Abstract
A previous US study reported poorer survival in children with acute lymphoblastic leukemia (ALL) exposed to extremely low-frequency magnetic fields (ELF–MF) above 0.3 μT, but based on small numbers. Data from 3073 cases of childhood ALL were pooled from prospective studies conducted in Canada, Denmark, Germany, Japan, UK and US to determine death or relapse up to 10 years from diagnosis. Adjusting for known prognostic factors, we calculated hazard ratios (HRs) and 95% confidence intervals (CI) for overall survival and event-free survival for ELF–MF exposure categories and by 0.1 μT increases. The HRs by 0.1 μT increases were 1.00 (CI, 0.93–1.07) for event-free survival analysis and 1.04 (CI, 0.97–1.11) for overall survival. ALL cases exposed to >0.3 μT did not have a poorer event-free survival (HR=0.76; CI, 0.44–1.33) or overall survival (HR=0.96; CI, 0.49–1.89). HRs varied little by subtype of ALL. In conclusion, ELF–MF exposure has no impact on the survival probability or risk of relapse in children with ALL.
Keywords: leukemia, children, survival, electromagnetic fields, adverse effects, pooled analyses
Introduction
Whether exposure to extremely low-frequency (ELF) magnetic fields (MF) either from power transmission and distribution or the use of electrical appliances is associated with an increased risk of leukemia in children has engendered scientific debate.1 In 2001, the International Agency for Research on Cancer classified ELF–MF as a possible carcinogen based on limited evidence in humans and inadequate evidence from experimental animal studies.2 The human evidence emerged from epidemiological studies on the etiology of childhood leukemia that observed weak associations between ELF–MF and leukemia risk at exposure levels above 0.3 and/or 0.4 μT. This was summarized in two pooled analyses published in 2000.3, 4 Subsequent pooled analyses confirmed these results.5, 6 However, despite the apparent consistency of the statistical association serious concerns remained, due to not only the lack of plausible biological mechanisms, but also the recognition that biases in the case-control studies could potentially have inflated risk estimates. In particular, the choice of controls coupled with their low participation rates could have led to an underestimate of exposure among controls.7 The more recent studies had also been unable to satisfactorily address the possible impact of bias.2, 6 Currently, therefore, there continues to be an unanswered question regarding the potential adverse health consequences of ELF–MF.8
Under the hypothesis that ELF–MF may promote growth of leukemia cells, investigators have studied the relationship between ELF–MF and length of remission and overall survival after childhood acute lymphoblastic leukemia (ALL). If the hypothesis is correct then one could expect a higher risk of recurrence of the disease. Demonstrating an effect of ELF–MF on disease progression would therefore directly support the biological plausibility. Indeed, a cohort study in the US, of children with ALL and subsequently another study in Germany following cases from a previous case-control study on ELF–MF and risk of ALL showed poorer survival from ALL related to ELF–MF exposures >0.3 μT (US) or ⩾0.2 μT (Germany), but based on small numbers.9, 10
To more rigorously evaluate a possible association between ELF–MF exposure and outcome in a large population of childhood ALL, a multinational collaboration was initiated. This report presents the results obtained from over 3000 children with ALL with ELF–MF exposure data from Canada, Denmark, Germany, Japan, the UK and the US, who were followed up for 10 years for relapse, second neoplasm and survival.
Materials and methods
Study population
The study population was derived from studies from Canada,11 Denmark,12 Germany,13 New Zealand,14 Sweden,15 the UK16 and the US17 previously combined in a pooled analysis of case-control studies by Ahlbom et al.,3 plus case-control studies from Germany and Japan.18, 19 Further, it included a US cohort study9 specifically designed to evaluate a possible association between ELF–MF exposure and ALL outcome. Selection of studies for this pooled analysis was based on fulfilling all of the following criteria: (i) the availability of either long-term measurements (24 h or longer) of the residential ELF–MF or ELF–MF estimations (so called ‘calculated fields') based on historical power load information of power lines and their distance to nearby residences; (ii) having a defined population-based study population; (iii) the feasibility of following the ALL cases for vital status; and (iv) being completed when the consortium was established in 2007.
For ALL cases from the various studies, we retrieved the date of diagnosis, diagnostic details on ALL subtype (later subdivided into B-precursor cell type ALL, T-cell type ALL, other ALL), diagnosis of Down syndrome, gender, date of birth, measures of socioeconomic status (country-specific categorization based on parents' highest attained education, disposable family income or small area based socioeconomic class predictor of the family's address), a prognostic risk designation (see below), white blood cell count, ELF–MF exposure, date of death, cause of death (disease-related or other), date when the child was last known to be alive, and, for the event-free survival analyses, information on date of relapse and date of diagnosis of a second malignant neoplasm. For a prognostic risk designation we used a dichotomous grouping defined at a workshop held by the US National Cancer Institute with the low risk group including children with age below 10 years and diagnostic white blood cell count below 50 000/μl, and the high risk group including children age 10 years or older or with a white blood cell count above or equal to 50 000/μl.20
The exclusion criteria for both overall and event-free survival were as follows: (i) exposure data from original study not found; (ii) vital status unknown; or (iii) child was less than 1 year of age at diagnosis (because of very poor overall survival in this age group together with short time to be potentially exposed to ELF–MF before diagnosis). There was an additional exclusion criterion for event-free survival, namely that date of diagnosis of second malignant neoplasm was unknown. Children with no information available on other prognostic factors or white blood cell count were generally also excluded from the analyses, but used for sensitivity analysis that were not adjusted for these factors. Most studies included cases coming from similar diagnostic time periods (mainly the 1990s), and because of poor survival of children with ALL from early time periods (before 1 January 1988) those were not included in our study, which applied to cases from Denmark and Sweden (see below).
Individual studies
Details on the individual studies are provided in Supplementary Appendix Table 1 and reasons for exclusions are provided in Supplementary Appendix Table 2.
The US POG (Pediatric Oncology Group) survival study was the only study specifically designed to investigate ELF–MF and ALL outcome.9 ALL cases were diagnosed from 1996–2001. Overall, 386 cases were provided with vital status; only one case excluded for missing outcome.
The US Children's Cancer Group case-control study included children with ALL diagnosed in the years 1989–1994.17, 21 In total, complete data were available for 481 of the 565 eligible ALL cases.
The UK case-control study covered all of England, Wales and Scotland, with cases diagnosed within the time interval 1991 and 1996, with slight variations by region.16 Overall, 906 ALL cases were eligible of which 806 were included in the analysis.
The first German case-control study was restricted to cases living in Lower Saxony (diagnosed 1988–1993) or Berlin (diagnosed 1991–1994).13 The second German case-control study covered all of former West Germany and included cases diagnosed 1992–1994.18 Overall, Germany provided 594 cases and 574 were included in the main analysis.
The Japanese case-control study included cases diagnosed from 1999–2001.19 Overall 244 cases were provided and 240 cases were used in the main survival analysis.
The British Columbia component of the Canadian case-control study included children diagnosed with ALL between 1990–1995.11 Exposure information from the different types of measurements was not available for all cases, and we therefore had to use varying exposure measurements for the children in this study (Supplementary Appendix Table 1). Eighty-four cases were eligible and 80 included in the overall analysis.
The original case-control study in Denmark included children diagnosed with ALL in 1968–1986.12 The data were later updated for the purpose of the present study with children diagnosed up to the end of 2003. Only children diagnosed after 1 January 1988 were included in the present study. This amounted to 520 cases with 507 included in the main analyses.
The case-control study in New Zealand included children diagnosed between 1990 and 1993.14 It was not practical to obtain information on white blood cell count at diagnosis or any other information to assess prognosis this long afterwards. This meant that New Zealand's data were not included in the main survival analysis, however, they were used in sensitivity analyses. Overall, New Zealand had provided 94 cases of whom one was exposed to ELF–MF >0.3 μT.
The case-control study in Sweden included children diagnosed with ALL during the years 1960–1985.15 The study did not include the whole of Sweden but focussed on children living within 300 m corridors of high-voltage power lines. Substantially lower survival rates were seen in Sweden compared with other studies because cases were diagnosed in an earlier time period.22 We therefore, decided not to include Sweden in the hazard analysis because of the difference in the baseline risk. As a stand-alone study the hazard rate associated with ELF–MF >0.3 μT compared with ⩽0.1 μT was 0.27 (95% confidence interval (CI), 0.04–2.14) in the overall survival analysis (see Statistical Methods).
Supplementary Appendix Table 2 provides an overview of study-specific exclusions. Overall, the majority of excluded cases had no information on white blood cell count (N=277). Otherwise, infants were excluded according to our eligibility criteria (N=72). Only 114 children were excluded because of missing information on vital status or missing information on relapse.
Statistical methods
There were two primary outcomes for this study: (i) overall survival with death from all causes counted as events; and (ii) event-free survival with the first, if any, of death from all causes, relapse and second malignant neoplasm considered as events. Children were followed from the date of diagnosis until the date of event, last date known to be alive, or date of 10 years of follow-up, whichever came first.
The associations between exposure to ELF–MF and overall survival and event-free survival were, respectively, assessed using Cox proportional hazards model. All analyses were stratified by study and adjusted for National Cancer Institute prognostic risk group, gender, year of diagnosis (included as a linear variable) and age at diagnosis (grouped into: 1–4 years, 5–9 years, and 10–14 years). Ethnicity and socioeconomic status were examined as an additional potential confounders using Kaplan-Meier curves with log-rank tests, but no associations were found and these variables not included in the final analyses. Exposure to ELF–MF was divided into four exposure categories (⩽0.1, 0.1–0.2, 0.2–0.3, and >0.3 μT) using the same categories assessed in the study by Foliart et al.9 Analyses were conducted for all subjects combined and separately for B-precursor ALL, T-cell ALL and others (the latter group consists mainly of ALL of unknown immunophenotype and therefore presumably includes a large proportion of B-precursor ALL). Furthermore, we modeled exposure as a continuous variable looking at the hazard ratio (HR) per 0.1 μT increase.
Sensitivity analyses were performed: (i) excluding the hypothesis-generating US POG study from the analyses; (ii) without adjusting for the prognostic factor allowing the New Zealand data to be included; (iii) excluding children who, before their date of diagnosis, had moved out from the residence where the measurements was taken (we additionally excluded children for which these information was not available); this applied to the cases from the case-control studies but not to cases from the US POG cohort study.
Results
Altogether, 3073 cases with ALL were included in the overall survival analyses and 3016 in the event-free survival analyses. Of the 3073, 56% were male, and 55% 1–4 years of age at diagnosis, 30% 5–9 year olds and 16% 10–14 year olds. Most children were diagnosed with B-precursor ALL (67%) with only 8% having T-cell ALL (25% not specified). The majority of cases were exposed to ELF–MF of ⩽0.1 μT (88%), with this proportion varying by country, ranging from 99% in Denmark to 69% in one of the US studies (Table 1). Sixty-eight ALL cases (2%) were in the high exposure group of ELF–MF >0.3 μT, the majority (N=42) coming from the two US studies.
Table 1. Exposure distribution by study for observations included in the main overall survival analysis.
|
⩽0.1 μT |
0.1 μT-0.2 μT |
0.2 μT—0.3 μT |
>0.3 μT |
Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | |
| US POGa | 297 | 77.1 | 63 | 16.4 | 11 | 2.9 | 14 | 3.6 | 385 |
| US CCGb | 330 | 68.6 | 90 | 18.7 | 33 | 6.9 | 28 | 5.8 | 481 |
| UK | 772 | 95.8 | 22 | 2.7 | 7 | 0.9 | 5 | 0.6 | 806 |
| Germany | 526 | 91.6 | 32 | 5.6 | 9 | 1.6 | 7 | 1.2 | 574 |
| Canada | 61 | 76.3 | 13 | 16.3 | 1 | 1.3 | 5 | 6.3 | 80 |
| Denmark | 503 | 99.2 | 1 | 0.2 | 1 | 0.2 | 2 | 0.4 | 507 |
| Japan | 214 | 89.2 | 13 | 5.4 | 6 | 2.5 | 7 | 2.9 | 240 |
| Total | 2703 | 88.0 | 234 | 7.6 | 68 | 7.6 | 68 | 2.2 | 3073 |
US Pediatric Oncology Group (POG) Survival Study.
Cases from the case-control study of the US Children's Cancer Group (CCG) (legacy group of the Children's Oncology Group).
For overall survival, comparison of the highest ELF–MF level >0.3 μT to the reference category ⩽0.1 μT demonstrated no increased risk of death (HR=0.96; 95% CI, 0.49–1.89) (Table 2). HRs in the intermediate exposure categories were elevated (1.42 for 0.1–0.2 μT and 1.27 for 0.2–0.3 μT) but had CIs that included the value 1.0. Exclusion of the hypothesis-generating US POG study did not change any of the HRs (Table 2). Stratification by type of ALL showed HRs for the highest ELF–MF level to be 1.35 (CI, 0.66–2.77) for B-precursor ALL and 0.90 (CI, 0.12–6.84) for other ALL, while no event was observed for T-cell ALL (Table 2). We observed a HR close to 1.0 when looking at the survival per 0.1 μT increase (HR 1.04; CI, 0.97–1.11).
Table 2. Overall survival of children with ALL exposed to residential ELF–MF.
| ⩽0.1 μT |
0.1 μT—0.2 μT |
0.2 μT—0.3 μT |
>0.3 μT |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (Events) | N (Events) | HR | 95% CI | N (Events) | HR | 95% CI | N (Events) | HR | 95% CI | |
| Main | 413 | 35 | 1.42 | 0.99–2.05 | 9 | 1.27 | 0.65–2.50 | 9 | 0.96 | 0.49–1.89 |
| US POG study not included | 393 | 29 | 1.42 | 0.96–2.12 | 8 | 1.28 | 0.63–2.61 | 6 | 0.70 | 0.31–1.59 |
| T-cell ALL | 61 | 9 | 2.35 | 1.09–5.08 | 0 | 0.00 | — | 0 | 0.00 | — |
| B-precursor ALL | 222 | 25 | 1.46 | 0.95–2.25 | 9 | 1.86 | 0.94–3.68 | 8 | 1.35 | 0.66–2.77 |
| Other ALL | 130 | 1 | 0.25 | 0.03–1.93 | 0 | 0.00 | — | 1 | 0.90 | 0.12–6.84 |
Abbreviations: ALL, acute lymphoblastic leukemia; CI, confidence interval; HR, hazard ratio; POG, Pediatric Oncology Group.
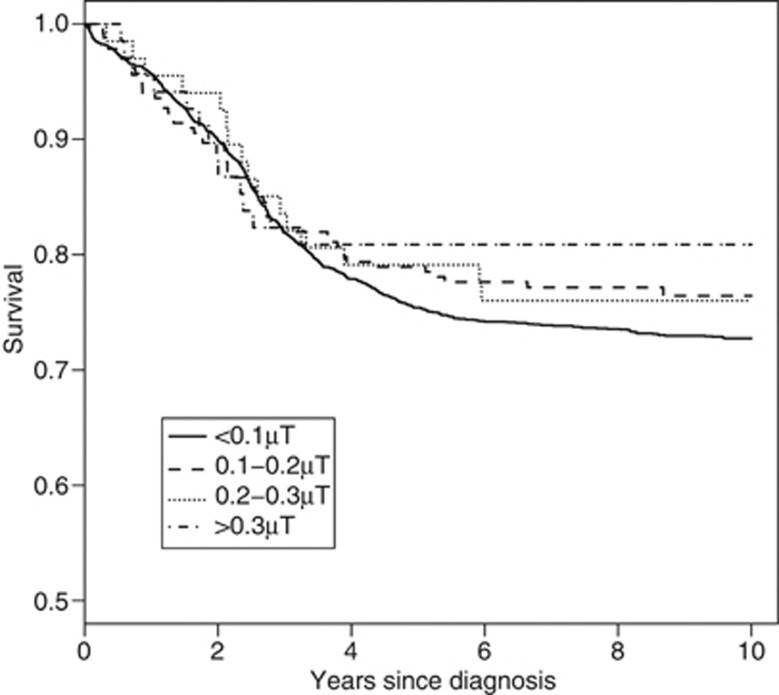
For the event-free survival analysis, no significantly elevated HRs were observed when considering ELF–MF exposure level (Table 3). We observed a HR of 1.0 when analyzing the survival per 0.1 μT increase (HR 1.00; CI, 0.93–1.07). Figure 1 shows the event-free survival curves by exposure categories.
Table 3. Event-free survival of children with ALL exposed to residential ELF–MF.
| ⩽0.1 μT |
0.1 μT—0.2 μT |
0.2 μT—0.3 μT |
>0.3 μT |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (Events) | N (Events) | HR | 95% CI | N (Events) | HR | 95% CI | N (Events) | HR | 95% CI | |
| Main | 712 | 54 | 1.10 | 0.82–1.46 | 16 | 1.14 | 0.69–1.89 | 13 | 0.76 | 0.44–1.33 |
| US POG study not included | 659 | 40 | 1.03 | 0.74–1.44 | 14 | 1.18 | 0.69–2.02 | 9 | 0.60 | 0.31–1.17 |
| T-cell ALL | 79 | 10 | 1.69 | 0.82–3.48 | 0 | 0.00 | — | 1 | 0.56 | 0.07–4.38 |
| B-precursor ALL | 452 | 43 | 1.14 | 0.83–1.58 | 15 | 1.44 | 0.85–2.43 | 11 | 0.93 | 0.51–1.70 |
| Other ALL | 181 | 1 | 0.15 | 0.02–1.07 | 1 | 0.43 | 0.06–3.16 | 1 | 0.42 | 0.06–3.12 |
Abbreviations: ALL, acute lymphoblastic leukemia; CI, confidence interval; HR, hazard ratio; POG Pediatric Oncology Group.
Figure 1.
Event-free survival of childhood ALL, by categories of residential exposure to ELF–MF.
Analysis not adjusting for prognostic risk (dichotomous score) or white blood cell count allowed the inclusion of additional cases, especially the study from New Zealand, but results changed little compared with the main analysis (based on N=3315 cases). For overall survival, HRs were 1.45 (CI, 1.04–2.02), 1.06 (CI, 0.54–2.07), and 0.97 (CI, 0.50–1.90) with increasing exposure levels of 0.1–0.2, 0.2–0.3, and >0.3 μT, while for the event-free survival analysis the respective HRs were 1.09 (CI, 0.82–1.45), 1.06 (CI, 0.64–1.76) and 0.84 (CI, 0.49–1.44).
Sensitivity analyses excluding children for whom ELF–MF exposures were measured in homes from which they moved out before the date of diagnosis, showed results similar to the main analyses (based on N=2783 cases). For overall survival, HRs were 1.30 (CI, 0.88–1.92), 1.24 (CI, 0.61–2.54) and 0.87 (CI, 0.41–1.87), with exposure categories of 0.1–0.2, 0.2–0.3 and >0.3 μT, and close to 1.0 per 0.1 μT increase (HR 1.03; CI, 0.96–1.11). For the event-free survival analysis the respective HRs were 1.07 (CI, 0.79–1.45), 1.19 (CI, 0.71–2.01), and 0.75 (CI, 0.41–1.37) in the categorical analyses, and 1.0 when analyzing event-free survival per 0.1 μT increase (HR 1.00; CI, 0.93–1.07).
Discussion
No statistically significant associations between exposure to ELF-EMF and poorer survival or higher risk of relapse were found in this pooled analysis of more than 3000 children with ALL from eight countries, all of whom were followed from diagnosis for 10 years, or until death or relapse. In both event-free and overall survival analyses, the HRs in the highest exposure groups of ELF–MF >0.3 μT as well as the HRs with increasing exposure by 0.1 μT were close to unity. These results do not confirm previous suggestions of a poorer survival in ALL survivors exposed to ELF–MF observed in studies in the US9 or Germany.10 Although both previous studies were included in the present analysis, the majority of the ALL cases in the high exposure category came from the other studies (47 of 68 or 69%). Although the hypothesis-generating study suggested an association in the highest exposure category,9 the CI of this estimate was very wide and therefore compatible with our overall finding (exclusion of the hypothesis-generating study did not materially change the pooled HRs); hence, random variation is the most likely explanation with the first study at the same time showing the highest HR of the individual studies. There was little indication of a differential effect of ELF–MF exposure on survival for different subtypes of ALL.
The reason for investigating ELF–MF in relation to ALL survival was that it was considered to be unlikely that ELF–MF were cancer initiators, due to the fact that experimental studies did not convincingly demonstrate DNA damage related to exposure, and because the quantum energy aligned with ELF–MF is, from theoretical considerations, not strong enough to break chemical bonds.8, 23 Therefore, it was hypothesized that ELF–MF might rather have a role in promoting cancer progression. Outcome in childhood ALL is dependent upon immunophenotype cytogenetic changes seen at diagnosis and treatment received. There is evidence of oligoclonality in a minority of children with ALL at diagnosis.24, 25, 26 Overall outcome is dependent upon the clone conferring the worst prognosis. It can be hypothesized that relapse is due to surviving clones (or their precursors) present at an undetectable level. These clones are likely to be subjected to the same environmental factors, which were present before initial diagnosis. In this case, poorer survival and higher rates of relapse in children with higher ELF–MF exposures, if observed, would support the suggestion that ELF–MFs may have an etiological role to play in childhood ALL development.
A major strength of our pooling project is the large data set compiled as a confirmatory source to test a hypothesis emerging from a previous smaller study. Further strengths were: (1) the systematic follow-up available through cancer registries and/or data from clinical trials; (2) availability of information to adjust for prognostic risk group; and (3) extended cumulative follow-up including the interval where risk of relapse and/or death is greatest.22 However, even with the sizable study population available, the proportion of cases exposed to >0.3 μT was relatively small. This in combination with the relatively limited number of events does impact the statistical power of the study, particularly for evaluation of relapse and survival among children with specific subtypes of ALL. Although the hypothesis-generating study and the case-control study were conducted for originally different purposes, the use of the case series from the case-control sets prospectively followed up for vital status and events in cancer registries or clinical trials lead to form a design perspective to very similar approaches, enabling us to pool the data.
The most important differences between the hypothesis-generating study9 and the ALL cases recruited in previous case-control studies to investigate risk related to ELF–MF exposure (all other studies) are differences in exposure assessment and information on residential history of the children after the date of diagnosis. The US POG study prospectively measured personal exposures of children in remission.9 For the case-control studies, exposure assessment was stationary measurements or calculation of ELF–MF (only Denmark) in residences occupied before diagnosis; however, the actual measurement was taken after diagnosis, in some studies even years after diagnosis. In the main analysis, as the etiologically relevant time period of exposure is unknown, all children were included, irrespective of whether they had moved. Under the assumption that exposure after diagnosis is most relevant, to reduce exposure misclassification in cases where the measurement was taken at a residence that was not the home at the date of diagnosis, data for these children was excluded in the sensitivity analyses, but the results from the main analysis were confirmed. However, we had no information on further residential history after diagnosis.
Another source of exposure uncertainty is that while most exposure data comes from long-term stationary measurements in the child's bedroom, for some children we had only spot measurements or calculated fields. The summary parameters from the long-term measurements also varied across studies (arithmetic mean, geometric mean, median). Bias could potentially be introduced by nonparticipation of cases with poorer survival than participating cases; however, we have no reason to believe this would also be associated with exposure to magnetic fields, and it may therefore be of lesser concern. Procedures for measurements for participating families with deceased children were not different.
Conclusions
In this large pooled analysis of more than 3000 children diagnosed with ALL in eight countries, no statistically significant associations were observed between exposure to ELF–MF and event-free survival or overall survival of ALL. These results provide no evidence that ELF–MF has a role in predicting outcome of childhood ALL.
Acknowledgments
This project was supported by a research contract from the Electric Power Research Institute (EPRI) to the Institute of Cancer Epidemiology, The Danish Cancer Society. The research agreement between EPRI and the Institute of Cancer Epidemiology guaranteed the complete freedom of the investigators to decide on study design, data collection methods, analysis plan, interpretation of the data, and the decision to submit the paper for publication. Dr Nick Douglas collected the survival data in New Zealand and Professor G Peter Herbison assisted with NZ data preparation. We thank Drs Masahiro Tsuchida (TCCSG), Masahito Tsurusawa (CCLSG), Keizo Horibe (JACLS), Jun Okamura (KYCCSG), and Tomohiro Saito (Funabashi Central Hospital) for their assistance to collect Japanese data.
Author Contributions
All authors contributed to the design of the study and to the writing of the paper.
GM is employed at the Electric Power Research Institute. CJ received funding from the Danish utility companies for other studies than the present one; all funds from Energy Denmark was provided via a firewall and CJ certify that his freedom to design, conduct, interpret, and publish research was not compromised by this or any other sponsor.
Footnotes
Supplementary Information accompanies the paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Schüz J, Ahlbom A. Exposure to electromagnetic fields and the risk of childhood leukaemia: a review. Radiat Prot Dosimetry. 2008;132:202–211. doi: 10.1093/rpd/ncn270. [DOI] [PubMed] [Google Scholar]
- IARC . IARC Monographs on the Evaluation of carcinogenic Risks to Humans, Volume 80, Non-Ionizing Radiation, Part 1: Static and extremely low-frequency (ELF) electric and magnetic fields. International Agency for Research on Cancer: Lyon; 2002. [PMC free article] [PubMed] [Google Scholar]
- Ahlbom A, Day N, Feychting M, Roman E, Skinner J, Dockerty J, et al. A pooled analysis of magnetic fields and childhood leukaemia. Br J Cancer. 2000;83:692–698. doi: 10.1054/bjoc.2000.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Sheppard AR, Kaune WT, Poole C, Kelsh MA. A pooled analysis of magnetic fields, wire codes, and childhood leukemia. Childhood Leukemia-EMF Study Group. Epidemiology. 2000;11:624–634. doi: 10.1097/00001648-200011000-00003. [DOI] [PubMed] [Google Scholar]
- Schüz J, Svendsen AL, Linet MS, McBride ML, Roman E, Feychting M, et al. Nighttime exposure to electromagnetic fields and childhood leukemia: an extended pooled analysis. Am J Epidemiol. 2007;166:263–269. doi: 10.1093/aje/kwm080. [DOI] [PubMed] [Google Scholar]
- Kheifets L, Ahlbom A, Crespi CM, Draper G, Hagihara J, Lowenthal RM, et al. Pooled analysis of recent studies on magnetic fields and childhood leukaemia. Br J Cancer. 2010;103:1128–1135. doi: 10.1038/sj.bjc.6605838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüz J. Implications from epidemiologic studies on magnetic fields and the risk of childhood leukemia on protection guidelines. Health Phys. 2007;92:642–648. doi: 10.1097/01.HP.0000243154.46491.7d. [DOI] [PubMed] [Google Scholar]
- Scientific Committee on Emerging and Newly Identified Health Risks Health Effects of Exposure to EMF. SCENIHR on EMF. 2009.
- Foliart DE, Pollock BH, Mezei G, Iriye R, Silva JM, Ebi KM, et al. Magnetic field exposure and long-term survival among children with leukaemia. Br J Cancer. 2006;94:161–164. doi: 10.1038/sj.bjc.6602916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen AL, Weihkopf T, Kaatsch P, Schüz J. Exposure to magnetic fields and survival after diagnosis of childhood leukemia: a German cohort study. Cancer Epidemiol Biomarkers Prev. 2007;16:1167–1171. doi: 10.1158/1055-9965.EPI-06-0887. [DOI] [PubMed] [Google Scholar]
- McBride ML, Gallagher RP, Thériault G, Armstrong BG, Tamaro S, Spinelli JJ, et al. Power-frequency electric and magnetic fields and risk of childhood leukemia in Canada. Am J Epidemiol. 1999;149:831–842. doi: 10.1093/oxfordjournals.aje.a009899. [DOI] [PubMed] [Google Scholar]
- Olsen JH, Nielsen A, Schulgen G. Residence near high voltage facilities and risk of cancer in children. BMJ. 1993;307:891–895. doi: 10.1136/bmj.307.6909.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis J, Schüz J, Meinert R, Zemann E, Grigat JP, Kaatsch P, et al. Combined risk estimates for two German population-based case-control studies on residential magnetic fields and childhood acute leukemia. Epidemiology. 1998;9:92–94. [PubMed] [Google Scholar]
- Dockerty JD, Elwood JM, Skegg DC, Herbison GP. Electromagnetic field exposures and childhood leukaemia in New Zealand. Lancet. 1999;354:1967–1968. doi: 10.1016/s0140-6736(99)05142-9. [DOI] [PubMed] [Google Scholar]
- Feychting M, Ahlbom A. Magnetic fields and cancer in children residing near Swedish high-voltage power lines. Am J Epidemiol. 1993;138:467–481. doi: 10.1093/oxfordjournals.aje.a116881. [DOI] [PubMed] [Google Scholar]
- UK Childhood Cancer Study Investigators, 1999 Exposure to power-frequency magnetic fields and the risk of childhood cancer. Lancet. 1999;354:1925–1931. [PubMed] [Google Scholar]
- Linet MS, Hatch EE, Kleinerman RA, Robison LL, Kaune WT, Friedman DR, et al. Residential exposure to magnetic fields and acute lymphoblastic leukemia in children. N Engl J Med. 1997;337:1–7. doi: 10.1056/NEJM199707033370101. [DOI] [PubMed] [Google Scholar]
- Schüz J, Grigat JP, Brinkmann K, Michaelis J. Residential magnetic fields as a risk factor for childhood acute leukaemia:results from a German population-based case-control study. Int J Cancer. 2001;91:728–735. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1097>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Kabuto M, Nitta H, Yamamoto S, Yamaguchi N, Akiba S, Honda Y, et al. Childhood leukemia and magnetic fields in Japan: a case-control study of childhood leukemia and residential power-frequency magnetic fields in Japan. Int J Cancer. 2006;119:643–650. doi: 10.1002/ijc.21374. [DOI] [PubMed] [Google Scholar]
- Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- Kleinerman RA, Linet MS, Hatch EE, Wacholder S, Tarone RE, Severson RK, et al. Magnetic field exposure assessment in a case-control study of childhood leukemia. Epidemiology. 1997;8:575–583. doi: 10.1097/00001648-199709000-00017. [DOI] [PubMed] [Google Scholar]
- Coebergh JW, Reedijk AM, de Vries E, Martos C, Jakab Z, Steliarova-Foucher E, et al. Leukaemia incidence and survival in children and adolescents in Europe during 1978-1997. report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2019–2036. doi: 10.1016/j.ejca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- WHO . Environmental Health Criteria Monograph n° 238. World Health Organization: Geneva; 2007. Extremely Low Frequency Fields. [Google Scholar]
- Deane M, Pappas H, Norton JD. Immunoglobulin heavy chain gene fingerprinting reveals widespread oligoclonality in B-lineage ALL. Leukemia. 1991;5:832–838. [PubMed] [Google Scholar]
- Szczepanski T, van der Velden VH, Hoogeveen PG, de Bie M, Jacobs D, van Wering ER, et al. Vdelta2-Jalpha arrangements are frequent in precursor-B-ALL but rare in normal lymphoid cells. Blood. 2004;103:3798–3804. doi: 10.1182/blood-2003-08-2952. [DOI] [PubMed] [Google Scholar]
- van der Velden VH, Szczepanski T, Wijkhuijs JM, Hart PG, Hoogeveen PG, Hop WC, et al. Age-related patterns of immunoglobulin and T-cell receptor gene rearrangements in precursor-B-ALL: implications for detection of minimal residual disease. Leukemia. 2003;17:1834–1844. doi: 10.1038/sj.leu.2403038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.