Abstract
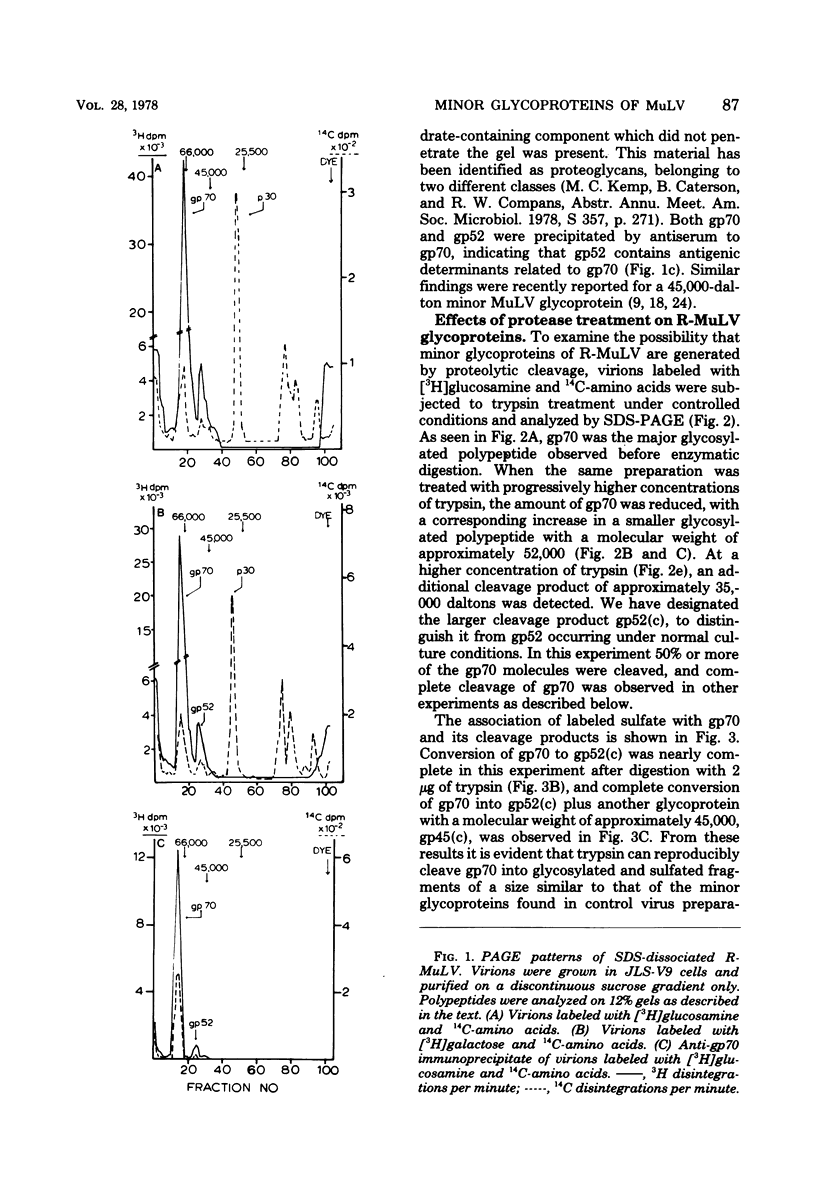
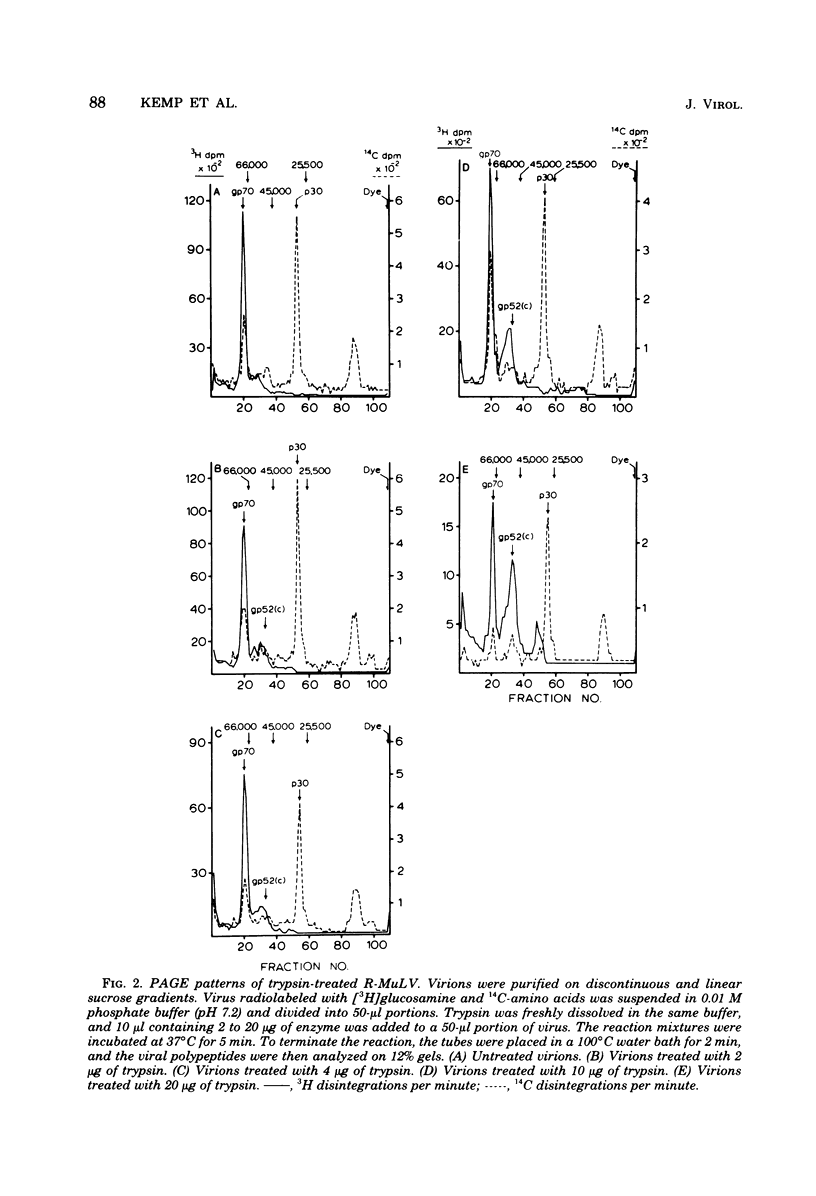
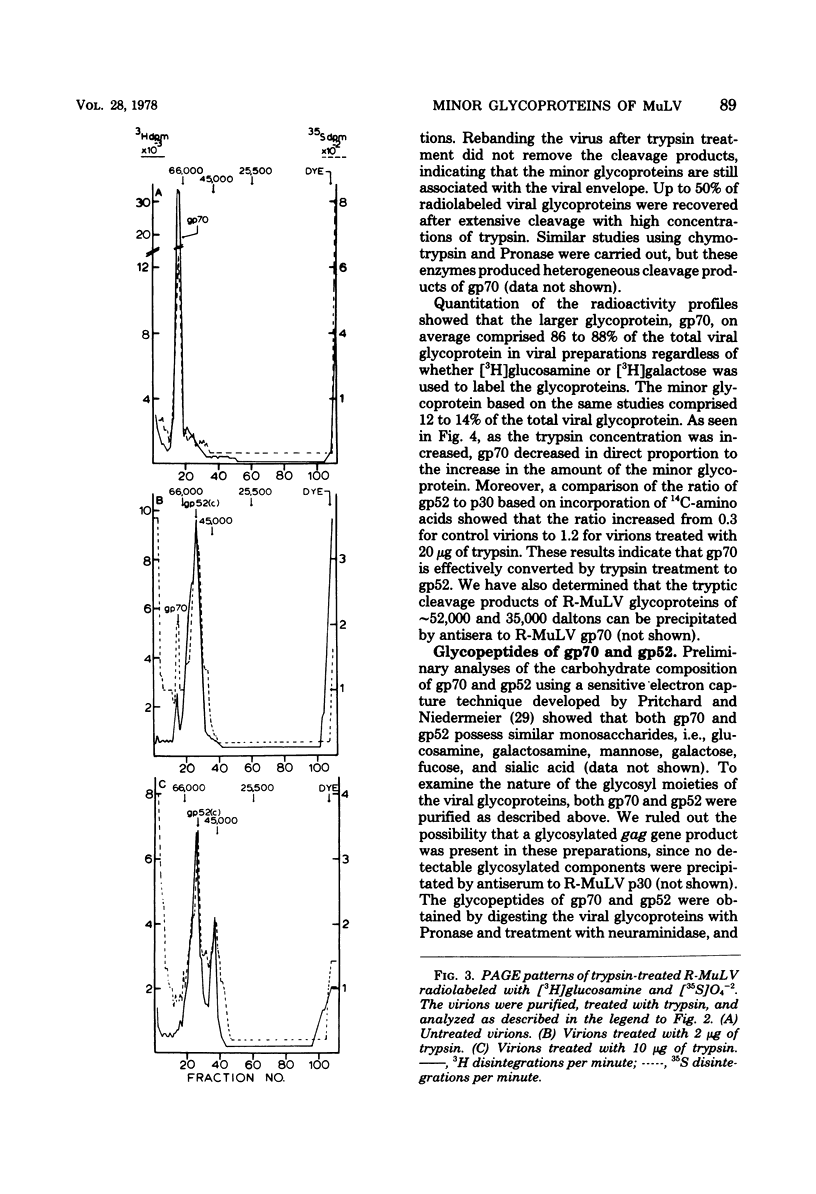
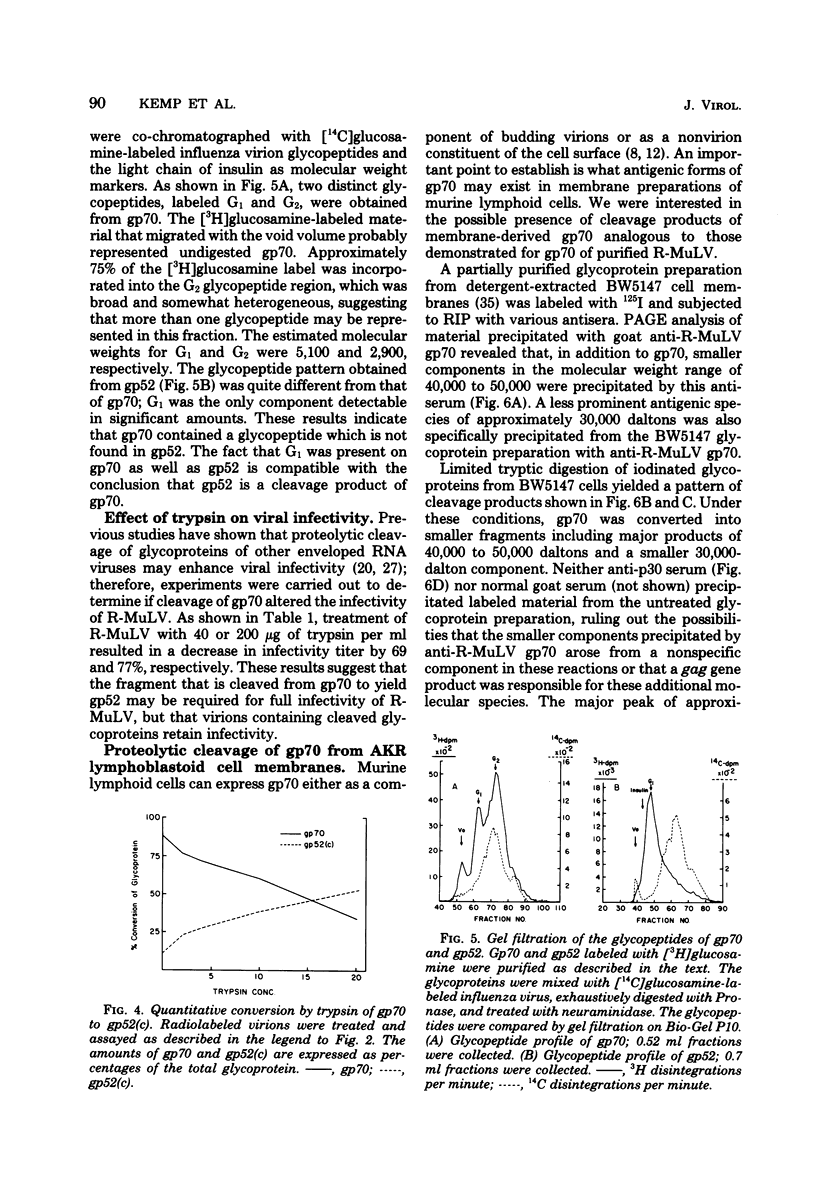
Polyacrylamide gel electrophoretic analysis and immunoprecipitation were used to study glycoproteins from purified Rauscher murine leukemia virus (R-MuLV) and from AKR thymic lymphoblastoid cell membranes. In addition to gp70, a minor glycoprotein of approximately 52,000 daltons (gp52) was demonstrated in purified R-MuLV preparations, which was antigenically related to gp70. Analysis of R-MuLV glycopeptides obtained after exhaustive Pronase digestion showed that gp70 has at least two different glycopeptide size classes with molecular weights of 5,100 and 2,900, respectively. gp52, however, contained only a single glycopeptide size class of approximately 5,100 daltons, indicating that the two glycoproteins contain distinct carbohydrate components. Trypsin treatment of R-MuLV converted gp70 into a product with a molecular mass of approximately 52,000 daltons as well as a 45,000-dalton minor product, with little effect on virus infectivity. Similarly, trypsin treatment of 125I-labeled glycoproteins derived from AKR mouse lymphoblastoid cell membranes generated fragments antigenically related to gp70 and similar in size to those obtained by trypsin treatment of R-MuLV. In both cases, the appearance of cleavage products was accompanied by a decrease in gp70 during trypsin treatment. The occurrence of glycosylated components antigenically related to gp70 in AKR membrane glycoprotein preparations and in purified R-MuLV preparations which were similar to those generated by trypsin treatment supports the concept that these minor components arise from proteolytic cleavage of gp70.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
- Boyse E. A. The Gx system in relation to C-type viruses and heredity. Immunol Rev. 1977 Jan;33:125–145. doi: 10.1111/j.1600-065x.1977.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Bubbers J. E., Lilly F. Selective incorporation of H-2 antigenic determinants into Friend virus particles. Nature. 1977 Mar 31;266(5601):458–459. doi: 10.1038/266458a0. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Distinct carbohydrate components of influenza virus glycoproteins in smooth and rough cytoplasmic membranes. Virology. 1973 Oct;55(2):541–545. doi: 10.1016/0042-6822(73)90199-2. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. J Immunol. 1976 Jul;117(1):136–142. [PubMed] [Google Scholar]
- Del Vellano B. C., Nave B., Croker B. P., Lerner R. A., Dixon F. J. The oncornavirus glycoprotein gp69/71: a constituent of the surface of normal and malignant thymocytes. J Exp Med. 1975 Jan 1;141(1):172–187. doi: 10.1084/jem.141.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Jensen F. C., Bryant M. L., Lerner R. A. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene family. Nature. 1977 May 5;267(5606):23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Smythers G. W., Marquardt H., Oroszlan S. Amino-terminal amino acid sequence and carboxyl-terminal analysis of Rauscher murine leukemia virus glycoproteins. Virology. 1978 Mar;85(1):319–322. doi: 10.1016/0042-6822(78)90437-3. [DOI] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsmann G., Moennig V., Pister L., Seifert E., Schäfer W. Properties of mouse leukemia viruses. VIII. The major viral glycoprotein of Friend leukemia virus. Seroimmunological, interfering and hemagglutinating capacities. Virology. 1974 Dec;62(2):307–318. doi: 10.1016/0042-6822(74)90394-8. [DOI] [PubMed] [Google Scholar]
- Kemp M. C., Perdue M. L., Rogers H. W., O'Callaghan D. J., Randall C. C. Structural polypeptides of the hamster strain of equine herpes virus type 1: products associated with purification. Virology. 1974 Oct;61(2):361–375. doi: 10.1016/0042-6822(74)90274-8. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Klemenz R., Diggelmann H. The generation of the two envelope glycoproteins of Rous sarcoma virus from a common precursor polypeptide. Virology. 1978 Mar;85(1):63–74. doi: 10.1016/0042-6822(78)90411-7. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Krantz M. J., Strand M., August J. T. Biochemical and immunological characterization of the major envelope glycoprotein gp69/71 and degradation fragments from Rauscher leukemia virus. J Virol. 1977 Jun;22(3):804–815. doi: 10.1128/jvi.22.3.804-815.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Proteolytic cleavage of the hemagglutinin polypeptide of influenza virus. Function of the uncleaved polypeptide HA. Virology. 1973 Mar;52(1):199–212. doi: 10.1016/0042-6822(73)90409-1. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Gilden R. V., Oroszlan S. Envelope glycoproteins of Rauscher murine leukemia virus: isolation and chemical characterization. Biochemistry. 1977 Feb 22;16(4):710–717. doi: 10.1021/bi00623a024. [DOI] [PubMed] [Google Scholar]
- McCarter J. A. Genetic studies of the ploidy of Moloney murine leukemia virus. J Virol. 1977 Apr;22(1):9–15. doi: 10.1128/jvi.22.1.9-15.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Hayami M. Analysis of precursors to the envelope glycoproteins of avian RNA tumor viruses in chicken and quail cells. J Virol. 1977 Jun;22(3):598–607. doi: 10.1128/jvi.22.3.598-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D. Activation of precursors to both glycoporteins of Newcastle disease virus by proteolytic cleavage. Virology. 1977 Mar;77(1):125–134. doi: 10.1016/0042-6822(77)90412-3. [DOI] [PubMed] [Google Scholar]
- Pinter A., Compans R. W. Sulfated components of enveloped viruses. J Virol. 1975 Oct;16(4):859–866. doi: 10.1128/jvi.16.4.859-866.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P. Retention of lymphocyte characteristics by myelomas and theta + -lymphomas: sensitivity to cortisol and phytohemagglutinin. J Immunol. 1973 Jun;110(6):1470–1475. [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Keegstra K. Glycoproteins of Sindbis virus: priliminary characterization of the oligosaccharides. J Virol. 1974 Sep;14(3):522–530. doi: 10.1128/jvi.14.3.522-530.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. F., Brown D. T. Envelopments of Sindbis virus: synthesis and organization of proteins in cells infected with wild type and maturation-defective mutants. J Virol. 1977 Jun;22(3):662–678. doi: 10.1128/jvi.22.3.662-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. S., Vitetta E. S., Fleissner E., Boyse E. A. Biochemical evidence linking the GIX thymocyte surface antigen to the gp69/71 envelope glycoprotein of murine leukemia virus. J Exp Med. 1975 Jan 1;141(1):198–205. doi: 10.1084/jem.141.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Reitz M. S., Paran M., Gallo R. C. Mechanism of stimulation of murine type-C RNA tumor virus production by glucocorticoids: post-transcriptional effects. J Virol. 1974 Oct;14(4):802–812. doi: 10.1128/jvi.14.4.802-812.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M. J., Compans R. W. Structural components of mouse mammary tumor virus. I. Polypeptides of the virion. Virology. 1977 Feb;76(2):751–766. doi: 10.1016/0042-6822(77)90256-2. [DOI] [PubMed] [Google Scholar]
- Zwerner R. K., Acton R. T. Growth properties and alloantigenic expression of murine lymphoblastoid cell lines. J Exp Med. 1975 Aug 1;142(2):378–390. doi: 10.1084/jem.142.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerner R. K., Barstad P. A., Acton R. T. Isolation and characterizaiton of murine cell surface components. I. Purification of milligram quantities of Thy-1.1. J Exp Med. 1977 Oct 1;146(4):986–1000. doi: 10.1084/jem.146.4.986. [DOI] [PMC free article] [PubMed] [Google Scholar]



