Abstract
Introduction
To systematically review the relationship between low pH in intervertebral discs and low back pain.
Material and methods
Electronic database (PubMed, ISI Web of Science, Cochrane Library, CINAHL, AMED, and China National Knowledge Infrastructure) searches and hand searching of conference proceedings were conducted. Two authors independently evaluated the methodological quality and abstracted relevant data according to standard criteria. Then the experimental methods and samples employed in the finally retrieved articles were assessed.
Results
We first retrieved 136 articles regarding pain and pH, and only 16 of them were mainly about low back pain and pH. Finally, 7 articles met our expectation to focus on the pathogenesis of low back pain caused by pH. In these 7 studies the authors held three opinions to explain the pathogenesis of low back pain in relation to low pH. First, low pH caused by lactate stimulates the muscle and increases the muscle tension, which causes low back pain. Second, low pH stimulates the nerve roots and produces the feeling of pain. Third, low pH changes the matrix metabolism, leading to neuronal death and low back pain.
Conclusions
In this systematic review we propose a new hypothesis that low back pain may be caused by low pH based on the previous literature. Further experimental studies are necessary to verify our hypothesis. This hypothesis will promote our understanding of the pathogenesis of low back pain and the development of novel diagnostic and therapeutic approaches for low back pain.
Keywords: low back pain, pH, acidity, intervertebral disc, systematic review
Introduction
Low back pain is one of the most frequent causes of morbidity and disability. Low back pain affects up to 50% to 80% of the population in developed countries and its recurrence rate amounts to 85%, resulting in an economic loss of approximately 50 to 100 billion dollars per year in the US [1, 2].
Currently, effective treatment of low back pain is severely hampered due to the fact that its pathogenesis remains elusive [3, 4]. In recent years, several hypotheses have been proposed to explain the pathogenesis of low back pain and most of them focus on the dysfunction of the spinal column and its components, such as injury and clinical instability [5–7], spinal column degeneration [8], inferior facet-tip impingement on the lamina [9], and Schmorl's nodes [10] and facet joint injury [11]. Other hypotheses focus on subfailure injury of the spinal muscles and ligaments and propose that spinal ligaments, disc annulus, facet capsules and thoracolumbar fascia may cause chronic back pain due to muscle control dysfunction [12–16]. In addition, the pain adaptation and pain-spasm-pain hypotheses have been proposed [17–19]. However, these hypotheses are largely speculative and need further experimental investigations.
The intervertebral disc (IVD) is composed of the nucleus pulposus (NP), the annulus fibrosus (AF), and the endplates (EP). The corpora vertebrae lie above and below the discs. The healthy disc is avascular, and its nutrition depends on diffusion via the AF and EP [20, 21]. The discs mainly produce ATP via anaerobic glycolysis; consequently lactate is produced and the pH is lower than other tissues. Low back pain is known to be related to intervertebral disc degeneration, and the pH would decrease in degenerated intervertebral discs [22, 23]. Therefore, low pH in the discs may be related to low back pain. Indeed, Hambly and Mooney [24] reported a close relationship between low back pain and low intradiscal pH in rabbits, while Krapf et al. [25] found that low pH could cause muscle spasm which was related to low back pain.
Based on the previous literature we propose a new hypothesis that low pH may cause low back pain. In this systematic review, we have collected and analysed the relevant literature regarding the relationship between low pH and low back pain to address the following questions: (1) What role does low pH play in low back pain? (2) Is the relationship obvious between low pH and low back pain? And (3), why are low pH and low back pain so relevant?
Material and methods
Electronic databases (PubMed, ISI Web of Science, Cochrane Library, CINAHL, AMED, and China National Knowledge Infrastructure), which were last updated on 26 Nov. 2011, were searched without limit by two independent investigators. The search used terms and Boolean operators as follows: (low back pain OR lower back pain OR low back ache OR low backaches OR lumbago OR recurrent low back pain OR postural low back pain or mechanical low back pain) AND (low pH OR lactate OR lactate OR hydrogen ion concentration). Reference lists of all the selected articles were hand-searched for any additional trials. Conference abstracts of key pain and orthopaedic journals were hand-searched to identify unpublished data. If necessary, we contacted the authors to get additional information.
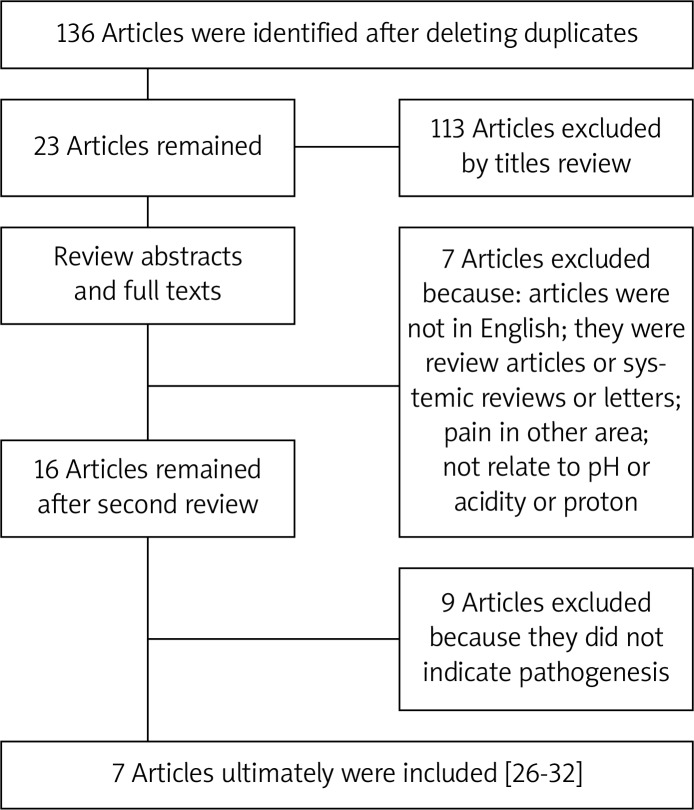
In total 136 articles were initially identified by literature search, and 113 articles were excluded after checking the titles and abstracts, which did not reach our expectation. Next we reviewed the full texts of the remaining articles and excluded the following articles: (1) articles not in English; (2) reviews, systematic reviews or letters; (3) pain in other tissues; (4) not related to pH, acidity or protons. As a result, 16 articles were retrieved and the references of these 16 articles were checked to ensure that other pertinent publications would not be missed. Finally, seven articles met our expectation to focus on the pathogenesis of low back pain caused by pH (Figure 1). The literature search was performed by two of the authors (CZL and HL) independently, and any disagreement was resolved by discussion.
Figure 1.
Flow diagram showing the selection of the relevant literature included in this systematic review
We scrutinized the seven articles with the focus on “the mechanisms by which pH causes low back pain”, and then assessed the experimental methods and samples employed in the seven articles.
Results
Seven articles met our expectation [26–32]. Then we evaluated the level of evidence for each article, according to the standard listed in Table I [33]. Five of them were level II, and two were level III. The characteristics of the seven studies are listed in Table II.
Table I.
Definition of the level of evidence
| Level of evidence | Characteristics of studies |
|---|---|
| I | 1. Randomized controlled trial |
| a. Significant difference | |
| b. No significant difference but confidence interval | |
| 2. Systematic review of level I randomized controlled trials (studies were homogeneous) | |
| II | 1. Prospective cohort study |
| 2. Poor quality randomized controlled trial | |
| 3. Systematic review (results from two or more previous studies) | |
| a. Level II studies | |
| b. Nonhomogeneous level I studies | |
| III | 1. Case-control study |
| 2. Retrospective cohort study | |
| 3. Systematic review of level III studies | |
| IV | Case series (no or historical control groups) |
| V | Expert opinion |
Table II.
Characteristics of the seven retrieved studies
| Study | Level of evidence | Number of patients | Male/female | Age [years] | Diseases |
|---|---|---|---|---|---|
| Diamant et al. (1968) [26] | II | 10 | 6/4 | 39 (average) | 10 Lumbar rhizopathies |
| Keshari et al. (2008) [27] | II | 18 | _ | 42 (average) | 9 Discogenic pain and 9 scoliosis |
| Bartels et al. (1998) [28] | II | 24 | _ | _ | 11 Back pain and 13 scoliosis |
| Bamrtann et al. (1996) [29] | III | 4 | 3/1 | 46-55 | 4 Chronic intractable pain |
| Strobel et al. (1997) [30] | II | 20 | _ | _ | 10 Low back pain and 10 fibromyalgia |
| Vormann et al. (2001) [31] | II | 82 | 30/52 | 46/50 (average) | 82 Low back pain |
| Moore et al. (1991) [32] | III | 18 | _ | _ | 18 Back pain |
Nerve roots
Three studies involving 32 patients [26, 27, 29] suggested that low pH would stimulate the nerve roots and cause low back pain.
Diamant et al. analysed the correlation between lactate level and pH in discs of patients with lumbar rhizopathy and found that low pH was caused by the increased lactate level due to the enhanced anaerobic glycolysis within the NP, which counteracts the decreased nutritional diffusion. The reaction of nerve roots in cases with low pH is related to increased production and leakage of acid metabolism. Sensitive structures such as the nerve roots could be irritated by the leakage of acid metabolites and it was shown that pain will arise in tissues with low pH [26, 34].
Keshari et al. used HR-MAS NMR spectroscopy to analyse snap frozen samples taken from 9 patients who underwent discectomy for painful disc degeneration [27, 35, 36]. They found that proteoglycan, collagen, and lactate may serve as metabolism markers of discogenic back pain. Therefore, they speculated that low pH was caused by increased lactate and increased lactate stimulated nerve fibres in granulation tissue associated with disc healing, which was correlated with discogenic pain [27, 35, 36].
Baumann et al. examined the responses of cultured adult human dorsal root ganglion (hDRG) neurons to low pH [29]. They found that low pH evoked, sustained depolarizations were due to more than one mechanism, and the inhibition of resting membrane conductance contributes to the responses to low pH in some hDRG neurons, which was related to low back pain [29].
Muscle tension and swelling of connective tissue
A previous study suggested that low pH would increase muscle tension, which could cause low back pain [30]. The authors examined 20 patients with chronic palpable tension of the erector muscles of the spine, and found that the pH decreased because of the enhanced anaerobic glycolysis in NP. The low pH was caused by the accumulation of lactate. Lactate would stimulate the multifidus muscle and increase the muscle tension. Simultaneously, myogelosis is induced, leading to low back pain [30]. Vormann et al. [31] showed that the simple and safe addition of an alkaline multimineral preparate was able to reduce the pain symptoms in these patients with chronic low back pain. These results suggest that a disturbed acid-base balance may contribute to the symptoms of low back pain.
Metabolism
Bartels et al. measured the oxygen and lactate concentrations in 11 patients with back pain and 13 patients with scoliosis, and found that in each case, the oxygen and lactate concentrations were the highest in the interior of the disc and fell toward the outer annulus [28]. Therefore, they speculated that the microcirculation through the endplate and the rate of cellular metabolism would influence the oxygen and lactate concentrations in the disc. For instance, the oxygen concentration would fall as cellular demand increases; consequently the lactate concentration would increase and the pH would decrease. It was observed that in some discs the concentration of oxygen was less than 40 mm Hg and that of lactate was more than 5 mmol/l, which would lead to cell death.
Another study also indicated that decreased pH, decreased PO2 and increased PCO2 may be related to the mechanisms of pain production in patients with back pain [32]. These abnormalities can be identified by magnetic resonance imaging. Further investigation is needed to determine whether therapeutic manipulation of these variables can be effective in relieving axial spinal pain.
Low pH would lead to a change in the matrix metabolism, which could strongly influence the cell activity and even cause cell death. It is well known that acid-sensing ion channels (ASICs) on the cell surface could be stimulated by protons. After cells die, the protons would increase and activate ASICs, which in turn mediate ischaemic neuronal death [37], and eventually cause low back pain [28, 38–40].
Discussion
After careful review of the seven articles we retrieved, we obtained a systematic view with regard to the relationship between low pH and low back pain, although the authors of the individual studies had proposed three different opinions.
If low pH directly stimulates the nerve roots, the pH is very important to the healing of low back pain. Lactate would cause low pH, stimulate the nerve roots, cause depolarization at the surface of the nerves, and modulate the nociceptors to let the patients feel pain. However, in order to establish a relationship between discogenic back pain and lactate, a much larger number of patients need to be studied and the changes in proteoglycans (PG)/collagen (col), PG/lactate peak (Lac), and Lac/col ratios should be correlated with visual pain scores or other pain indexes [27, 29].
The second opinion holds that low pH would act on the muscle but not nerve roots. If the oxygen tension falls below 5 mm Hg, the muscle tension would increase, and even result in myogelosis. Muscle contraction depends solely on the chemical energy of ATP. If the oxygen tension decreased, the cells would undergo anaerobic glycolysis and produce much lactate, leading to decreased pH. However, it remains elusive what level of pH would cause pain [30].
The third opinion claims that disc energy and matrix metabolism are crucially involved in low back pain [38–40]. This provides a valuable insight into the pathogenesis of low back pain. Nevertheless, the detailed cellular and molecular mechanisms by which disc energy and matrix metabolism disruption lead to neuronal death and eventually pain development are not completely understood.
This systematic review had several limitations. First, the heterogeneity between individual studies was substantial. Second, there are only small number patients in several prospective cohort studies of selected articles. Third, there may be some selection bias because the retrieved articles were confined to limited databases.
In conclusion, in this systematic review we propose a new hypothesis that low back pain may be caused by low pH based on previous literature, in which three opinions have been proposed by the authors to explain the pathogenesis of low back pain in relation to low pH. First, low pH caused by lactate stimulates the muscle and increases the muscle tension, which would cause low back pain. Second, low pH stimulates the nerve roots and produces the feeling of pain. Third, low pH changes the matrix metabolism, leading to neuronal death and low back pain. These different opinions are not exclusive but may be complementary. Further experimental studies are necessary to verify our hypothesis that low pH causes low back pain. This hypothesis will promote our understanding of the pathogenesis of low back pain and the development of novel diagnostic and therapeutic approaches for low back pain.
Acknowledgments
This study was partly supported by a grant from the National Nature Science Foundation of China (81171756) and the Science and Technology Planning Project of Zhejiang Province (2012C13G2010083).
References
- 1.Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–64. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 2.DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011;12:224–33. doi: 10.1111/j.1526-4637.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- 3.Gracely RH. Pain measurement. Acta Anaesthesiol Scand. 1999;43:897–908. doi: 10.1034/j.1399-6576.1999.430907.x. [DOI] [PubMed] [Google Scholar]
- 4.Ghahreman A, Bogduk N. Predictors of a favorable response to transforaminal injection of steroids in patients with lumbar radicular pain due to disc herniation. Pain Med. 2011;12:871–9. doi: 10.1111/j.1526-4637.2011.01116.x. [DOI] [PubMed] [Google Scholar]
- 5.Panjabi MM. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5:390–6. doi: 10.1097/00002517-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Depalma M, Ketchum J, Saullo T, Schofferman J. Structural etiology of chronic low back pain due to motor vehicle collision. Pain Med. 2011;12:1622–7. doi: 10.1111/j.1526-4637.2011.01246.x. [DOI] [PubMed] [Google Scholar]
- 7.Raczkowski JW, Daniszewska B, Zolynski K. Functional scoliosis caused by leg length discrepancy. Arch Med Sci. 2010;6:393–8. doi: 10.5114/aoms.2010.14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, Reilly J. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine. 1978;3:319–28. doi: 10.1097/00007632-197812000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Yang KH, King AI. Mechanism of facet load transmission as a hypothesis for low-back pain. Spine. 1984;9:557–65. doi: 10.1097/00007632-198409000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Lipson SJ, Fox DA, Sosman JL. Symptomatic intravertebral disc herniation (Schmorl's node) in the cervical spine. Ann Rheum Dis. 1985;44:857–9. doi: 10.1136/ard.44.12.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farfan HF, Sullivan JD. The relation of facet orientation to intervertebral disc failure. Can J Surg. 1967;10:179–85. [PubMed] [Google Scholar]
- 12.Schleip R, Vleeming A, Lehmann-Horn F, Klingler W. Letter to the Editor concerning “A hypothesis of chronic back pain: ligament subfailure injuries lead to muscle control dysfunction” (M. Panjabi) Eur Spine J. 2007;16:1733–5. doi: 10.1007/s00586-006-0298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panjabi MM. A hypothesis of chronic back pain: ligament subfailure injuries lead to muscle control dysfunction. Eur Spine J. 2006;15:668–76. doi: 10.1007/s00586-005-0925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DePalma MJ, Ketchum JM, Saullo TR. Etiology of chronic low back pain in patients having undergone lumbar fusion. Pain Med. 2011;12:732–9. doi: 10.1111/j.1526-4637.2011.01098.x. [DOI] [PubMed] [Google Scholar]
- 15.Karabekir HS, Yildizhan A, Atar EK, et al. Effect of ligamenta flava hypertrophy on lumbar disc herniation with contralateral symptoms and signs: a clinical and morphometric study. Arch Med Sci. 2010;6:617–22. doi: 10.5114/aoms.2010.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petronic I, Nikolic D, Cirovic D, et al. Distribution of affected muscles and degree of neurogenic lesion in patients with spina bifida. Arch Med Sci. 2011;7:1049–54. doi: 10.5114/aoms.2011.26619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dieen JH, Selen LP, Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J Electromyogr Kinesiol. 2003;13:333–51. doi: 10.1016/s1050-6411(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 18.Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69:683–94. doi: 10.1139/y91-102. [DOI] [PubMed] [Google Scholar]
- 19.Maigne JY, Vautravers P. Mechanism of action of spinal manipulative therapy. Joint Bone Spine. 2003;70:336–41. doi: 10.1016/s1297-319x(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 20.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–4. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 21.Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8:18–44. doi: 10.1111/j.1533-2500.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 22.Kitano T, Zerwekh JE, Usui Y, et al. Biochemical changes associated with the symptomatic human intervertebral disk. Clin Orthop Relat Res. 1993;293:372–7. [PubMed] [Google Scholar]
- 23.Wuertz K, Godburn K, Iatridis JC. MSC response to pH levels found in degenerating intervertebral discs. Biochem Biophys Res Commun. 2009;379:824–9. doi: 10.1016/j.bbrc.2008.12.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hambly MF, Mooney V. Effect of smoking and pulsed electromagnetic fields on intradiscal pH in rabbits. Spine. 1992;17:S83–5. doi: 10.1097/00007632-199206001-00004. [DOI] [PubMed] [Google Scholar]
- 25.Krapf MW, Muller S, Mennet P, et al. Recording muscle spasm in the musculus erector spinae using in vivo 31P magnetic resonance spectroscopy in patients with chronic lumbalgia and generalized tendomyopathies. Z Rheumatol. 1992;51:229–37. [PubMed] [Google Scholar]
- 26.Diamant B, Karlsson J, Nachemson A. Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies. Experientia. 1968;24:1195–6. doi: 10.1007/BF02146615. [DOI] [PubMed] [Google Scholar]
- 27.Keshari KR, Lotz JC, Link TM, et al. Lactic acid and proteoglycans as metabolic markers for discogenic back pain. Spine. 2008;33:312–7. doi: 10.1097/BRS.0b013e31816201c3. [DOI] [PubMed] [Google Scholar]
- 28.Bartels EM, Fairbank JC, Winlove CP, Urban JP. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine. 1998;23:1–7. doi: 10.1097/00007632-199801010-00001. [DOI] [PubMed] [Google Scholar]
- 29.Baumann TK, Burchiel KJ, Ingram SL, Martenson ME. Responses of adult human dorsal root ganglion neurons in culture to capsaicin and low pH. Pain. 1996;65:31–8. doi: 10.1016/0304-3959(95)00145-X. [DOI] [PubMed] [Google Scholar]
- 30.Strobel ES, Krapf M, Suckfull M, et al. Tissue oxygen measurement and 31P magnetic resonance spectroscopy in patients with muscle tension and fibromyalgia. Rheumatol Int. 1997;16:175–80. doi: 10.1007/BF01330292. [DOI] [PubMed] [Google Scholar]
- 31.Vormann J, Worlitschek M, Goedecke T, Silver B. Supplementation with alkaline minerals reduces symptoms in patients with chronic low back pain. J Trace Elem Med Biol. 2001;15:179–83. doi: 10.1016/S0946-672X(01)80064-X. [DOI] [PubMed] [Google Scholar]
- 32.Moore MR, Brown CW, Brugman JL, et al. Relationship between vertebral intraosseous pressure, pH, PO2, pCO2, and magnetic resonance imaging signal inhomogeneity in patients with back pain. An in vivo study. Spine. 1991;16:S239–42. doi: 10.1097/00007632-199106001-00012. [DOI] [PubMed] [Google Scholar]
- 33.Prommahachai A, Wittayapirot K, Jirarattanaphochai K, Sae-Jung S. Correction with instrumented fusion versus non-corrective surgery for degenerative lumbar scoliosis: a systematic review. J Med Assoc Thai. 2010;93:920–9. [PubMed] [Google Scholar]
- 34.Menkin V. Biochemical mechanisms in inflammation. Br Med J. 1960;1:1521–8. doi: 10.1136/bmj.1.5185.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoki Y, Akeda K, An H, et al. Nerve fiber ingrowth into scar tissue formed following nucleus pulposus extrusion in the rabbit anular-puncture disc degeneration model: effects of depth of puncture. Spine. 2006;31:E774–80. doi: 10.1097/01.brs.0000238681.71537.41. [DOI] [PubMed] [Google Scholar]
- 36.Ozawa T, Ohtori S, Inoue G, et al. The degenerated lumbar intervertebral disc is innervated primarily by peptide-containing sensory nerve fibers in humans. Spine. 2006;31:2418–22. doi: 10.1097/01.brs.0000239159.74211.9c. [DOI] [PubMed] [Google Scholar]
- 37.Wang YZ, Xu TL. Acidosis, acid-sensing ion channels, and neuronal cell death. Mol Neurobiol. 2011;44:350–8. doi: 10.1007/s12035-011-8204-2. [DOI] [PubMed] [Google Scholar]
- 38.Ohshima H, Urban JP. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine. 1992;17:1079–82. doi: 10.1097/00007632-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829–35. doi: 10.1002/jor.1100170607. [DOI] [PubMed] [Google Scholar]
- 40.Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–19. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]