intervention on inflammation in HFD-induced obese rats (Table 5). TNF-α and IL-6 were unchanged by HFD. Exercise (P < 0.001) and Exercise+AGE groups had lower CRP levels than the ND, HFD, and AGE groups. Contrary to our expectations, HFD did not induce a significant increase in blood MDA levels (Table 4).
Table 5.
Effect of AGE and exercise on inflammation factors changes in high fat diet-induces obese rats1)
1)Valuses are means ± SD.
HFD, high-fat diet; AGE, aged garlic extract.
Experimental values were compared with those for the high fat diet group using Duncan's multiple comparison test after one way ANOVA.
*P < 0.05 vs HFD, ***P < 0.001 vs HFD. NSnot significant. a,b,cdenoting a significant post hoc difference from the other groups (P < 0.05).
Discussion
This study demonstrates that a 4-week regimen of supplementation with AGE with and without exercise on body weight, fat accumulation, and inflammatory cytokines in HFD-induced obese rats. This result suggested that the major finding was that AGE supplementation showed anti-obesity effects; it attenuated the increase in body weight, visceral fat, liver weight, T-C, and LDL-C. Exercise with/without AGE supplementation also significantly improved obesity status, but the combination of exercise and AGE had an additive effect on body weight, visceral fat, and epididymal fat gain, as well as on TG levels.
High-energy diets are widely used to induce obesity and fat deposition in animals. Most studies found that HFD results in increased body weight, fat mass, and the development of hyperlipidemia and metabolic syndrome [28,29]. We found that the HFD group had less food intake and a higher food efficiency ratio than the ND group. These findings indicate that the decrease in food intake was due to the increased amount of energy in the HFD, which has been reported previously [30]. As expected, we found that the HFD significantly increased body weight and, fat mass, which led to obesity and hyperlipidemia [31].
Exercise training is routinely suggested to prevent lifestyle-related diseases that are associated with obesity. It attenuates increase in body weight and visceral fat and reduces metabolic risk factors by increasing energy expenditure [32]. In our HFD-induced obesity model, exercise significantly attenuated body weight and visceral fat increases observed during HFD consumption. These results indicate that increased physical activity was the main reason for reduced body fat accumulation. Our findings are similar to those of Gollisch et al. [7], who demonstrated that exercise training led to reductions in body weight and visceral adipose tissue in rats. Similarly, we found that AGE supplementation also reduced the effect of HFD on weight and visceral fat gain. As expected, the combination of exercise and AGE supplementation was more effective than either intervention alone; the combined regimen attenuated the effect of the HFD on body and liver weight, as well as visceral and epididymal fat. Although the combined intervention group had the lowest liver weight, this result was not significantly different from the single interventions.
Food intake regulation, which may be modulated by neuroendocrine mechanisms, is an exceedingly complex biological process that involves a large number of cues and biological substrates [33]. Energy expenditure by exercise and suppression of food intake may be related to altered appetite due to hypothalamic signals [34]. In the present study, AGE supplementation and exercise either alone or as combined treatments significantly inhibited food intake. These interventions had a cumulative effect on reducing body weight, epididymal fat, liver weight, and TG levels. It is unclear whether AGE consumption directly changes appetite; therefore, this effect requires further investigation.
The exact mechanism by which increased adipose tissue mass induces metabolic dysfunction and atherogenic dyslipidemia is unclear. Adipose tissue lipolysis significantly promotes circulating fatty acids and is associated with hepatic steatosis [35,36]. Our findings demonstrate that HFD significantly increased plasma TC, LDL-C, and TG levels in comparison with the ND. It is well known that increased plasma cholesterol increases the risk of developing atherosclerosis [37]. Some studies have shown that exercise training inhibits the development of hepatic steatosis and reduces serum TG in obese rats [38,39]. A previous study showed that garlic extract consumption attenuated serum TG in humans with high blood cholesterol [40]. Surprisingly, exercise or AGE alone did not affect TG, but the combination of exercise with AGE supplementation attenuated HFD-induced hypertriglyceridemia. These results may indicate decreased TG levels were available for liver-induced lipid synthesis [41], and insulin action in the liver was improved [42] with Exercise+AGE. The present study demonstrates that AGE supplementation reduced T-C and LDL-C levels, effects that were not observed following Exercise or Exercise+AGE. This may be because AGE lower cholesterol in part by inhibiting hepatic cholesterol synthesis [43].
Obesity leads to chronic low-grade inflammation that is associated with white adipose tissue, which produces and secretes a wide range of inflammatory molecules [44]. Previous studies have demonstrated elevated IL-6 and TNF-α mRNA levels in obese subjects, which decrease following weight loss [45]. Garlic extract has been shown to decrease IL-6 and TNF-α, suggesting an anti-inflammatory effect [46,47]. In our study, we did not find any significant changes in IL-6, TNF-α, and CRP following HFD. However, exercise with and without AGE administration decreased plasma IL-6 levels, but the result was not statistically significant. Based on previous reports, we expected increased levels of pro-inflammatory cytokines levels with HFD [48]. However, it is possible that a 10-week HFD regimen was not sufficient to elicit an appreciable increase. Although CRP was not significantly increased by HFD, exercise alone and in combination with AGE reduced CRP levels. These findings confirm the anti-inflammatory effect of exercise, and this effect was greater when AGE was not included in the regimen. Previous studies have been controversial, and some researchers reported that exercise training may decrease inflammatory cytokines [44,49]. However, the anti-inflammatory mechanism of exercise training during HFD is unclear and requires further investigation.
In conclusion, this study demonstrated that AGE supplementation and exercise have beneficial effects on reducing weight and visceral fat gain in rats fed with HFD. AGE with and without exercise, but not exercise alone, effectively lowered blood lipids levels. In addition, AGE with exercise is more effective in controlling obesity and TG levels than either intervention alone. However, more extensive research is needed before recommending AGE supplementation to obese humans.
Footnotes
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0020224, and R13-2007-023-00000-0).
References
- 1.Goran MI, Treuth MS. Energy expenditure, physical activity, and obesity in children. Pediatr Clin North Am. 2001;48:931–953. doi: 10.1016/s0031-3955(05)70349-7. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy RL, Chokkalingham K, Srinivasan R. Obesity in the elderly: who should we be treating, and why, and how? Curr Opin Clin Nutr Metab Care. 2004;7:3–9. doi: 10.1097/00075197-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Steinberger J, Daniels SR American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young); American Heart Association Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Circulation. 2003;107:1448–1453. doi: 10.1161/01.cir.0000060923.07573.f2. [DOI] [PubMed] [Google Scholar]
- 4.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297:E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansell BJ, Watson KE, Fogelman AM, Navab M, Fonarow GC. High-density lipoprotein function recent advances. J Am Coll Cardiol. 2005;46:1792–1798. doi: 10.1016/j.jacc.2005.06.080. [DOI] [PubMed] [Google Scholar]
- 6.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 7.Gollisch KS, Brandauer J, Jessen N, Toyoda T, Nayer A, Hirshman MF, Goodyear LJ. Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am J Physiol Endocrinol Metab. 2009;297:E495–E504. doi: 10.1152/ajpendo.90424.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Cheng M, Zhao M, Ge A, Guo F, Zhang M, Yang Y, Liu L, Yang N. Differential effects of high-fat-diet rich in lard oil or soybean oil on osteopontin expression and inflammation of adipose tissue in diet-induced obese rats. Eur J Nutr. 2012 doi: 10.1007/s00394-012-0428-z. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 9.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szekanecz Z. Pro-inflammatory cytokines in atherosclerosis. Isr Med Assoc J. 2008;10:529–530. [PubMed] [Google Scholar]
- 11.Quak SH, Furnes R, Lavine J, Baur LA Obesity Working Group. Obesity in children and adolescents. J Pediatr Gastroenterol Nutr. 2008;47:254–259. doi: 10.1097/MPG.0b013e318181b2cd. [DOI] [PubMed] [Google Scholar]
- 12.Tzotzas T, Evangelou P, Kiortsis DN. Obesity, weight loss and conditional cardiovascular risk factors. Obes Rev. 2011;12:e282–e289. doi: 10.1111/j.1467-789X.2010.00807.x. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Wang D, Song G, Zuo C, Qiao X, Qin S. The effect of combination therapy of allicin and fenofibrate on high fat diet-induced vascular endothelium dysfunction and liver damage in rats. Lipids Health Dis. 2010;9:131. doi: 10.1186/1476-511X-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karmakar S, Das D, Maiti A, Majumdar S, Mukherjee P, Das AS, Mitra C. Black tea prevents high fat diet-induced non-alcoholic steatohepatitis. Phytother Res. 2011;25:1073–1081. doi: 10.1002/ptr.3466. [DOI] [PubMed] [Google Scholar]
- 15.Cao ZH, Gu DH, Lin QY, Xu ZQ, Huang QC, Rao H, Liu EW, Jia JJ, Ge CR. Effect of pu-erh tea on body fat and lipid profiles in rats with diet-induced obesity. Phytother Res. 2011;25:234–238. doi: 10.1002/ptr.3247. [DOI] [PubMed] [Google Scholar]
- 16.Seo EY, Ha AW, Kim WK. Alpha-lipoic acid reduced weight gain and improved the lipid profile in rats fed with high fat diet. Nutr Res Pract. 2012;6:195–200. doi: 10.4162/nrp.2012.6.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee SK, Maulik SK. Effect of garlic on cardiovascular disorders: a review. Nutr J. 2002;1:4. doi: 10.1186/1475-2891-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 19.Yeh YY, Yeh SM. Garlic reduces plasma lipids by inhibiting hepatic cholesterol and triacylglycerol synthesis. Lipids. 1994;29:189–193. doi: 10.1007/BF02536728. [DOI] [PubMed] [Google Scholar]
- 20.Ide N, Lau BH. Aged garlic extract attenuates intracellular oxidative stress. Phytomedicine. 1999;6:125–131. doi: 10.1016/S0944-7113(99)80047-6. [DOI] [PubMed] [Google Scholar]
- 21.Zare A, Farzaneh P, Pourpak Z, Zahedi F, Moin M, Shahabi S, Hassan ZM. Purified aged garlic extract modulates allergic airway inflammation in BALB/c mice. Iran J Allergy Asthma Immunol. 2008;7:133–141. [PubMed] [Google Scholar]
- 22.Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY, Tzan K, Wang A, Parthasarathy S, He G, Rajagopalan S, Sun Q. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1115–R1125. doi: 10.1152/ajpregu.00806.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Touati S, Meziri F, Devaux S, Berthelot A, Touyz RM, Laurant P. Exercise reverses metabolic syndrome in high-fat diet-induced obese rats. Med Sci Sports Exerc. 2011;43:398–407. doi: 10.1249/MSS.0b013e3181eeb12d. [DOI] [PubMed] [Google Scholar]
- 24.Torrens C, Hanson MA, Gluckman PD, Vickers MH. Maternal undernutrition leads to endothelial dysfunction in adult male rat offspring independent of postnatal diet. Br J Nutr. 2009;101:27–33. doi: 10.1017/S0007114508988760. [DOI] [PubMed] [Google Scholar]
- 25.Moriguchi T, Saito H, Nishiyama N. Aged garlic extract prolongs longevity and improves spatial memory deficit in senescence-accelerated mouse. Biol Pharm Bull. 1996;19:305–307. doi: 10.1248/bpb.19.305. [DOI] [PubMed] [Google Scholar]
- 26.Morihara N, Ushijima M, Kashimoto N, Sumioka I, Nishihama T, Hayama M, Takeda H. Aged garlic extract ameliorates physical fatigue. Biol Pharm Bull. 2006;29:962–966. doi: 10.1248/bpb.29.962. [DOI] [PubMed] [Google Scholar]
- 27.Boor P, Celec P, Behuliak M, Grancic P, Kebis A, Kukan M, Pronayová N, Liptaj T, Ostendorf T, Sebeková K. Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metabolism. 2009;58:1669–1677. doi: 10.1016/j.metabol.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Iyer A, Kauter K, Alam MA, Hwang SH, Morisseau C, Hammock BD, Brown L. Pharmacological inhibition of soluble epoxide hydrolase ameliorates diet-induced metabolic syndrome in rats. Exp Diabetes Res. 2012;2012:758614. doi: 10.1155/2012/758614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Handa P, Tateya S, Rizzo NO, Cheng AM, Morgan-Stevenson V, Han CY, Clowes AW, Daum G, O'Brien KD, Schwartz MW, Chait A, Kim F. Reduced vascular nitric oxide-cGMP signaling contributes to adipose tissue inflammation during high-fat feeding. Arterioscler Thromb Vasc Biol. 2011;31:2827–2835. doi: 10.1161/ATVBAHA.111.236554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burneiko RC, Diniz YS, Galhardi CM, Rodrigues HG, Ebaid GM, Faine LA, Padovani CR, Cicogna AC, Novelli EL. Interaction of hypercaloric diet and physical exercise on lipid profile, oxidative stress and antioxidant defenses. Food Chem Toxicol. 2006;44:1167–1172. doi: 10.1016/j.fct.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Schölmerich J, Bollheimer LC. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol. 2006;36:485–501. doi: 10.1677/jme.1.01909. [DOI] [PubMed] [Google Scholar]
- 32.Jakicic JM. Exercise in the treatment of obesity. Endocrinol Metab Clin North Am. 2003;32:967–980. doi: 10.1016/s0889-8529(03)00075-6. [DOI] [PubMed] [Google Scholar]
- 33.Hagan S, Niswender KD. Neuroendocrine regulation of food intake. Pediatr Blood Cancer. 2012;58:149–153. doi: 10.1002/pbc.23376. [DOI] [PubMed] [Google Scholar]
- 34.Flores MB, Fernandes MF, Ropelle ER, Faria MC, Ueno M, Velloso LA, Saad MJ, Carvalheira JB. Exercise improves insulin and leptin sensitivity in hypothalamus of Wistar rats. Diabetes. 2006;55:2554–2561. doi: 10.2337/db05-1622. [DOI] [PubMed] [Google Scholar]
- 35.Maljaars J, Peters HP, Masclee AM. Review article: The gastrointestinal tract: neuroendocrine regulation of satiety and food intake. Aliment Pharmacol Ther. 2007;26(Suppl 2):241–250. doi: 10.1111/j.1365-2036.2007.03550.x. [DOI] [PubMed] [Google Scholar]
- 36.Mastorakos G, Zapanti E. The hypothalamic-pituitary-adrenal axis in the neuroendocrine regulation of food intake and obesity: the role of corticotropin releasing hormone. Nutr Neurosci. 2004;7:271–280. doi: 10.1080/10284150400020516. [DOI] [PubMed] [Google Scholar]
- 37.Kawaguchi H, Miyoshi N, Miura N, Fujiki M, Horiuchi M, Izumi Y, Miyajima H, Nagata R, Misumi K, Takeuchi T, Tanimoto A, Yoshida H. Microminipig, a non-rodent experimental animal optimized for life science research:novel atherosclerosis model induced by high fat and cholesterol diet. J Pharmacol Sci. 2011;115:115–121. doi: 10.1254/jphs.10R17FM. [DOI] [PubMed] [Google Scholar]
- 38.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G619–G626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 39.Gauthier MS, Couturier K, Latour JG, Lavoie JM. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J Appl Physiol. 2003;94:2127–2134. doi: 10.1152/japplphysiol.01164.2002. [DOI] [PubMed] [Google Scholar]
- 40.Durak I, Kavutcu M, Aytaç B, Avci A, Devrim E, Ozbek H, Oztürk HS. Effects of garlic extract consumption on blood lipid and oxidant/antioxidant parameters in humans with high blood cholesterol. J Nutr Biochem. 2004;15:373–377. doi: 10.1016/j.jnutbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Estadella D, Oyama LM, Dâmaso AR, Ribeiro EB, Oller Do Nascimento CM. Effect of palatable hyperlipidic diet on lipid metabolism of sedentary and exercised rats. Nutrition. 2004;20:218–224. doi: 10.1016/j.nut.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol. 1988;254:E248–E259. doi: 10.1152/ajpendo.1988.254.3.E248. [DOI] [PubMed] [Google Scholar]
- 43.Yeh YY, Liu L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: human and animal studies. J Nutr. 2001;131:989S–993S. doi: 10.1093/jn/131.3.989S. [DOI] [PubMed] [Google Scholar]
- 44.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 45.Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, Kaser S, Kaser A, Tilg H. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–1264. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- 46.Hodge G, Hodge S, Han P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry. 2002;48:209–215. doi: 10.1002/cyto.10133. [DOI] [PubMed] [Google Scholar]
- 47.Colín-González AL, Ortiz-Plata A, Villeda-Hernández J, Barrera D, Molina-Jijón E, Pedraza-Chaverrí J, Maldonado PD. Aged garlic extract attenuates cerebral damage and cyclooxygenase-2 induction after ischemia and reperfusion in rats. Plant Foods Hum Nutr. 2011;66:348–354. doi: 10.1007/s11130-011-0251-3. [DOI] [PubMed] [Google Scholar]
- 48.Glatz JF, de Groot RH, Hesselink MK, Schrauwen P. Lipids in metabolic health and disease. Prostaglandins Leukot Essent Fatty Acids. 2011;85:195. doi: 10.1016/j.plefa.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]