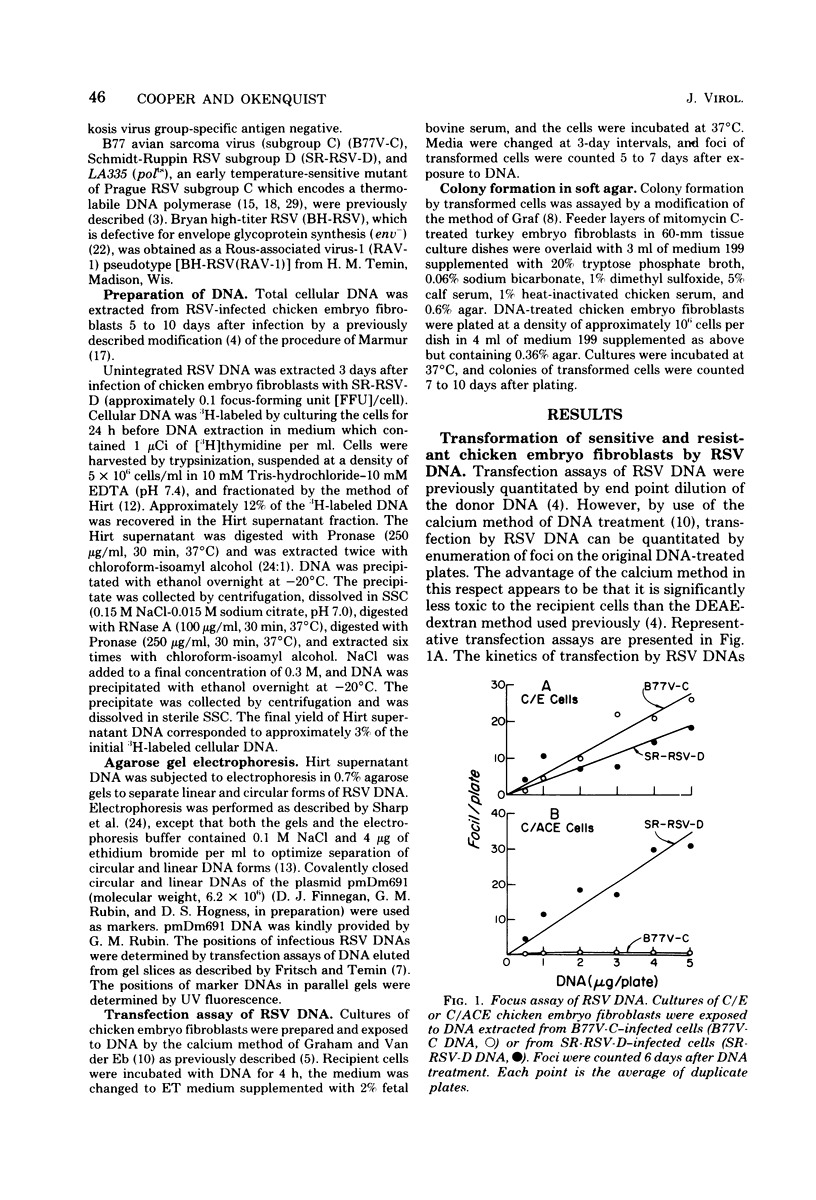
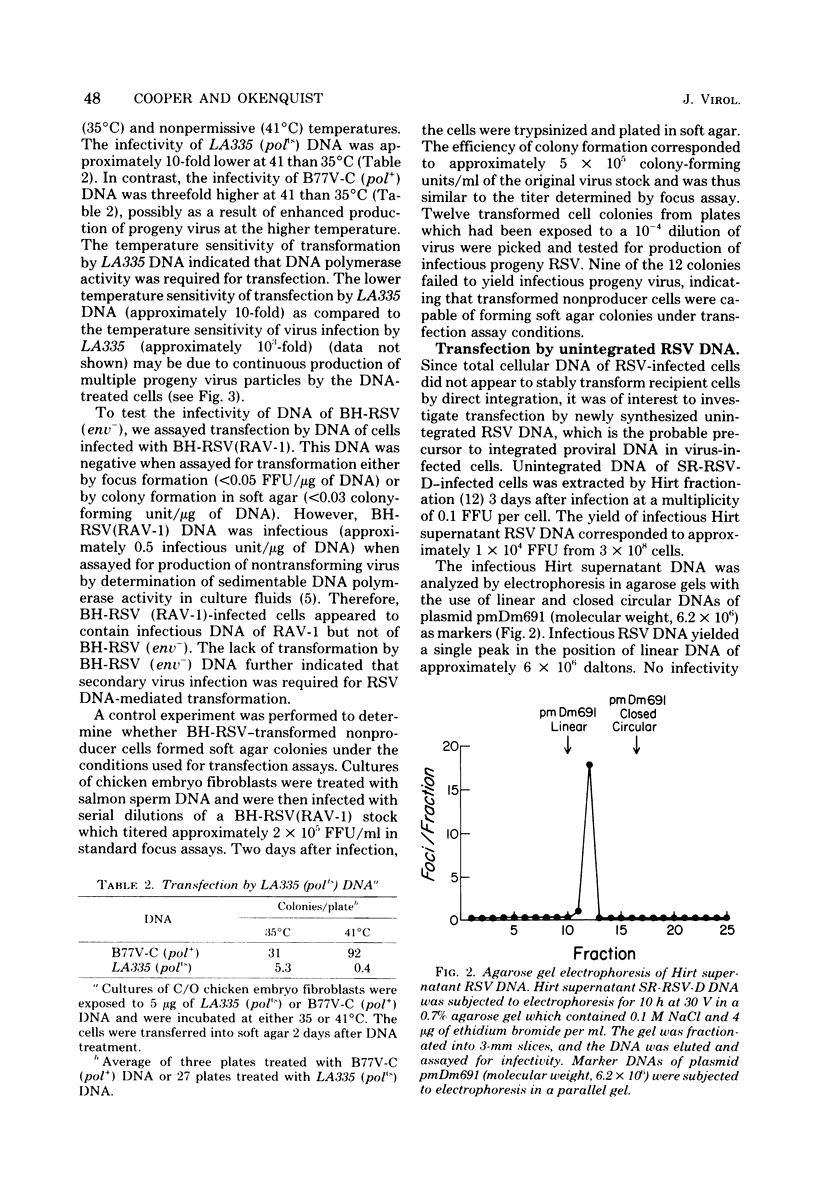
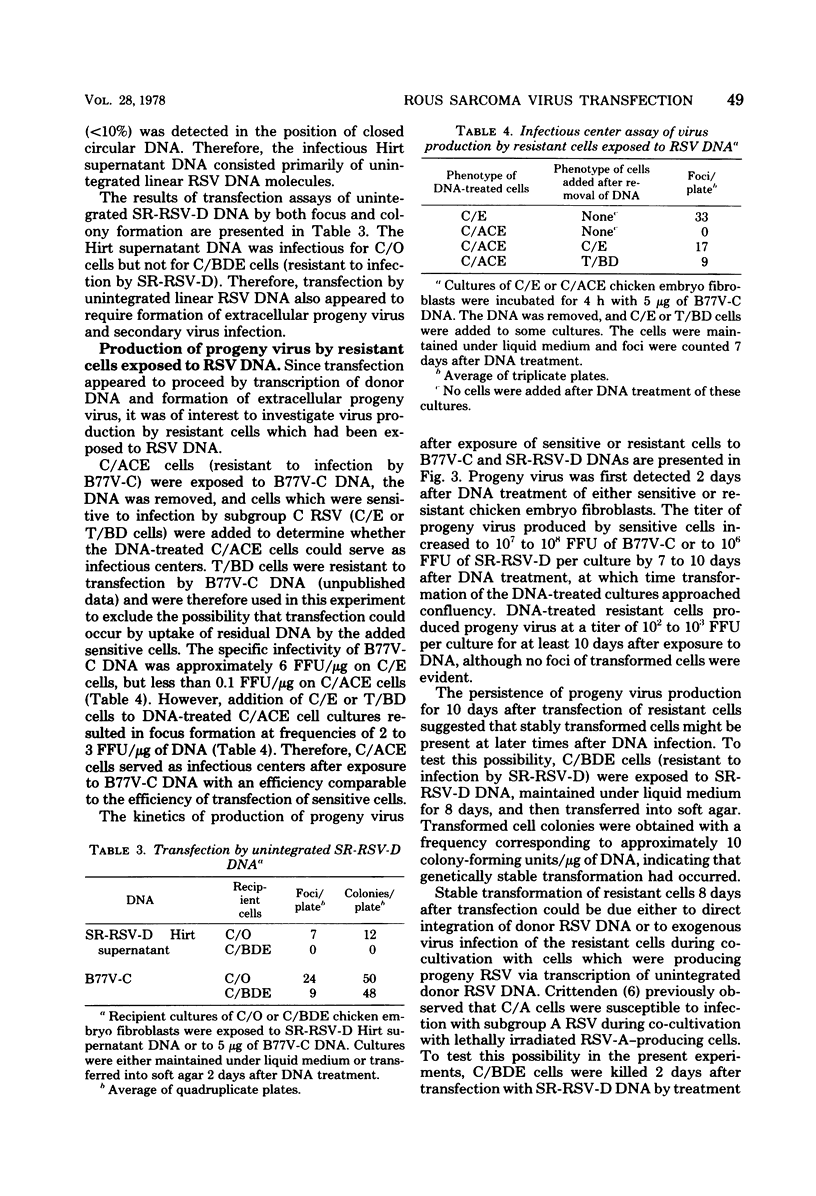
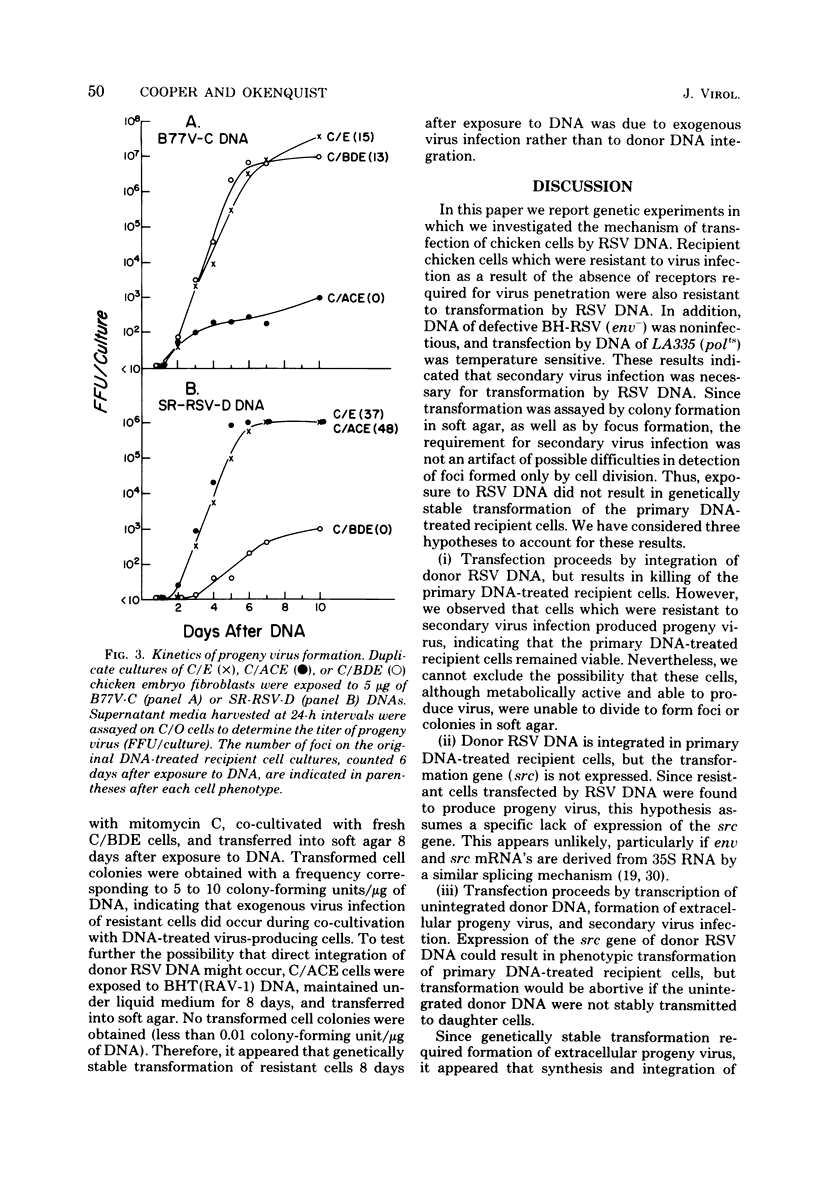
Abstract
The mechanism of transfection by Rous sarcoma virus DNA was investigated by assaying DNA-mediated transformation under conditions which restricted secondary virus infection. Chicken embryo fibroblasts which were genetically resistant to virus infection as a result of the absence of receptors for virus penetration were also resistant to transformation by integrated or unintegrated Rous sarcoma virus DNA. In addition, DNA of replication-defective Bryan hightiter Rous sarcoma virus was noninfectious, and transformation by DNA of a temperature-sensitive DNA polymerase mutant was temperature sensitive. These results indicated that secondary virus infection was necessary for transformation by Rous sarcoma virus DNA. Since transformation was assayed by colony formation in soft agar, as well as by focus formation, the requirement for secondary virus infection was not an artifact of potential difficulty in detection of foci formed by division of single transformed cells. Therefore, it appeared that donor DNA did not stably transform recipient cells by direct integration. Instead, the results were consistent with the hypothesis that transfection of chicken embryo fibroblasts by Rous sarcoma virus DNA proceeded by transcription of donor DNA, formation of extracellular progeny virus, and secondary virus infection of sensitive cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canaani E., Helm K. V., Duesberg P. Evidence for 30-40S RNA as precursor of the 60-70S RNA of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1973 Feb;70(2):401–405. doi: 10.1073/pnas.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Castellot S. B. Assay of noninfectious fragments of DNA of avian leukosis virus-infected cells by marker rescue. J Virol. 1977 May;22(2):300–307. doi: 10.1128/jvi.22.2.300-307.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Infectious rous sarcoma virus and reticuloendotheliosis virus DNAs. J Virol. 1974 Nov;14(5):1132–1141. doi: 10.1128/jvi.14.5.1132-1141.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Lack of infectivity of the endogenous avian leukosis virus-related genes in the DNA of uninfected chicken cells. J Virol. 1976 Feb;17(2):422–430. doi: 10.1128/jvi.17.2.422-430.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden L. B. Observations on the nature of a genetic cellular resistance to avian tumor viruses. J Natl Cancer Inst. 1968 Jul;41(1):145–153. [PubMed] [Google Scholar]
- Fritsch E., Temin H. M. Formation and structure of infectious DNA of spleen necrosis virus. J Virol. 1977 Jan;21(1):119–130. doi: 10.1128/jvi.21.1.119-130.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T. Two types of target cells for transformation with avian myelocytomatosis virus. Virology. 1973 Aug;54(2):398–413. doi: 10.1016/0042-6822(73)90152-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L. Biological activity of tumor virus DNA. Adv Cancer Res. 1977;25:1–51. doi: 10.1016/s0065-230x(08)60631-4. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Richards O. C., Shank P. R., Kung H. J., Davidson N. Covalently closed circular DNA of avian sarcoma virus: purification from nuclei of infected quail tumor cells and measurement by electron microscopy and gel electrophoresis. J Mol Biol. 1976 Sep 15;106(2):337–357. doi: 10.1016/0022-2836(76)90090-5. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Johnson P. H., Grossman L. I. Electrophoresis of DNA in agarose gels. Optimizing separations of conformational isomers of double- and single-stranded DNAs. Biochemistry. 1977 Sep 20;16(19):4217–4225. doi: 10.1021/bi00638a014. [DOI] [PubMed] [Google Scholar]
- Karpas A., Milstein C. Recovery of the genome of murine sarcoma virus (MSV) after infection of cells with nuclear DNA from MSV transformed non-virus producing cells. Eur J Cancer. 1973 Apr;9(4):295–299. doi: 10.1016/0014-2964(73)90097-2. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Friis R. R., Linial M., Vogt P. K. Determination of the defective function in two mutants of Rous sarcoma virus. Virology. 1974 Oct;61(2):559–574. doi: 10.1016/0042-6822(74)90290-6. [DOI] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Proteins of helper-dependent RSV. Virology. 1971 Aug;45(2):401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Varmus H. E. Virus-specific DNA in the cytoplasm of avian sarcoma virus-infected cells is a precursor to covalently closed circular viral DNA in the nucleus. J Virol. 1978 Jan;25(1):104–104. doi: 10.1128/jvi.25.1.104-104.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Smith E. J., Crittenden L. B., Brinsfield T. H., Jr Status of the endogenous avian leukosis virus in resistant cells from a producing line. Virology. 1974 Oct;61(2):594–596. doi: 10.1016/0042-6822(74)90293-1. [DOI] [PubMed] [Google Scholar]
- Smotkin D., Gianni A. M., Rozenblatt S., Weinberg R. A. Infectious viral DNA of murine leukemia virus. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4910–4913. doi: 10.1073/pnas.72.12.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Yoshimura F. K., Weinberg R. A. Infectious, linear, unintegrated DNA of Moloney murine leukemia virus. J Virol. 1976 Dec;20(3):621–626. doi: 10.1128/jvi.20.3.621-626.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Shank P. R. Unintegrated viral DNA is synthesized in the cytoplasm of avian sarcoma virus-transformed duck cells by viral DNA polymerase. J Virol. 1976 May;18(2):567–573. doi: 10.1128/jvi.18.2.567-573.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Mason W. S., Drost S. D., Baltimore D. DNA polymerase activity from two temperature-sensitive mutants of Rous sarcoma virus is thermolabile. Nature. 1974 Sep 6;251(5470):27–31. doi: 10.1038/251027a0. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]


