Abstract
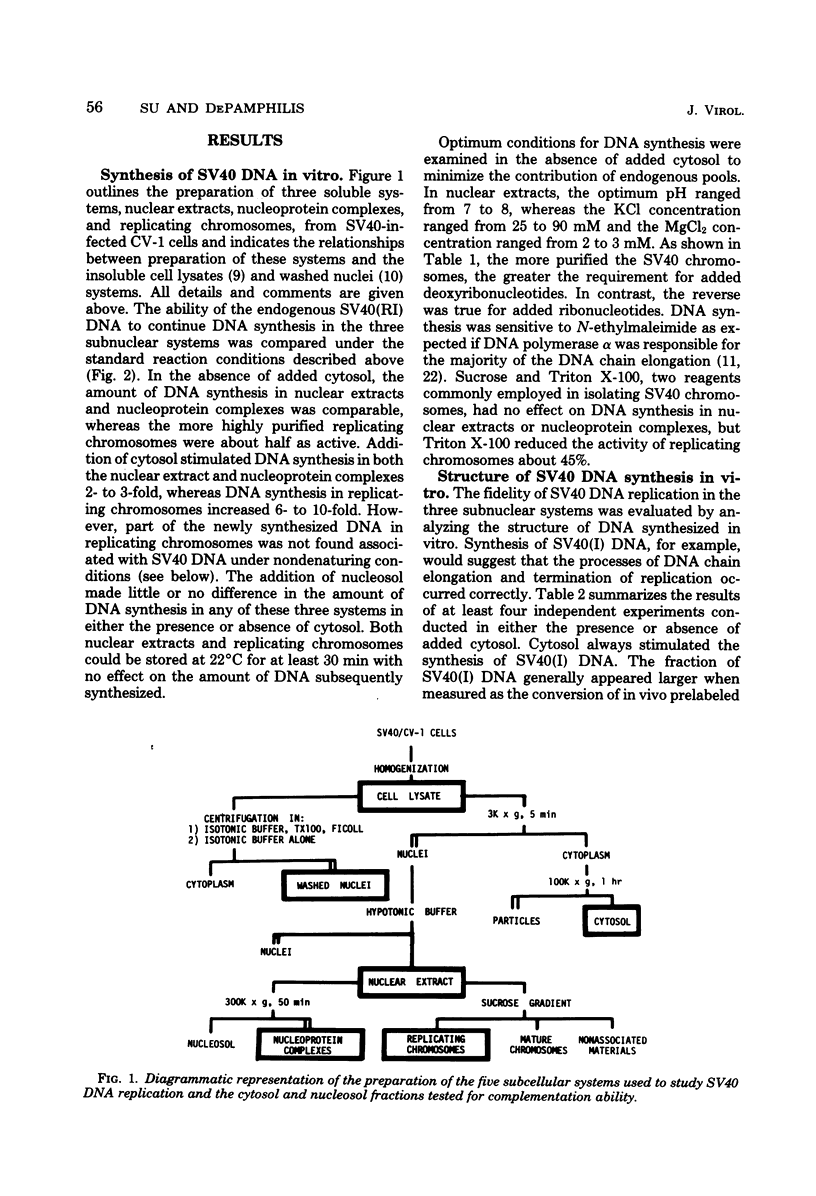
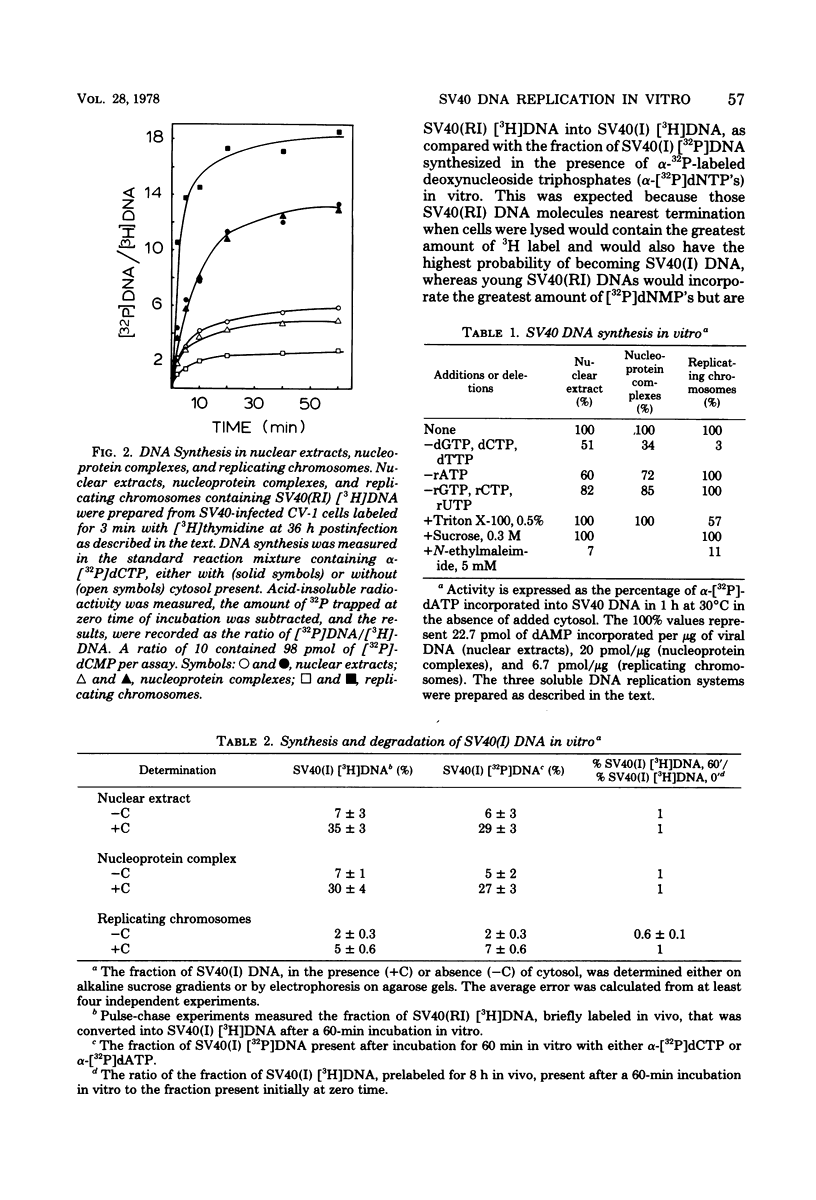
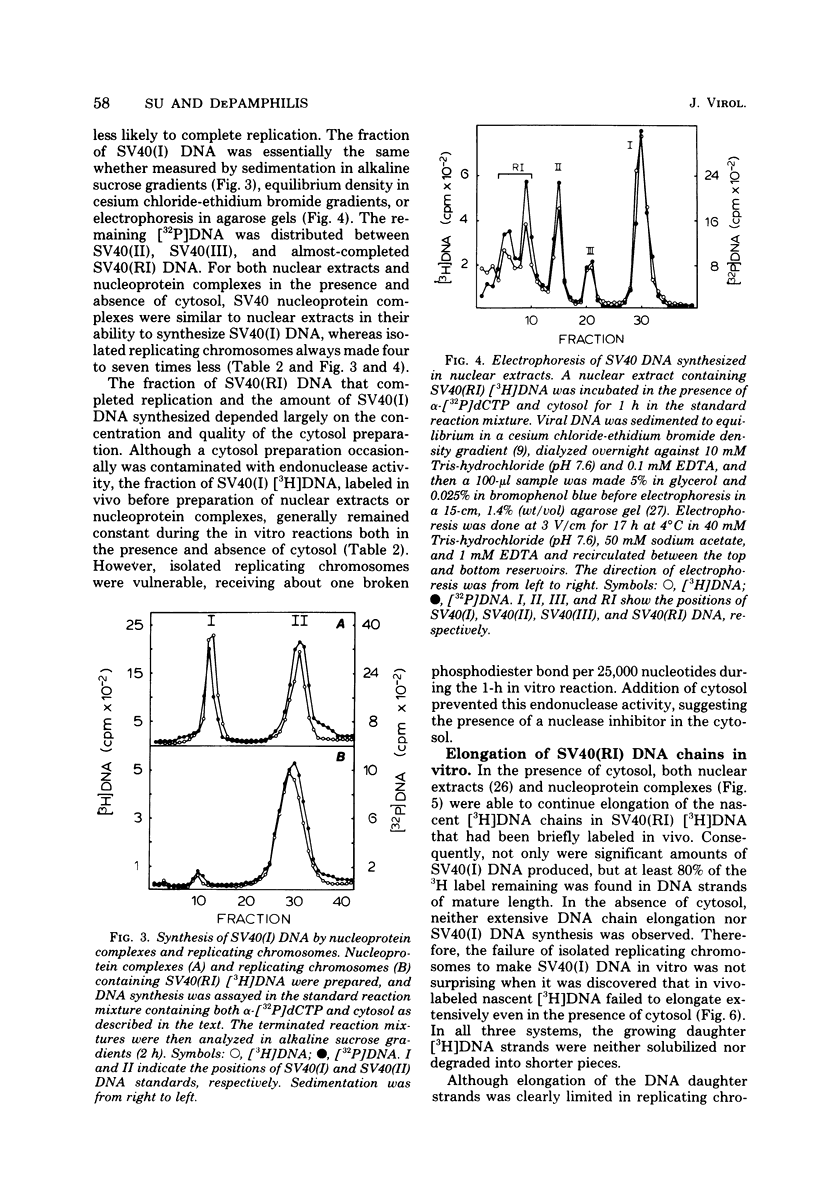
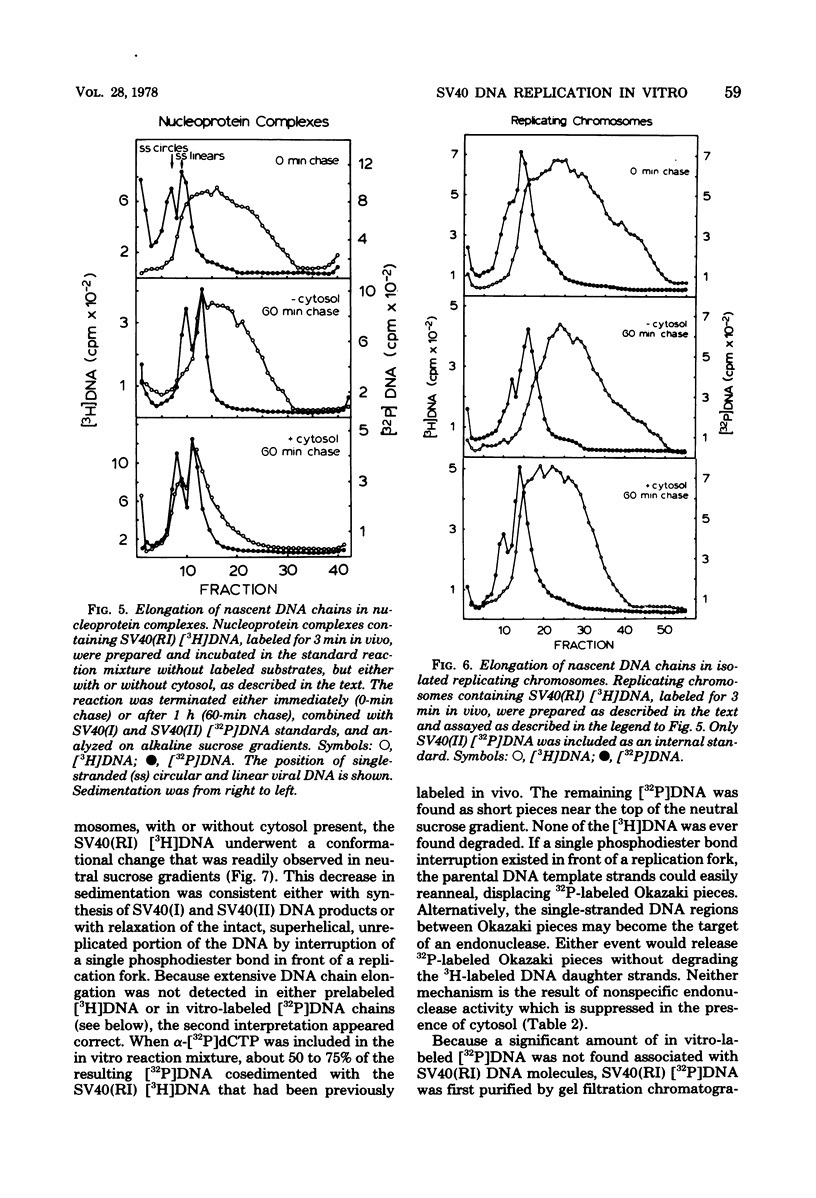
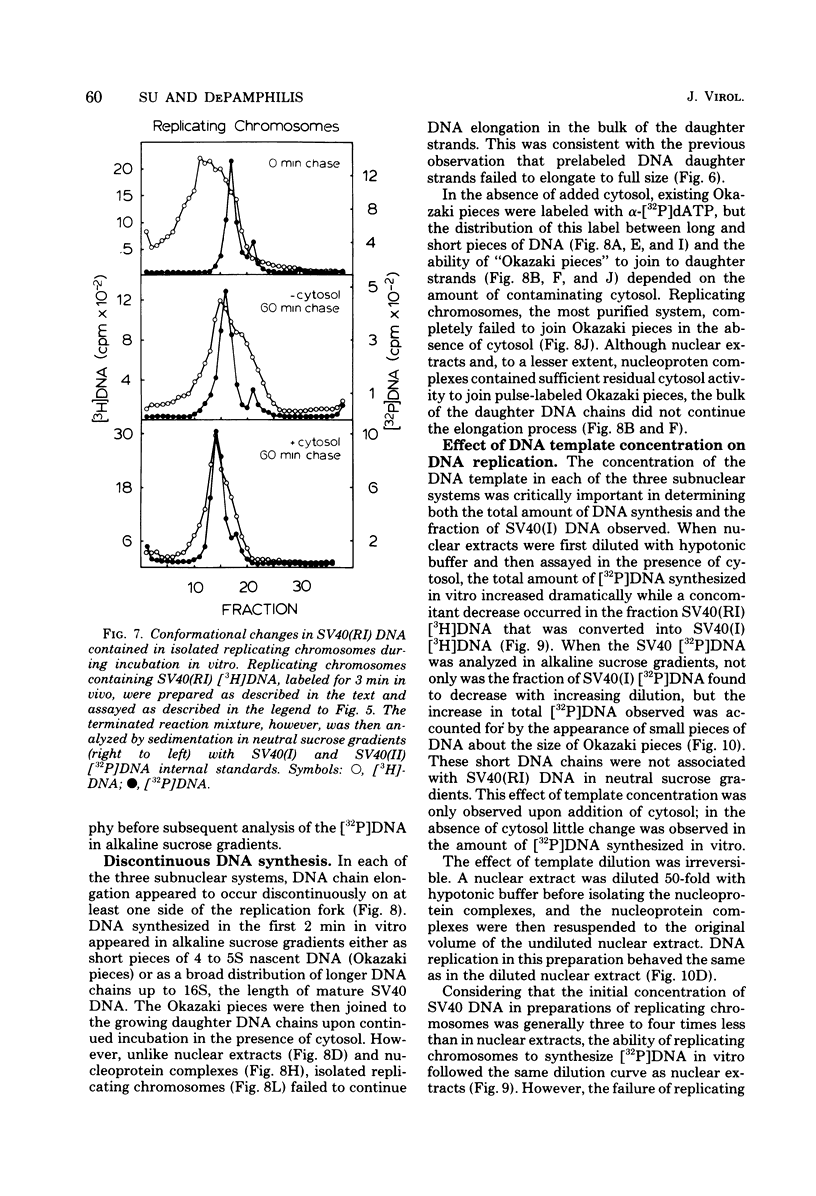
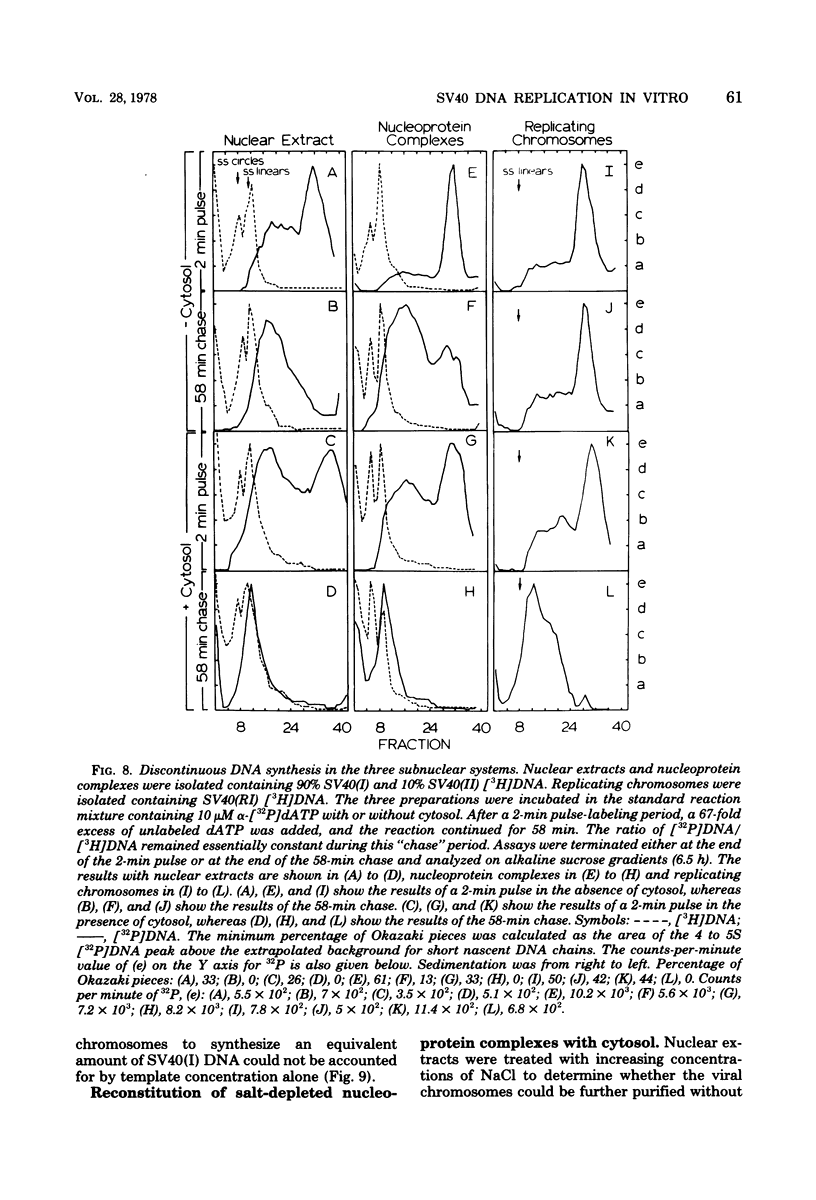
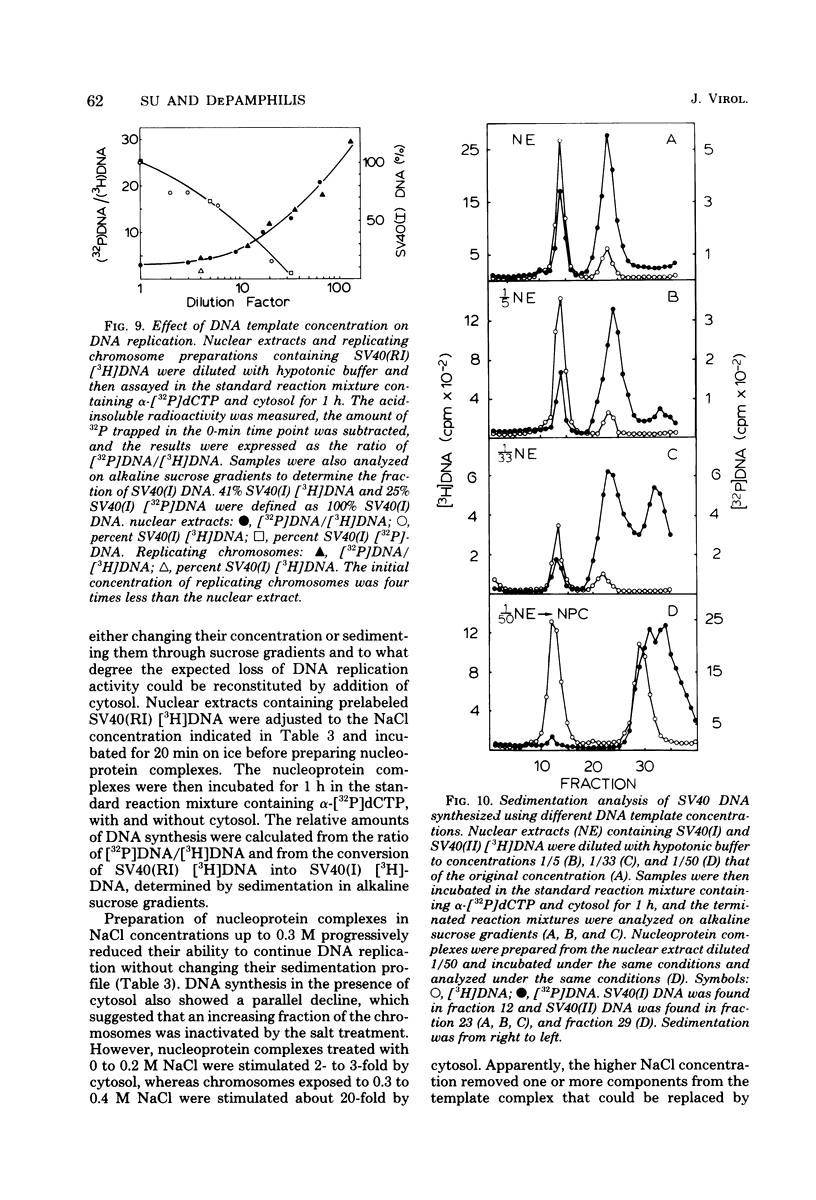
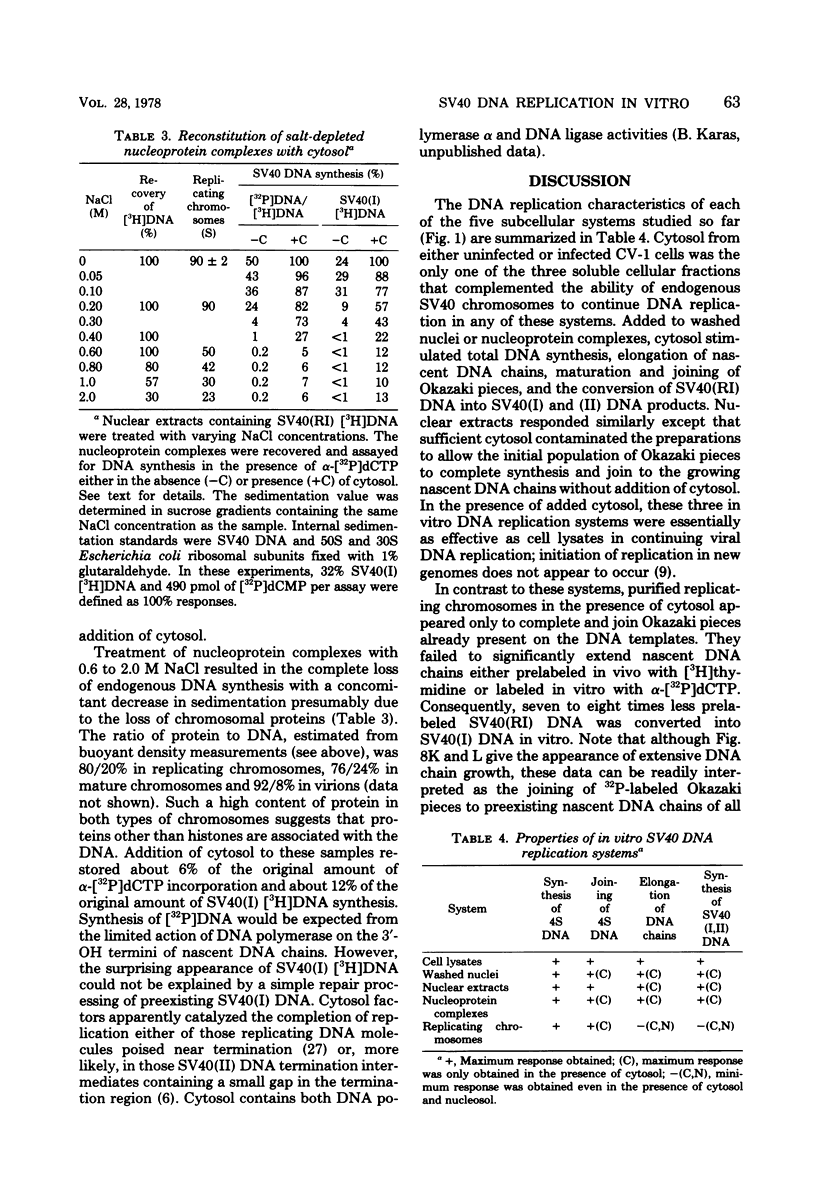
Three subnuclear systems capable of continuing many aspects of simian virus 40 (SV40) DNA replication were characterized in an effort to define the minimum requirements for “normal” DNA replication in vitro. Nuclear extracts, prepared by incubating nuclei isolated from SV40-infected CV-1 cells in a hypotonic buffer to release both SV40 replicating and mature chromosomes, were either centrifuged to separate the total SV40 nucleoprotein complexes from the soluble nucleosol or fractionated on sucrose gradients to provide purified SV40 replicating chromosomes. With nuclear extracts, CV-1 cell cytosol stimulated total DNA synthesis, elongation of nascent DNA chains, maturation and joining of “Okazaki pieces,” and the conversion of replicating viral DNA into covalently closed, superhelical DNA. Nucleoprotein complexes responded similarly, but frequently the response was reduced by 10 to 30%. In contrast, isolated replicating chromosomes in the presence of cytosol appeared only to complete and join Okazaki pieces already present on the template; without cytosol, Okazaki pieces incorporated α-32P-labeled deoxynucleoside triphosphates but failed to join. Consequently, replicating chromosomes failed to extensively continue nascent DNA chain growth, and the conversion of viral replicating DNA into mature DNA was seven to eight times less than that observed in nuclear extracts. Addition of neither cytosol nor nucleosol corrected this problem. In the presence of cytosol, nonspecific endonuclease activity was not a problem in any of the three in vitro systems. Extensive purification of replicating chromosomes was limited by three as yet irreversible phenomena. First, replicating chromosomes isolated in a low-ionic-strength medium had a limited capability to continue DNA synthesis. Second, diluting either nuclear extracts or replicating chromosomes before incubation in vitro stimulated total DNA synthesis but was accompanied by the simultaneous appearance of small-molecular-weight nascent DNA not associated with intact viral DNA templates and a decrease in the synthesis of covalently closed viral DNA. Although this second phenomenon appeared similar to the first, template concentration alone could not account for the failure of purified replicating chromosomes to yield covalently closed DNA. Finally, preparation of nucleoprotein complexes in increasing concentrations of NaCl progressively decreased their ability to continue DNA replication. Exposure to 0.3 M NaCl removed one or more factors required for DNA synthesis which could be replaced by addition of cytosol. However, higher NaCl concentrations yielded nucleoprotein complexes that had relatively no endogenous DNA synthesis activity and that no longer responded to cytosol. These data demonstrate that continuation of endogenous DNA replication in vitro requires both the soluble cytosol fraction and a complex nucleoprotein template whose ability to continue DNA synthesis depends on its concentration and ionic environment during its preparation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B., Sternglanz R. Recent excitement in the DNA replication problem. Nature. 1977 Oct 20;269(5630):655–661. doi: 10.1038/269655a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., Kaufman G., DePamphilis M. L. RNA primers in SV40 DNA replication: identification of transient RNA-DNA covalent linkages in replicating DNA. Biochemistry. 1977 Nov 15;16(23):4990–4998. doi: 10.1021/bi00642a009. [DOI] [PubMed] [Google Scholar]
- BLACK P. H., CRAWFORD E. M., CRAWFORD L. V. THE PURIFICATION OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:381–387. doi: 10.1016/0042-6822(64)90175-8. [DOI] [PubMed] [Google Scholar]
- Bellard M., Oudet P., Germond J. E., Chambon P. Subunit structure of simian-virus-40 minichromosome. Eur J Biochem. 1976 Nov 15;70(2):543–553. doi: 10.1111/j.1432-1033.1976.tb11046.x. [DOI] [PubMed] [Google Scholar]
- Brison O., Kedinger C., Wilhelm J. Enzymatic properties of viral replication complexes isolated from adenovirus type 2-infected HeLa cell nuclei. J Virol. 1977 Nov;24(2):423–435. doi: 10.1128/jvi.24.2.423-435.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. C., Birkenmeier E., Salzman N. P. Simian virus 40 DNA replication: characterization of gaps in the termination region. J Virol. 1976 Feb;17(2):614–621. doi: 10.1128/jvi.17.2.614-621.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G., Griffith J. Salt and divalent cations affect the flexible nature of the natural beaded chromatin structure. Nucleic Acids Res. 1977 Jun;4(6):1837–1851. doi: 10.1093/nar/4.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crémisi C., Chestier A., Yaniv M. Preferential association of newly synthesized histones with replicating SV40 DNA. Cell. 1977 Dec;12(4):947–951. doi: 10.1016/0092-8674(77)90159-3. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Berg P. Requirement of a Cytoplasmic Fraction for Synthesis of SV40 Deoxyribonucleic Acid in Isolated Nuclei*. J Biol Chem. 1975 Jun 10;250(11):4348–4354. [PubMed] [Google Scholar]
- Edenberg H. J., Anderson S., DePamphilis M. L. Involvement of DNA polymerase alpha in simian virus 40 DNA replication. J Biol Chem. 1978 May 10;253(9):3273–3280. [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Waqar M. A., Huberman J. A. DNA synthesis by partially purified replicating simian virus 40 chromosomes. Nucleic Acids Res. 1977 Sep;4(9):3083–3095. doi: 10.1093/nar/4.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Waqar M. A., Huberman J. A. Subnuclear systems for synthesis of simian virus 40 DNA in vitro. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4392–4396. doi: 10.1073/pnas.73.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Davoli D. Molecular biology of papovaviruses. Annu Rev Biochem. 1977;46:471–522. doi: 10.1146/annurev.bi.46.070177.002351. [DOI] [PubMed] [Google Scholar]
- Francke B., Hunter T. In vitro polyoma DNA synthesis: requirement for cytoplasmic factors. J Virol. 1975 Jan;15(1):97–107. doi: 10.1128/jvi.15.1.97-107.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel G. D. Adenovirus DNA synthesis in vitro in an isolated complex. J Virol. 1978 Jan;25(1):459–463. doi: 10.1128/jvi.25.1.459-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L. M., Kelinman R. E., Horwitz M. S. Replication of adenovirus type 2 DNA in vitro. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4425–4429. doi: 10.1073/pnas.74.10.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann G., Anderson S., DePamphilis M. L. RNA primers in Simian virus 40 DNA replication. II. Distribution of 5' terminal oligoribonucleotides in nascent DNA. J Mol Biol. 1977 Nov 5;116(3):549–567. doi: 10.1016/0022-2836(77)90083-3. [DOI] [PubMed] [Google Scholar]
- Krokan H., Wist E., Prydz H. Effect of cytosol on DNA synthesis in isolated HeLa cell nuclei. Biochem Biophys Res Commun. 1977 Mar 21;75(2):414–419. doi: 10.1016/0006-291x(77)91058-0. [DOI] [PubMed] [Google Scholar]
- Morrison J. M., Keir H. M., Subak-Sharpe H., Crawford L. V. Nearest neighbour base sequence analysis of the deoxyribonucleic acids of a further three mammalian viruses: Simian virus 40, human papilloma virus and adenovirus type 2. J Gen Virol. 1967 Jan;1(1):101–108. doi: 10.1099/0022-1317-1-1-101. [DOI] [PubMed] [Google Scholar]
- Otto B., Fanning E. DNA polymerase alpha is associated with replicating SV40 nucleoprotein complexes. Nucleic Acids Res. 1978 May;5(5):1715–1728. doi: 10.1093/nar/5.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B., Reichard P. Replication of polyoma DNA in isolated nuclei. V. Complementation of in vitro DNA replication. J Virol. 1975 Feb;15(2):259–267. doi: 10.1128/jvi.15.2.259-267.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder B. A., Crew F., Crawford L. V. Comparison of nuclease digestion of polyoma virus nucleoprotein complex and mouse chromatin. J Virol. 1978 Jan;25(1):175–186. doi: 10.1128/jvi.25.1.175-186.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton E. R., Kang J., Wassarman P. M., DePamphilis M. L. Chromatin assembly in isolated mammalian nuclei. Nucleic Acids Res. 1978 Feb;5(2):349–362. doi: 10.1093/nar/5.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. In vitro replication of simian virus 40 DNA in a nucleoprotein complex. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3466–3470. doi: 10.1073/pnas.73.10.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Discontinuous DNA replication: accumulation of Simian virus 40 DNA at specific stages in its replication. J Mol Biol. 1978 Apr 15;120(3):401–422. doi: 10.1016/0022-2836(78)90427-8. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Goulian M. DNA synthesis in human lymphocyts: intermediates in DNA synthesis, in vitro and in vivo. J Mol Biol. 1975 Dec 5;99(2):317–337. doi: 10.1016/s0022-2836(75)80149-5. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Goulian M. Initiator RNA of discontinuous DNA synthesis in human lymphocytes. Cell. 1977 Oct;12(2):483–489. doi: 10.1016/0092-8674(77)90124-6. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Chumackov P. M., Georgiev G. P. Minichromosome of simian virus 40: presence of histone HI. Nucleic Acids Res. 1976 Aug;3(8):2101–2113. doi: 10.1093/nar/3.8.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Arens M., Green M. Adenovirus deoxyribonucleic acid replication. Isolation of a soluble replication system and analysis of the in vitro DNA product. J Biol Chem. 1977 Nov 25;252(22):7940–7946. [PubMed] [Google Scholar]


