Abstract
Cowden syndrome (CS) is a difficult-to-recognize multiple hamartoma syndrome with high risks of breast, thyroid, and other cancers. Germline mutations in PTEN on 10q23 were found to cause 85% of CS when accrued from tertiary academic centers, but prospective accrual from the community over the last 12 years has revealed a 25% PTEN mutation frequency. PTEN is the phosphatase that has been implicated in a heritable cancer syndrome and subsequently in multiple sporadic cancers and developmental processes. PTEN antagonizes the AKT1/PI3K signaling pathway and has roles in cell cycle, migration, cell polarity, and apoptosis. We report that 8 of 91 (8.8%) unrelated CS individuals without germline PTEN mutations carried 10 germline PIK3CA mutations (7 missense, 1 nonsense, and 2 indels) and 2 (2.2%) AKT1 mutations. These mutations result in significantly increased P-Thr308-AKT and increased cellular PIP3. Our observations suggest that PIK3CA and AKT1 are CS susceptibility genes.
Main Text
Germline mutations in PTEN (OMIM 176920) on 10q23 were found in 85% of families with Cowden syndrome (CS) (OMIM 158350) when accrued from tertiary academic centers.1,2 Prospective accrual from the community over the last 12 years has revealed that 25% of CS cases are due to PTEN mutations;3 10% of CS individuals without a detected PTEN mutation carry germline SDHx variants4 and ∼30% have germline KLLN (OMIM 612105) hypermethylation.5 CS is a clinical mimic and difficult to recognize with broad phenotypic presentations and reduced penetrance. In this context, identification of additional predisposition genes would facilitate molecular diagnosis, predictive testing, genetic counseling, and medical management. We created a PTEN Cleveland Clinic (CC) Risk Calculator based on prospective accrual of >3,000 CS and Cowden-like (CSL) individuals and multiple logistic regression weighted by neoplasia risk in PTEN mutation positive versus individuals without a detected PTEN mutation in our cohort compared to that in the general population and by age of neoplasia onset in our cohort.3 Increasing CC score correlates with higher prior probability of finding PTEN mutations. For clinical purposes, we suggest a threshold of 10 (>3%–5% prior probability) for consideration of PTEN testing. Based on this rationale, we selected 91 CS/CSL individuals without mutations in PTEN or SDHx, or hypermethylation of the KLLN promoter, and with CC scores ranging from 8 to 54 (adults), with >80% having scores >8 or who met pediatric criteria. We sought to determine whether individuals with high CC scores without alterations in known genes have mutations in genes encoding proteins immediately downstream of PTEN.
Germline genomic DNA from 91 unrelated consenting (IRB-8458-PTEN) CS/CSL probands, without PTEN/SDHx/KLLN mutations/alterations, were analyzed by Sanger sequencing (ABI3730xl) of AKT1 (OMIM 164730), PIK3CA (OMIM 171834), PIK3R1 (OMIM 171833), and PIK3R2 (OMIM 603157) (see Table S1 available online). While no germline mutations were found in PIK3R1 (NM_181523.2) and PIK3R2 (NM_005027.2), ten (10.99%) probands were found to carry germline PIK3CA and AKT1 mutations (NM_006218.2, NM_001014431.1) (Table 1; Figure 1; Table S2). None of the mutations were found in 96 population controls, dbSNP, or the available data set in 1000 Genomes Project. To predict the functionality of the germline mutations, we used MutPred software6 and three-dimensional (3D) protein modeling (Discovery Studio-3.1, Accelrys Inc) of the mutations within their respective domains. The MutPred software, which calculates the probability of a deleterious mutation and corresponding hypothesis of disrupted molecular mechanism, revealed values between 0.46 and 0.88 for our detected variants/mutations. MutPred values between 0.45 and 0.75 predict for benign variations and values >0.75 pathogenic mutations. Three-dimensional modeling revealed that all of the mutations altered polarity and conformation and/or stability of specific domains, leading to or resulting in inappropriate exposure or hiding of key amino acid functional domains. Two mutations in PIK3CA c.353G>A and c.1145G>A that result in p.Gly118Asp (MutPred = 0.496) and p.Arg382Lys (MutPred = 0.464) alterations, respectively, had 3D plots showing significant structural alterations (Figures S1 and S2); furthermore, the germline p.Gly118Asp has been reported as a somatic change with functionality in a sporadic malignancy.
Table 1.
CS/CSL Individuals with Germline AKT1 and PIK3CA Mutations
| Sample CCF IDs | Gene | Exon | Mutations | MutPred | Gender | Age | CC Score |
|---|---|---|---|---|---|---|---|
| CCF03121-01-001DG | AKT1 | 2 | c.73C>T, p.Arg25Cys | 0.879 | F | 38 | 8 |
| CCF04333-01-001LM | AKT1 | 12 | c.1303A>C, p.Thr435Pro | 0.595 | F | 47 | 22 |
| CCF03451-01-001DC | PIK3CA | 2 | c.353G>A, p.Gly118Asp | 0.496 | M | 32 | 12 |
| CCF04880-01-001MQ | PIK3CA | 2 | c.403G>A, p.Glu135Lys | 0.677 | F | 54 | 13 |
| CCF00102-01-001GG | PIK3CA | 3 | c.652G>A, p.Glu218Lys | 0.598 | F | 44 | 22 |
| CCF03330-01-001LM | PIK3CA | 5 | c.1066G>A, p.Val356Ile | 0.503 | F | 35 | 10 |
| CCF02069-01-001CS | PIK3CA | 5 | c.1145G>A, p.Arg382Lys | 0.464 | M | 47 | 22 |
| CCF04477-01-001FN | PIK3CA | 9 | c.1634A>C, p.Glu545Ala and c.1658_1659 delGTinsC, p. Ser553Thrfs∗7 | 0.769 | F | 71 | 13 |
| CCF05779-01-001SC | PIK3CA | 9 | c.1634A>C, p.Glu545Ala and c.1658_1659 delGTinsC p. Ser553Thrfs∗7 | 0.769 | F | 27 | 22 |
| CCF00494-01-001WG | PIK3CA | 11 | c.1895T>G, p.Leu632∗ | N/A | M | 59 | 7 |
CC Score, PTEN Cleveland Clinic Clinical Score for a priori probability of finding PTEN mutations. Accession numbers used are NM_001014431.1 (AKT1) and NM_006218.2 (PIK3CA).
Figure 1.
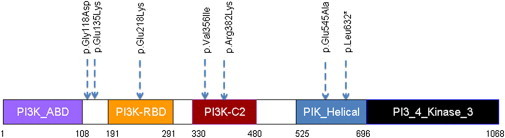
Spectrum of Germline PIK3CA Mutations in CS/CSL Individuals without PTEN Germline Mutations
A display of amino acid changes that result from different germline PIK3CA mutations in CS/CSL individuals (blue arrows) relative to PIK3CA functional domains.
PIK3CA encodes p110α, the catalytic subunit of PI3K, which adds a phosphate to phosphatidylinositol-4,5-biphosphate (PIP2) to form phosphatidylinositol-3,4,5-triphosphate (PIP3) at the cellular membrane.7–10 PIP3 recruits PH domain-containing proteins, e.g., AKT1 to the cell membrane.11 We performed western blot analysis for phospho(P)-AKT1 and p110α in germline protein lysates extracted from CS-derived lymphoblastoid cells from AKT1 and PIK3CA mutation-positive individuals and four controls (Figure 2), using antibodies against P-AKT1Ser473 (Epitomics Inc., Burlingame, CA), P-AKT1Thr308 (Santa Cruz Biotechnology, CA), p110alpha (Cell Signaling Technology, Danvers, MA), and actin (Santa Cruz Biotechnology). All mutation-positive protein lysates showed a significant increase of P-Thr308-AKT1 levels (Figures 2A and 2B). No obvious differences in P-Ser473-AKT1 were noted between controls and individuals with the mutation, corroborating the specificity of the mutations because Thr308-AKT1, but not Ser473-AKT, is a phosphorylation target of p110α12. To assess the impact of PIK3CA mutations on PI3K activity, we used immunofluorescence to compare PIP3 in lymphoblastoid cell lines derived from two individuals that carry germline PIK3CA mutations to that from WT individuals (as described,13 Figure 3). Lymphoblastoid cells were incubated on poly-L-lysine-coated slides for 30 min. Cells were then washed with PBS and then fixed with 100% ice-cold methanol (for 1 min) on slides. Slides were then blocked in 5% goat serum PBS and incubated sequentially with anti-phosphatidylinositol PIP3 antibody (MBL International, Woburn, MA, Cat# D145-3) and Alexa Fluor secondary antibody. Slides were counterstained with DAPI and visualized on a Leica TCS-SP spectral laser scanning confocal microscope. Counterstaining with DAPI revealed increased levels of PIP3 in PIK3CA-p.Glu218Lys and moderately increased levels in PIK3CA-p.Arg382Lys cells compared to wild-type (WT) (Figure 3). Cells bearing PTEN-p.Arg335∗ served as a positive control (Figure 3). Treating the PTEN-p.Arg335∗ cells with Wortmannin (PI3K inhibitor) decreased PIP3 levels.
Figure 2.
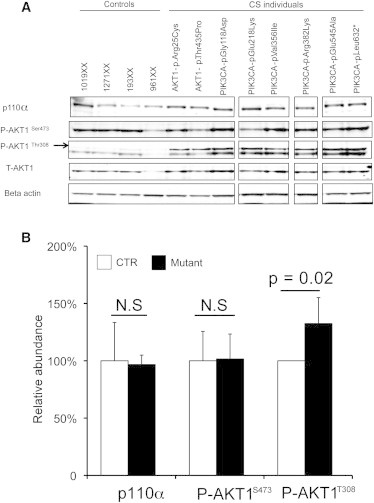
AKT1 and PIK3CA Mutations Result in Upregulation of Phosphorylated P-Thr308-AKT1
(A) Western analysis of peripheral blood protein lysates from CS/CSL individuals with germline AKT1 and PIK3CA mutations. Mutation-positive individuals showed modest increase in p110α levels and marked increased levels of P-Thr308-AKT1 (arrow) but no detectable difference in P-Ser473-AKT1 and total (T)-AKT1 protein levels compared to controls.
(B) Densitometric analysis of specific signals using Image J software. Signals were normalized to actin. A value of 100% was arbitrarily assigned to the ratio obtained in the controls (n = 4) and the relative ratio was made in the individuals with mutations (n = 8). Two-tailed Student’s t test was utilized. N.S., not significant.
Figure 3.
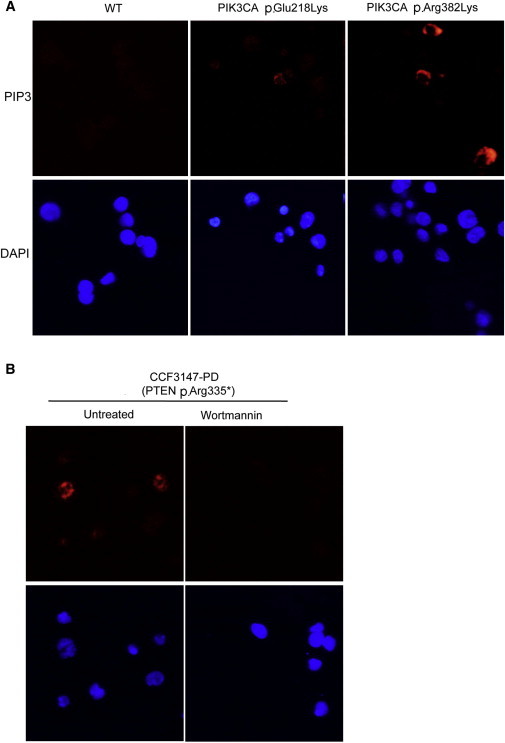
Increased PIP3 Levels in Lymphoblastoid Cell Lines (LCLs) Derived from PIK3CA Mutation-Positive Individuals
(A) Indirect immunofluorescence staining of PIP3 (red) and DAPI (blue) in LCLs derived from an unaffected control (WT) and two CS individuals with PIK3CA germline mutations.
(B) LCLs isolated from a CS individual harboring a PTEN germline mutation (p.Arg335∗) that were either treated or not treated with the PI3K inhibitor, Wortmannin (100 nM for 30 min), serve as positive control (left) and negative control (right) for PIP3 levels, respectively.
We initially predicted that germline AKT1 mutations would be more frequent than those in PIK3CA based on the report that frequent postzygotic somatic AKT1 mutations occur in affected tissues of Proteus syndrome, also rarely associated with PTEN mutations.14,15 Instead, we found that 2.2% of CS/CSL probands carried germline AKT1 mutations (c.73C>T and c.1303A>C, Table 1). The PH-domain p.Arg25Cys germline change has previously been reported as a somatic alteration in noninherited cancers, with insulin-dependent increased kinase activity16 but abrogation of estradiol-related kinase activity.17 In vitro insulin exposure results in AKT1-p.Arg25Cys stabilizing p110α and increasing the latter’s activity phosphorylating and upregulating AKT1.16 Our western blot supports this observation, showing tendency toward increased p110α and a significantly increased phosphorylation of AKT1 at Thr308 (Figures 2A and 2B). The p.Thr435Pro mutation has not been described before but the end result is increased P-AKT1.
We found that >8.8% CS/CSL probands, without detected PTEN germline mutation, have heterozygous germline PIK3CA mutations but not in genes encoding the regulatory subunits of PI3K, PIK3R1/PIK3R2 (Table 1). Somatic mutations in these three genes have been amply described in solid tumors, including those of the breast, thyroid, and endometrium18–20. Overall, the somatic mutational spectra and the germline spectra in PIK3CA appear different, with the majority of CS/CSL mutations within the C2-domain. C2 is responsible for p110α recruitment to cellular membranes. C2 mutations have been shown in vitro to alter charge or conformation enhancing recruitment of the mutant-p110α to cell membranes.21 Corroborating this, western analysis reveals slightly increased p110α and markedly increased P-Thr308-AKT (Figure 2A). Recently, postzygotic and mosaic mutations in the genes encoding various proteins in the AKT/PI3K signaling pathway have been described in rare nonneoplasia hemihypertrophy-segmental overgrowth syndromes.13,22,23 Note that C2-domain PIK3CA mutations are prominent in megalencephaly-capillary malformation syndrome as well. Among all these syndromes, including CS/CSL, involvement of the brain and vascular malformation are not infrequent. Just as we proposed the term PTEN hamartoma tumor syndrome (PHTS) for any individual irrespective of clinical presentation carrying a germline PTEN mutation, we would like to propose the term PTEN/AKT/PI3K-opathy to encompass this broad range of phenotypes.
In summary, 11% of Cowden and Cowden-like syndrome individuals without detected PTEN/SDHx/KLLN mutation/alteration carry germline gain-of-function AKT1 or PIK3CA mutations. Together, the PIK3CA and AKT1 pathways represent a novel driver of predisposition to hamartoma-neoplasia syndromes that may have diagnostic, genetic counseling, and therapeutic implications.
Acknowledgments
We are deeply grateful to all of our research participants and their families, clinicians, and genetic professionals who have contributed one way or the other to our studies over the many years, especially the last 6 years. This study is funded, in part, by NCI grant P01CA124570 and the American Cancer Society Clinical Research Professorship (both to C.E.). C.E. is the Sondra J. and Stephen R. Hardis Chair in Cancer Genomic Medicine at the Cleveland Clinic.
Supplemental Information
Web Resources
The URLs for data presented herein are as follows:
OMIM, www.omim.org
PTEN Cleveland Clinic (CC) Score and Risk Calculator, http://www.lerner.ccf.org/gmi/ccscore
References
- 1.Liaw D., Marsh D.J., Li J., Dahia P.L., Wang S.I., Zheng Z., Bose S., Call K.M., Tsou H.C., Peacocke M. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 2.Marsh D.J., Coulon V., Lunetta K.L., Rocca-Serra P., Dahia P.L., Zheng Z., Liaw D., Caron S., Duboué B., Lin A.Y. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum. Mol. Genet. 1998;7:507–515. doi: 10.1093/hmg/7.3.507. [DOI] [PubMed] [Google Scholar]
- 3.Tan M.H., Mester J., Peterson C., Yang Y., Chen J.L., Rybicki L.A., Milas K., Pederson H., Remzi B., Orloff M.S., Eng C. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am. J. Hum. Genet. 2011;88:42–56. doi: 10.1016/j.ajhg.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ni Y., He X., Chen J., Moline J., Mester J., Orloff M.S., Ringel M.D., Eng C. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependant destabilization of p53. Hum. Mol. Genet. 2012;21:300–310. doi: 10.1093/hmg/ddr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett K.L., Mester J., Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA. 2010;304:2724–2731. doi: 10.1001/jama.2010.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li B., Krishnan V.G., Mort M.E., Xin F., Kamati K.K., Cooper D.N., Mooney S.D., Radivojac P. Automated inference of molecular mechanisms of disease from amino acid substitutions. Bioinformatics. 2009;25:2744–2750. doi: 10.1093/bioinformatics/btp528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanhaesebroeck B., Leevers S.J., Ahmadi K., Timms J., Katso R., Driscoll P.C., Woscholski R., Parker P.J., Waterfield M.D. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 8.Whitman M., Kaplan D., Roberts T., Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem. J. 1987;247:165–174. doi: 10.1042/bj2470165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitman M., Kaplan D.R., Schaffhausen B., Cantley L., Roberts T.M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- 10.Whitman M., Melton D.A. Involvement of p21ras in Xenopus mesoderm induction. Nature. 1992;357:252–254. doi: 10.1038/357252a0. [DOI] [PubMed] [Google Scholar]
- 11.Furnari F.B., Huang H.J., Cavenee W.K. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 1998;58:5002–5008. [PubMed] [Google Scholar]
- 12.Rodríguez-Escudero I., Andrés-Pons A., Pulido R., Molina M., Cid V.J. Phosphatidylinositol 3-kinase-dependent activation of mammalian protein kinase B/Akt in Saccharomyces cerevisiae, an in vivo model for the functional study of Akt mutations. J. Biol. Chem. 2009;284:13373–13383. doi: 10.1074/jbc.M807867200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivière J.B., Mirzaa G.M., O’Roak B.J., Beddaoui M., Alcantara D., Conway R.L., St-Onge J., Schwartzentruber J.A., Gripp K.W., Nikkel S.M., Finding of Rare Disease Genes (FORGE) Canada Consortium De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat. Genet. 2012;44:934–940. doi: 10.1038/ng.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X., Hampel H., Thiele H., Gorlin R.J., Hennekam R.C., Parisi M., Winter R.M., Eng C. Association of germline mutation in the PTEN tumour suppressor gene and Proteus and Proteus-like syndromes. Lancet. 2001;358:210–211. doi: 10.1016/s0140-6736(01)05412-5. [DOI] [PubMed] [Google Scholar]
- 15.Smith J.M., Kirk E.P., Theodosopoulos G., Marshall G.M., Walker J., Rogers M., Field M., Brereton J.J., Marsh D.J. Germline mutation of the tumour suppressor PTEN in Proteus syndrome. J. Med. Genet. 2002;39:937–940. doi: 10.1136/jmg.39.12.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohn A.D., Takeuchi F., Roth R.A. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J. Biol. Chem. 1996;271:21920–21926. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- 17.Stoica G.E., Franke T.F., Moroni M., Mueller S., Morgan E., Iann M.C., Winder A.D., Reiter R., Wellstein A., Martin M.B., Stoica A. Effect of estradiol on estrogen receptor-alpha gene expression and activity can be modulated by the ErbB2/PI 3-K/Akt pathway. Oncogene. 2003;22:7998–8011. doi: 10.1038/sj.onc.1206769. [DOI] [PubMed] [Google Scholar]
- 18.Cheung L.W., Hennessy B.T., Li J., Yu S., Myers A.P., Djordjevic B., Lu Y., Stemke-Hale K., Dyer M.D., Zhang F. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011;1:170–185. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du L., Shen J., Weems A., Lu S.L. Role of phosphatidylinositol-3-kinase pathway in head and neck squamous cell carcinoma. J. Oncol. 2012;2012:450179. doi: 10.1155/2012/450179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelman J.A., Luo J., Cantley L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 21.Gymnopoulos M., Elsliger M.A., Vogt P.K. Rare cancer-specific mutations in PIK3CA show gain of function. Proc. Natl. Acad. Sci. USA. 2007;104:5569–5574. doi: 10.1073/pnas.0701005104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J.H., Huynh M., Silhavy J.L., Kim S., Dixon-Salazar T., Heiberg A., Scott E., Bafna V., Hill K.J., Collazo A. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat. Genet. 2012;44:941–945. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindhurst M.J., Parker V.E., Payne F., Sapp J.C., Rudge S., Harris J., Witkowski A.M., Zhang Q., Groeneveld M.P., Scott C.E. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat. Genet. 2012;44:928–933. doi: 10.1038/ng.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


