Abstract
Various forms of fermented soybean products are well documented for their health benefits. The efficacy of anti-obesogenic effect of Doenjang, one of the most commonly used seasonings in Korean cuisine, has been reported only in animal models; thus, an evaluation of Doenjang needs to be conducted in human studies. We aimed to test the hypothesis that Doenjang supplementation reduces body weight and changes body composition in overweight adults. A total of 51 overweight adults participated in this study. A group of males with BMI ≥ 23 kg/m2 and waist to hip ratio (WHR) ≥ 0.90, and a group of females with BMI ≥ 23 kg/m2 and WHR ≥ 0.85 were randomly assigned to either a Doenjang supplement (9.9 g dry/day) group or a placebo group for a 12-week randomized, double-blind and placebo-controlled study. Anthropometric parameters, abdominal fat distribution by computerized tomography (CT) and blood components were measured before and after the intervention period. After the 12-week study, the Doenjang supplementation group had significant reductions in body weight (kg), body fat mass (kg) and body fat (%) compared to the placebo group, the supplementation of Doenjang resulted in a significant reduction in visceral fat (cm2), although no changes were observed in total and subcutaneous fat are as (cm2), serum lipid profiles and dietary intakes. The present study demonstrated that daily supplementation of 9.9 g dry/day of Doenjang for 12 weeks reduces body weight and visceral fat in overweight adults.
Keywords: Doenjang, body weight, visceral fat, abdominal fat, overweight adults
Introduction
Overweight and obesity have become a worldwide epidemic with growing prevalence across all age groups around the world [1]. The dramatic increase in the number of overweight and obese adults has been attributed in part by changes in dietary and lifestyle patterns in both developed and developing countries [2]. Overweight and obesity are well-documented risk factors for diabetes, hypertension, dyslipidemia, asthma, arthritis and coronary heart disease [3].
Several animal and cellular studies have provided evidences of soy proteins and soy products in maintaining optimal body weight [4]. Soy products also potentiate insulin secretion and insulin sensitivity in murine diabetic models [5]. Fermentation of soybean products has also been reported to increase the bioactivity of the functional elements [6-9].
Korea has experienced major changes in dietary habits in recent years due to increased affluence and western cultural influences. These changes have resulted in a greater variety of foods with low cost food supplies, resulting in an increase in metabolic diseases such as obesity and diabetes [10]. Although the increased energy consumption is responsible for much of the increases in the prevalence of obesity, changes in dietary choices favoring western-style foods with less traditional foods contribute to the risks of metabolic diseases [11]. The traditional Korean diet is characterized by a variety of fermented soybean and vegetable-based foods that contain several bioactive compounds promoting cardiovascular health and glucose regulation [12]. Doenjang, a fermented soybean paste, has traditionally been one of the most widely used seasonings in Korean cuisine. Doenjang is manufactured from meju, a product of cooked and crushed soybeans, fermented primarily by Bacillus subtilis and molds, such as Rhizopus, Mucor and Aspergillus [13]. Traditionally prepared Doenjang thus contains most of the nutritive and biologically active functional components found in soybeans, and are often in more biologically available forms than those in raw soybeans. For instance, Doenjang contains trypsin inhibitors, free isoflavones, saponins, phytic acid and linoleic acid, which are produced during fermentation [7]. Soybean isoflavones, in mostly their glucoside forms (genistin, daidzin, glycitin), are metabolized into aglycones (genistein, daidzein, glycitein) during fermentation. Isoflavonoids from legumes are reported to be hydrolyzed by microorganisms in the large intestine prior to absorption. It has been suggested that the aglycone forms of isoflavones are more effectively absorbed than the glycosides [7]. Accordingly, Doenjang has received attention from both the public and the industry, as many studies have reported its healthful physiological effects.
In the past decade, Doenjang has been documented as having anti-mutagenic [14], hypotensive [15], anticancer [16], anti-oxidative [17,18] and anti-inflammatory properties [19]. Recently, anti-obesity effects of Doenjang and genistein have been observed in animals [9,20-22]. In five published reports, genistein enhanced the transcription of carnitine palmitoyltransferase (CPT)-I, a rate regulating an enzyme for fatty acid oxidation, and also activated peroxisome proliferator-activated receptor (PPAR)-alpha target genes involved in fatty acid beta-oxidation [9,20-23].
To date, the efficacy of Doenjang as an anti-obesogenic functional food has been documented in animal models; however, it has yet been evaluated inhuman studies. We aimed to test the hypothesis that Doenjang supplementation for 12 weeks reduces body weight and body fat composition as assessed by anthropometric measurements and CT scans in overweight adults using a randomized, double-blind, placebo-controlled design.
Subjects and Methods
Subjects
The subjects for this study were recruited from the Clinical Trial Center for Functional Foods (CTCF2) in Chonbuk National University Hospital in Jeonju, Korea during the period between June-August 2008. A total of 83 healthy men and women (19-60 years) agreed to participate. Only those subjects who were overweight (both genders: BMI ≥ 23 kg/m2; WHR ≥ 0.90 for males; WHR ≥ 0.85 for females) and who had no other diagnosed illnesses were invited to participate in the study.
Sixty subjects who met the study criteria were randomly divided either into the Doenjang (n = 30, dry 9.9 g/d) group or the placebo group (n = 30, dry 9.9 g/d with inactive ingredients). The exclusion criteria for the study were as follows: subjects with lipid metabolism disorders, > 10% change in body weight in the past 3 months, cardiovascular disease (e.g., history of arrhythmia, heart failure, myocardial infarction), wearing a pacemaker, allergy or hypersensitivity to any of the ingredients in the test products, gastrointestinal diseases (e.g., Crohn's disease) or gastrointestinal surgery (e.g., a caecum or enterocele surgery), participation in other clinical trials within the past 2 months, abnormal hepatic liver function, renal disease such as acute/chronic renal failure, nephrotic syndrome, use of anti-psychosis drug therapy within the previous 2 months, laboratory tests, medical or psychological conditions deemed by the investigators to interfere with successful participation in the study, history of alcohol or substance abuse, pregnancy and/or breastfeeding a child. Sixty potentially eligible subjects were invited to the screening examination and were enrolled in this study. All subjects signed written informed consent forms before participating in the study. The research protocol used in this study was approved by the Institutional Review Board (IRB) of CTCF2, Chonbuk National University Hospital in Jeonju, Korea.
Study design
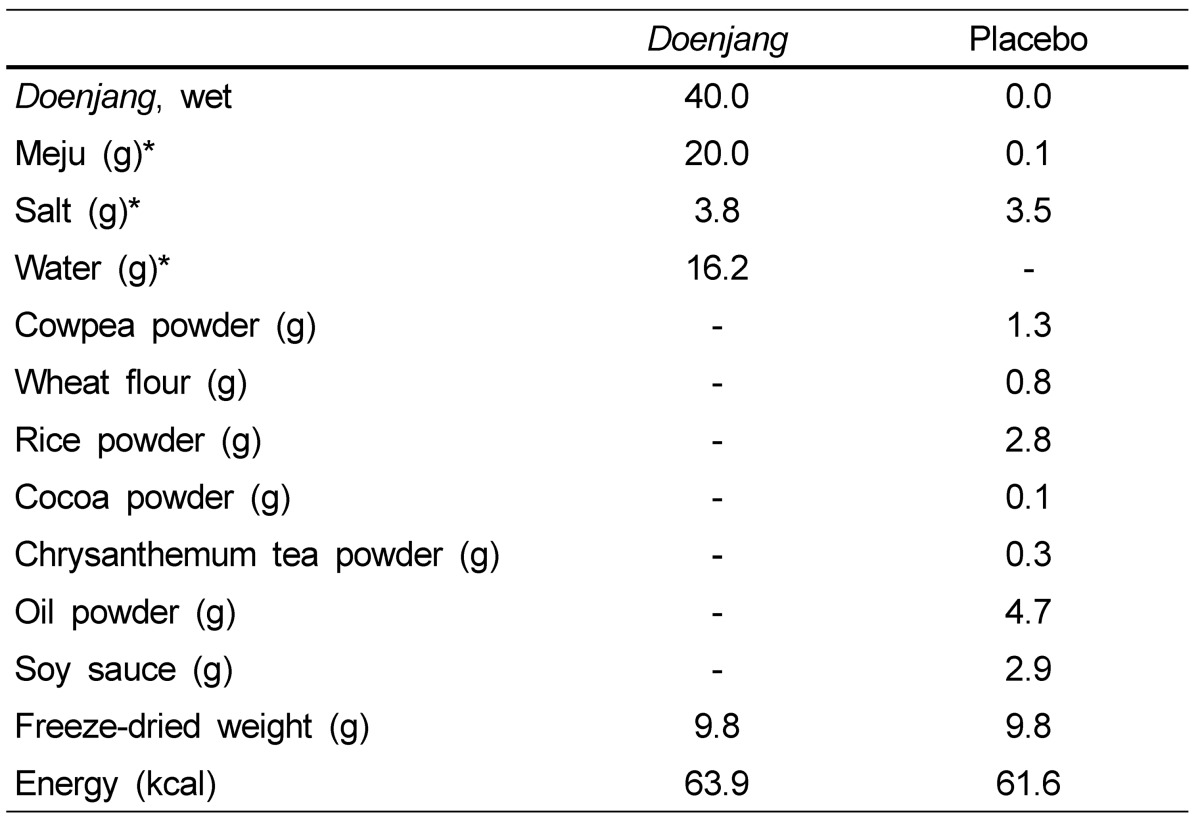
This study was a 12 week, randomized, double-blind, placebo controlled clinical trial that followed a screening period. Subjects who responded and met all of the selection criteria during a telephone interview made a screening visit. After the informed consent form was reviewed and signed, a physical examination, electrocardiogram and blood tests followed. Random numbers between 1 and 60 were generated by the computer to be assigned to each subject. The enrolled subjects were scheduled for the baseline visit when they were randomly assigned into either the Doenjang group (n = 30) or the placebo group (n = 30). The Doenjang group took 9.9 g/d (3.3 g pills/pack, three times/d) of freeze-dried Doenjang for 12 weeks, which was equivalent to 40 g of fresh Doenjang. The placebo group took the same amount of isocaloric placebo pills that were indistinguishable by shape, size, color and flavor, but without the active ingredients present in Doenjang (Table 1). Doenjang produced by the traditional method was purchased and freeze-dried using a freeze dryer (model PVTFD 100R, Ilsinlab, Yangu, Republic of Korea), and were then made into pills (Imshil Herbal Medicine Co, Imsil, Republic of Korea). The subjects were provided with Doenjang or placebo pills every 4 weeks. They were also instructed to maintain their usual dietary habits and activity levels, and use no other functional foods or dietary supplements during the 12-week intervention period.
Table 1.
Composition of Doenjang and placebo supplements (day)
*Ingredients of Doenjang before fermentation
Assessments
The subjects were asked to make a total number of five visits to the clinic: The initial screening, week 0, and at weeks 4, 8 and 12. At the initial screening visit, information on demographics, smoking, alcohol drinking, medical history, dietary intakes and vital signs were obtained; a urinary pregnancy test was conducted for women of child-bearing age. At week 0 and at the end of the 12-week intervention, anthropometric, biochemical parameters and computed tomography were measured for both groups. Anthropometric parameters (weight, BMI, body fat mass, % body fat, lean body mass and WHR) were measured using bioelectrical impedance analysis (Inbody 3.0, Biospace Co., Seoul, Korea). Fasting blood samples (> 12 hr) were analyzed for the blood lipid profile: total cholesterol, triglycerides, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), free fatty acid, apolipoprotein AI (Apo AI) and apolipoprotein B (Apo B). Blood tests were conducted with a Hitachi 7600-110 analyzer (Hitachi High-Technologies Corp., Tokyo, Japan) by the standard methods used at the clinical laboratory of Chonbuk National University Hospital. At both times, visceral fat area, subcutaneous fat area and total fat area were also measured using multi detector-row computed tomography scanning (SomatomSensation16, Siemens, Forchheim, Germany); visceral to subcutaneous ratios (VSR) were also calculated. At weeks 4 and 8 of the study, the subjects were interviewed for their current medication use, self-reported symptoms or side effects, changes in physical activities, lifestyles and eating patterns and pill compliances.
All participants completed a 3-day dietary record including two week days and one weekend day in order to evaluate the energy intake and diet quality at each clinic visit. Dietary intake data were analyzed by the same dietitian, using Can-Pro 3.0 software (The Korean Nutrition Society, Seoul, Republic of Korea).
Safety measurements
Safety assessments at the baseline and conclusion of the study also included an electrocardiogram, a hematology test and complete blood chemistry: white blood cells, red blood cells, hemoglobin, hematocrit, platelet count, total protein, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN) and creatinine. At each of the five visits, the pulse and blood pressure were measured after a 5minrest, using the digital blood pressure monitor (OMRONT4 Corp., Tokyo, Japan).
Statistical analysis
The statistical analyses were performed using the SAS version 9.0 for Windows (SAS Institute, Cary, NC, USA). Data are expressed as mean and standard errors (SE).
The sample size for the study was based on the mean (standard error) cm2 of visceral fat difference between treatments in the previous study [25], -7.8 (3.6) cm2 for the experimental group and +3.9 (6.4) cm2 for the placebo group. It estimated to provide 80% power in order to detect a difference between groups in visceral fat of 11.7 (standard deviation; 20.5) cm2 with α = 0.05, using a 2-tailed t-test of the difference between the means. A sample size of forty eight participants (24 per group), determined by calculation when considering a 20% dropout rate of the total 60 participants, were selected.
Between subjects t-tests were calculated for all variables measures in order to determine whether there were changes associated with the treatment group. Within each treatment group, paired comparison t-tests were calculated to test whether there was a change from 0 week to 12 weeks, or whether the last available value was significantly different from zero. Repeated measures analysis of variance was performed in order to see whether there were effects associated with time (with-person variable), treatment group (between-group variable) or with the interaction of time and the treatment group. The estimated outcome for the absolute change at 12 weeks (95% confidence interval [95CIs]) were calculated for the mean absolute change in the treatment and placebo groups at 12 weeks and for a global test for the treatment effect over the entire trial period. Changes in measurements over the 12-week study period were obtained by calculating the difference between the 0 week (pre) and post-intervention measurement in each group.
Results
Recruitment and study subjects
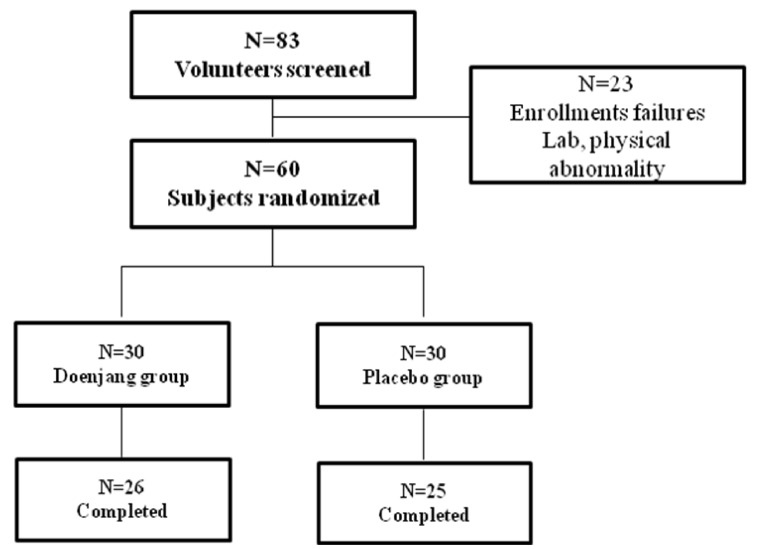
The sampling and trial profiles for the number of subjects initially screened and for those who completed or withdrew from the study are summarized in Fig. 1. Of the 83 pre-screened subjects at the onset, 23 subjects were excluded for not meeting the selection criteria either by laboratory tests and/or physical examinations. The remaining 60 subjects were divided equally and randomly between the Doenjang (n = 30) and placebo (n = 30) groups.
Fig. 1.
Flow chart for the enrollment of study subjects
Four participants from the Doenjang group (13%) and five participants from the placebo group (16%) failed to complete the study. Seven participants were disqualified because of their inadequate intake of the prescribed supplements or by not participating in other aspects of the study; two participants voluntarily withdrew. At the end, 51 subjects (Doenjang = 26, placebo = 25) remained in the study.
General characteristics and dietary assessment of subjects
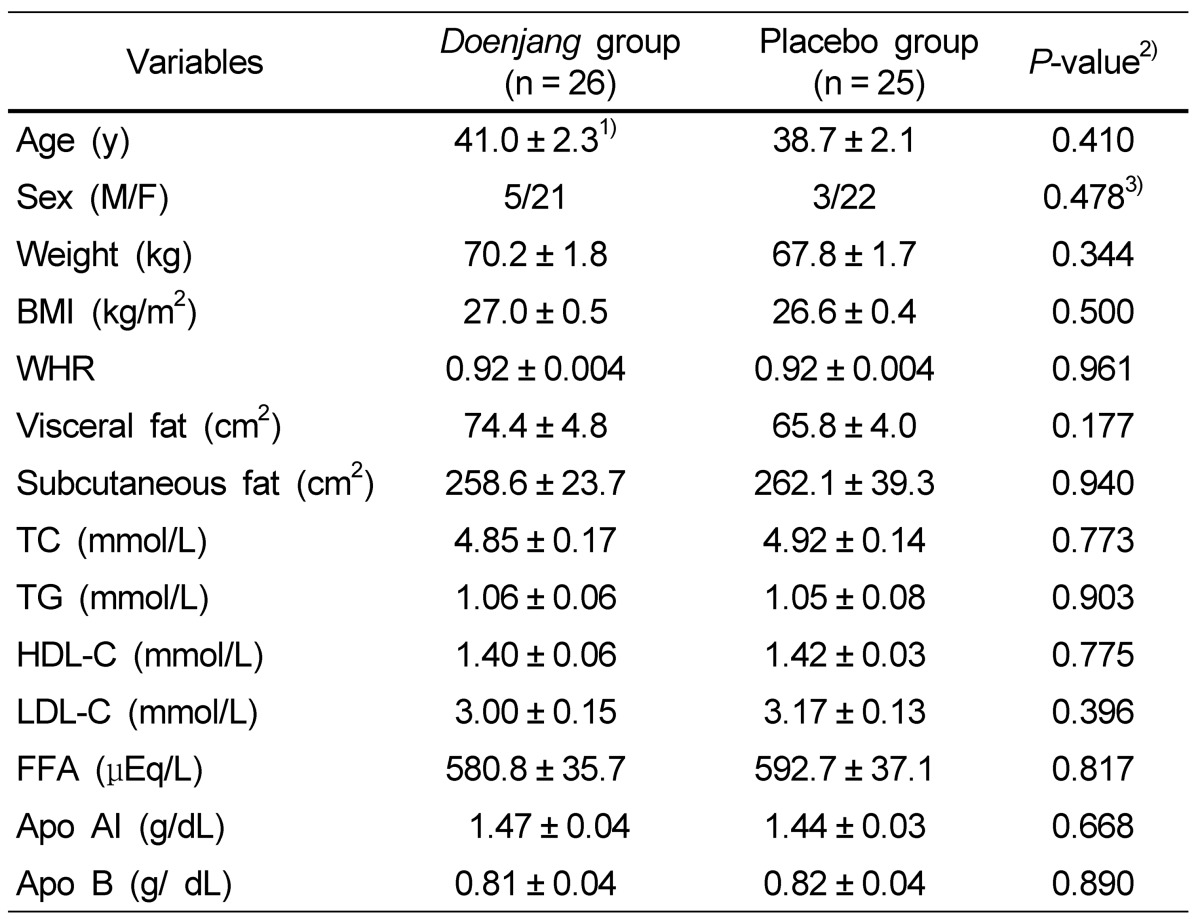
The experimental and placebo groups were similar in socio-demographic characteristics (Table 2). The dietary intakes of macro- and micro-nutrients did not change over time nor did they differ between the two groups over the course of the 12-week intervention (Data not shown).
Table 2.
Baseline characteristics of the subjects
1)Values are expressed as means ± SE.
2)By independent t test
3)Chi-square test
BMI, body mass index; WHR, waist to hip ratio; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; FFA, free fatty acid; Apo AI, Apolipoprotein AI; Apo B, Apolipoprotein B.
Body weight and components
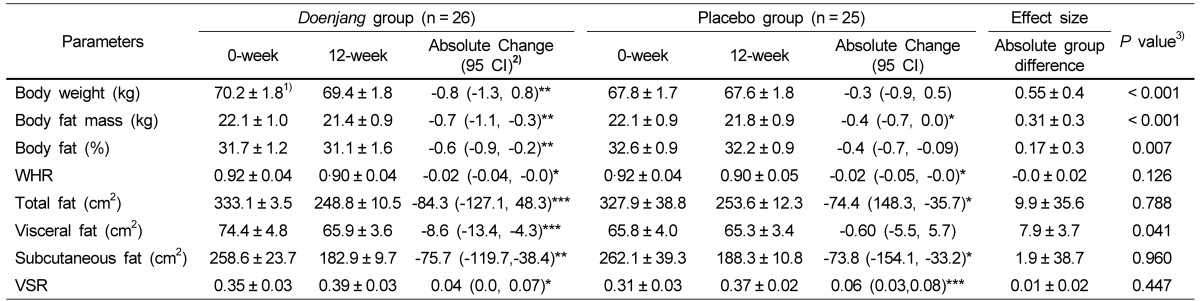
Body weight and composition data are summarized in Table 3. After 12 weeks of supplementation, subjects in the Doenjang group showed a significant reduction in body weight (P < 0.001), body fat mass (P < 0.001) and % body fat (P < 0.01), without showing changes in the waist-hip-ratio (WHR) compared with those of the placebo group. The decreases in the mean values for body weight (kg) and body fat (%) in the Doenjang group over the 12 weeks were 0.8 and 0.6, respectively.
Table 3.
Change of body weight and composition andabdominal fat area measurements at the 0-week and 12-week of the study
1)Values are expressed as means ± SE.
WHR, waist to hip ratio; VSR, Visceral to subcutaneous ratio.
2)Values in this column represent the difference between the mean changes scores of the Doenjang group and those of the placebo group;95% CIs are in parentheses.
3)Pvalues derived from repeated measures analysis (per protocol) after adjusting for age, gender and BMI.
*P < 0.05, **P < 0.01, ***P < 0.001 : P-values indicate significant differences in the variables between 0 week and 12 weeks, which were evaluated by the paired t-test.
Abdominal fat distribution by computerized tomography
Abdominal fat distribution data quantified by computerized tomography are presented in Table 3. After the 12-week intervention, the Doenjang group exhibited significant reduction in total fat area (-84.3 cm2), visceral fat area (-8.6 cm2) and subcutaneous fat area (-75.7 cm2). The placebo group also had reductions in total fat area (-74.4 cm2), visceral fat area (-0.9 cm2) and subcutaneous fat area (-73.8 cm2). The Doenjang group showed significantly reduced visceral fat area compared to the placebo group (P < 0.05).
Lipid profiles
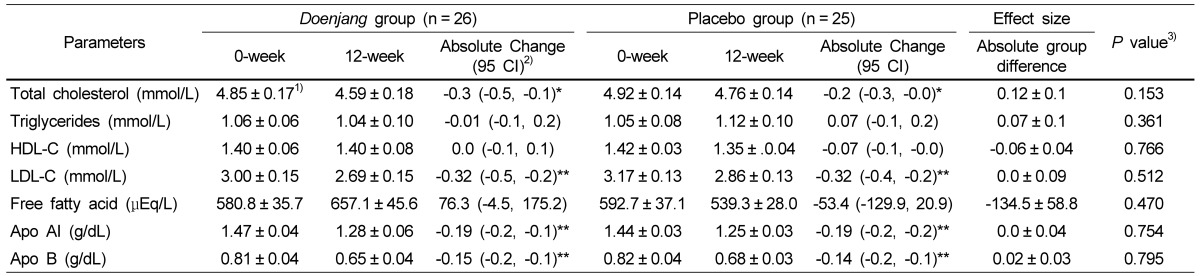
As portrayed in Table 4, after the 12-week intervention, the serum concentration of total cholesterol, LDL-C, Apo AI and Apo B decreased significantly in the Doenjang group (P < 0.001). The degree of reduction in the Doenjang group however did not differ from those observed in the placebo group.
Table 4.
Serum lipid profiles of Doenjang and placebo groups at the 0-week and at the 12-week of the study
1)Values are expressed as means ± SE.
HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; Apo AI, Apolipoprotein AI; Apo B, Apolipoprotein B.
2)Values in this column represent the difference between the mean changes scores of the Doenjang group and those of the placebo group; 95% CIs are in parentheses.
3)P values derived from repeated measures analysis (per protocol) after adjusting for age, gender and BMI.
*P < 0.05, **P < 0.01: P-values indicate significant differences in the variables between 0 week and 12 weeks, which were evaluated by the paired t-test.
Safety measurements
At each clinic visit, the investigators probed the subjects to observe if they experienced any side effects since the previous visit. No moderate or serious adverse events were reported during the 12-week study period. The evaluation was also expanded to include laboratory tests, electrocardiogram and vital signs (blood pressure, pulse) during the subjects' visits. The results of the clinical tests were in the normal range; hence, no subjects withdrew due to adverse effects.
Discussion
Many studies evaluate the effects of dietary interventions on weight loss in subjects on a low calorie diet, and therefore, assess the additive effect of the intervention in a group of subjects following a weight loss program. This study took a different approach; we evaluated the effect of adding a traditional food, Doenjang, to the diet of overweight subjects following a normal diet and lifestyle.
Doenjang is one of the most widely used fermented seasonings in Korean food culture. Anti-obesogenic effects of Doenjang and genistein have recently been reported in both cellular and animal studies [9,20-23]. It is generally accepted that the beneficial effects of genistein are mainly mediated by the changes in the expression of genes involved in cholesterol metabolism, such as hydroxymethylglutaryl-CoA (HMG-CoA) reductase and LDL receptor [21]. Furthermore, genistein and daidzein both increasehepatic CPT-1 enzyme activity by up-regulating CPT-1 transcription, a rate regulating the enzyme of fatty acid oxidation. The increase in fatty acid oxidation results in increased energy expenditure, leading to decreased body fat and weight [9,20]. Other research findings have shown that Doenjang reduces hyperlipidemia in rats fed with a high fat/high cholesterol diet [21]. Green tea-Doenjang was shown to exert anti-obesity activity in rats fed with high fat diets [22]. Among Korean traditional fermented soybean products, Doenjang shows the greatest efficacy for reducing body weight and exhibits lipid lowering activities in rats [24]. Animal experiments have consistently shown that Doenjang-containing food products are effective for weight loss [9,22-24]. These studies have suggested that Doenjang has more positive effects on weight management than any other soybean products, partially due to its longer fermentation and ripening process. However, the doses used in many of the cited animal studies were higher than would ever be used for human diets. Therefore, this study was designed to evaluate its effects in humans at dietary levels that are realistic for human consumption. We thus hypothesized that Doenjang (Korean fermented soybean paste) supplementation will result in favorable changes in anthropometric parameters and abdominal fat distribution as assessed by CT scans and lipid profiles in overweight subjects (BMI ≥ 23 kg/m2).
We found that subjects in the Doenjang group had decreased mean body weight, body fat mass and % body fat without changing WHR when compared to the baseline data. Although the placebo group experienced decreases in two of the four above mentioned parameters, reduction in body weight (P < 0.001), body fat mass (P < 0.001) and % body fat (P < 0.01) were significantly less than the Doenjang group and did reach a statistical significance. In a previous study, L-carnitine and isoflavone supplements given to overweight/obese women for 12 weeks resulted in a significant decrease in body weight and percent body fat in the treatment group, whereas in the placebo group, body weight and percent body fat remained unchanged [25]. Of the obese subjects, those who tended to have a higher visceral fat percentage than subcutaneous fat, and higher abdominal fat percentages than posterior fat, were at increased risk for atherosclerosis. The risk for cardiovascular diseases is common in people with abnormal metabolic activities. Previous reports have demonstrated that visceral fat accumulation is specifically associated with metabolic alterations (e.g., insulin resistance, elevated serum triglycerides) of obesity [26,27].
In this study, CT scans showed that the abdominal fat area, i.e., total fat area and subcutaneous fat area were significantly reduced in both groups after the intervention period; however, the differences were not significant between the groups. On the other hand, the visceral fat area was significantly reduced by -8.6 cm2 (P < 0.001) in the Doenjang group, whereas no change was realized in the placebo group (-0.6 cm2). In particular, the Doenjang group showed significantly reduced visceral fat area compared with the placebo group (P < 0.05). Other investigators have reported that taking 75 mg/day of isoflavone for one year resulted in no improvements in BMI or body fat distribution; however, trunk fat mass significantly decreased [28]. Gwak et al. [25] found that L-carnitine and isoflavone supplements given to overweight women for 12 weeks significantly decreased both the total fat area at the L4 level and the visceral fat area, which was similar to the findings of this study. Therefore, prolonged intake of isoflavone-rich soybeans and fermented bean products, such as Doenjang, seemed to decrease abdominal fat, particularly visceral fat, which is important for preventing age-related chronic diseases. The dosage used in this study was equivalent to 40 g wet weight of Doenjang (dry weight 9.9 g), roughly equivalent to 3 bowls of Doenjang soup (similar to Japanese miso soup), which contains approximately 13.3 g of Doenjang each, thereby supplying about 20-30 mg of isoflavones (containing 8.57 mg aglycones [29]) per day. This level produces plasma concentrations of isoflavones ranging around 200-300 nmol/L, which is typical of many orally-ingested therapeutics [30].
In a previous study, cholesterol-lowering substances were isolated from Doenjang and were identified as aglycones: genistein, daidzein, glycitein. Since isoflavone aglycones inhibit HMG-CoA reductase, Doenjang may also help prevent cardiovascular diseases [31]. Several animal studies have shown that Doenjang decreases blood triglyceride and cholesterol levels [9,21,22,24]. In this study, lipid profiles and apolipoprotein concentrations did not significantly differ between the Doenjang and placebo groups. The lack of Doenjang treatment effect may be explained by the fact that the study subjects were healthy subjects with no known abnormalities in serum cholesterol and triglycerides. The serum lipid and lipoprotein concentrations of all participants fell within a normal range, therefore, the results seem to be insufficient for assessing those particular effects.
In the present study, the limitations are also its strengths. Since Doenjang, as a whole food, was used in the study, it is not possible to ascertain which compounds were responsible for the decreased body weight and visceral adiposity or by what mechanism due to the complex composition of Doenjang. However, the presence of a high level of isoflavones in Doenjang may be the cause. The subjects were instructed to maintain their normal dietary habits and physical activities during the 12-week intervention period; however, compliance to the instruction could not be validated. Yet, this study revealed that a whole food can have beneficial effects on body composition in a free living environment. To the best of our knowledge, no other studies, to date, have reported the effects of Doenjang supplementation for decreasing the risk of obesity, particularly abdominal fat, in overweight people.
The results of this study showed that 40 g (dried weight, 9.9 g) of Doenjang supplement for 12 weeks significantly improved body weight and abdominal obesity compared with the placebo group. We thus conclude that the intake of Doenjang, along with a controlled diet and exercise, would be effective for reducing abdominal fat and weight management in overweight people. Additional research on the effect of prolonged intake of Doenjang in a larger study and longer-term study is needed.
Footnotes
This study was supported by grants from the Ministry for Food, Agriculture, Forestry and Fisheries (20080410496-00).
References
- 1.de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 2.Jones-Smith JC, Gordon-Larsen P, Siddiqi A, Popkin BM. Cross-national comparisons of time trends in overweight inequality by socioeconomic status among women using repeated cross-sectional surveys from 37 developing countries, 1989-2007. Am J Epidemiol. 2011;173:667–675. doi: 10.1093/aje/kwq428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonçalves FB, Koek M, Verhagen HJ, Niessen WJ, Poldermans D. Body-mass index, abdominal adiposity, and cardiovascular risk. Lancet. 2011;378:227. doi: 10.1016/S0140-6736(11)61121-5. [DOI] [PubMed] [Google Scholar]
- 4.Velasquez MT, Bhathena SJ. Role of dietary soy protein in obesity. Int J Med Sci. 2007;4:72–82. doi: 10.7150/ijms.4.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon DY, Daily JW, 3rd, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res. 2010;30:1–13. doi: 10.1016/j.nutres.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Namgung HJ, Park HJ, Cho IH, Choi HK, Kwon DY, Shim SM, Kim YS. Metabolite profiling of doenjang, fermented soybean paste, during fermentation. J Sci Food Agric. 2010;90:1926–1935. doi: 10.1002/jsfa.4036. [DOI] [PubMed] [Google Scholar]
- 7.Kwon DY, Hong SM, Ahn IS, Kim MJ, Yang HJ, Park S. Isoflavonoids and peptides from meju, long-term fermented soybeans, increase insulin sensitivity and exert insulinotropic effects in vitro. Nutrition. 2011;27:244–252. doi: 10.1016/j.nut.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima N, Nozaki N, Ishihara K, Ishikawa A, Tsuji H. Analysis of isoflavone content in tempeh, a fermented soybean, and preparation of a new isoflavone-enriched tempeh. J Biosci Bioeng. 2005;100:685–687. doi: 10.1263/jbb.100.685. [DOI] [PubMed] [Google Scholar]
- 9.Kwak CS, Park SC, Song KY. Doenjang, a fermented soybean paste, decreased visceral fat accumulation and adipocyte size in rats fed with high fat diet more effectively than nonfermented soybeans. J Med Food. 2012;15:1–9. doi: 10.1089/jmf.2010.1224. [DOI] [PubMed] [Google Scholar]
- 10.Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, Choi SH, Cho SI, Park KS, Lee HK, Jang HC, Koh KK. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care. 2011;34:1323–1328. doi: 10.2337/dc10-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee MJ, Popkin BM, Kim S. The unique aspects of the nutrition transition in South Korea: the retention of healthful elements in their traditional diet. Public Health Nutr. 2002;5:197–203. doi: 10.1079/PHN2001294. [DOI] [PubMed] [Google Scholar]
- 12.Song Y, Joung H. A traditional Korean dietary pattern and metabolic syndrome abnormalities. Nutr Metab Cardiovasc Dis. 2012;22:456–462. doi: 10.1016/j.numecd.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Kim YS, Kim MC, Kwon SW, Kim SJ, Park IC, Ka JO, Weon HY. Analyses of bacterial communities in meju, a Korean traditional fermented soybean bricks, by cultivation-based and pyrosequencing methods. J Microbiol. 2011;49:340–348. doi: 10.1007/s12275-011-0302-3. [DOI] [PubMed] [Google Scholar]
- 14.Park KY, Jung KO, Rhee SH, Choi YH. Antimutagenic effects of doenjang (Korean fermented soypaste) and its active compounds. Mutat Res. 2003;523-524:43–53. doi: 10.1016/s0027-5107(02)00320-2. [DOI] [PubMed] [Google Scholar]
- 15.Aoki H, Furuya Y, Endo Y, Fujimoto K. Effect of gamma-aminobutyric acid-enriched tempeh-like fermented soybean (GABA-Tempeh) on the blood pressure of spontaneously hypertensive rats. Biosci Biotechnol Biochem. 2003;67:1806–1808. doi: 10.1271/bbb.67.1806. [DOI] [PubMed] [Google Scholar]
- 16.Jung KO, Park SY, Park KY. Longer aging time increases the anticancer and antimetastatic properties of doenjang. Nutrition. 2006;22:539–545. doi: 10.1016/j.nut.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Fan J, Zhang Y, Chang X, Saito M, Li Z. Changes in the radical scavenging activity of bacterial-type douchi, a traditional fermented soybean product, during the primary fermentation process. Biosci Biotechnol Biochem. 2009;73:2749–2753. doi: 10.1271/bbb.90361. [DOI] [PubMed] [Google Scholar]
- 18.Chen YC, Sugiyama Y, Abe N, Kuruto-Niwa R, Nozawa R, Hirota A. DPPH radical-scavenging compounds from dou-chi, a soybean fermented food. Biosci Biotechnol Biochem. 2005;69:999–1006. doi: 10.1271/bbb.69.999. [DOI] [PubMed] [Google Scholar]
- 19.Choi J, Kwon SH, Park KY, Yu BP, Kim ND, Jung JH, Chung HY. The anti-inflammatory action of fermented soybean products in kidney of high-fat-fed rats. J Med Food. 2011;14:232–239. doi: 10.1089/jmf.2010.1039. [DOI] [PubMed] [Google Scholar]
- 20.Yang JY, Lee SJ, Park HW, Cha YS. Effect of genistein with carnitine administration on lipid parameters and obesity in C57Bl/6J mice fed a high-fat diet. J Med Food. 2006;9:459–467. doi: 10.1089/jmf.2006.9.459. [DOI] [PubMed] [Google Scholar]
- 21.Pyo YH, Seong KS. Hypolipidemic effects of Monascus-fermented soybean extracts in rats fed a high-fat and -cholesterol diet. J Agric Food Chem. 2009;57:8617–8622. doi: 10.1021/jf901878c. [DOI] [PubMed] [Google Scholar]
- 22.Park JH, Ha AW, Cho JS. Effects of green tea-soybean paste on weights and serum lipid profiles in rats fed high fat diet. Korean J Food Sci Technol. 2005;37:806–811. [Google Scholar]
- 23.Kim S, Shin HJ, Kim SY, Kim JH, Lee YS, Kim DH, Lee MO. Genistein enhances expression of genes involved in fatty acid catabolism through activation of PPARalpha. Mol Cell Endocrinol. 2004;220:51–58. doi: 10.1016/j.mce.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Kwon SH, Lee KB, Im KS, Kim SO, Park KY. Weight reduction and lipid lowering effects of korean traditional soybean fermented products. J Korean Soc Food Sci Nutr. 2006;35:1194–1199. [Google Scholar]
- 25.Gwak JH, Lee JH, Lee SJ, Park HW, Kim Y, Hyun YJ. The effect of L-carnitine and isoflavone supplementation on weight reduction and visceral fat accumulation in overweight women. Korean J Nutr. 2007;40:630–638. [Google Scholar]
- 26.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 27.Demerath EW, Reed D, Rogers N, Sun SS, Lee M, Choh AC, Couch W, Czerwinski SA, Chumlea WC, Siervogel RM, Towne B. Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levels. Am J Clin Nutr. 2008;88:1263–1271. doi: 10.3945/ajcn.2008.26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Oka J, Tabata I, Higuchi M, Toda T, Fuku N, Ezaki J, Sugiyama F, Uchiyama S, Yamada K, Ishimi Y. Effects of isoflavone and exercise on BMD and fat mass in postmenopausal Japanese women: a 1-year randomized placebo-controlled trial. J Bone Miner Res. 2006;21:780–789. doi: 10.1359/jbmr.060208. [DOI] [PubMed] [Google Scholar]
- 29.Jang CH, Park CS, Lim JK, Kim JH, Kwon DY, Kim YS, Shin DH, Kim JS. Metabolism of isoflavone derivatives during manufacturing of traditional Meju and Doenjang. Food Sci Biotechnol. 2008;17:442–445. [Google Scholar]
- 30.Busby MG, Jeffcoat AR, Bloedon LT, Koch MA, Black T, Dix KJ, Heizer WD, Thomas BF, Hill JM, Crowell JA, Zeisel SH. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr. 2002;75:126–136. doi: 10.1093/ajcn/75.1.126. [DOI] [PubMed] [Google Scholar]
- 31.Sung JH, Choi SJ, Lee SW, Park KH, Moon TW. Isoflavones found in Korean soybean paste as 3-hydroxy-3-methylglutaryl Coenzyme A reductase inhibitors. Biosci Biotechnol Biochem. 2004;68:1051–1058. doi: 10.1271/bbb.68.1051. [DOI] [PubMed] [Google Scholar]