Abstract
Introduction
Subclinical hypothyroidism has been reported to be associated with disturbed cognitive function. In this study, changes of subtests of the Wechsler Memory Scale and memory quotient were investigated in subjects with subclinical hypothyroidism following treatment with levothyroxine. The aim of the study was a randomized double blind placebo-controlled clinical trial.
Material and methods
Sixty subjects (51 females and 9 males) with subclinical hypothyroidism were enrolled. Memory quotient was evaluated at the beginning of the study and three months after enrollment, using Wechsler's memory test. Subclinical hypothyroidism was defined as serum TSH level between 4.5 mU/l and 10 mU/l in the presence of normal free-T4 (0.8-2 ng/dl) and positive anti-TPO-Ab. The intervention and control groups received levothyroxine and placebo respectively for 3 months. Re-evaluation was done using the Wechsler Memory Scale at the end of the study.
Results
The mean age was 34 ±10 years, mean TSH level was 8.25 ±3.64 muIU/l. Memory quotient was similar in both groups at the beginning of the study: 105.70 ±2.1 in intervention group vs. 105.87 ±2.1 in control group (p = 0.89). At the end of the study, the memory quotient rose by 9.3 points in the intervention group and by 3.23 in the controls (p = 0.002). Analysis of the scores of Wechsler Memory subtests in the intervention group indicated significant improvement of mental control (p = 0.002), logical memory (p < 0.001), associate learning (p = 0.014), age corrected score (p = 0.002), and memory quotient (p < 0.001).
Conclusions
This study showed the efficacy of levothyroxine for cognitive function of subjects with subclinical hypothyroidism.
Keywords: Memory Quotient, Subclinical Hypothyroidism, Wechsler Memory Test, levothyroxine
Introduction
Subclinical hypothyroidism (SCH) is defined biochemically as a normal serum free thyroxin (T4) concentration in the presence of increased thyroid stimulating hormone (TSH). One of the criteria to begin treatment in these subjects is probable progression of the disease to clinical hypothyroidism, which accounts for 3-8% of cases per year [1]. Higher titers of anti-thyroperoxidase (TPO) antibody and TSH levels are reported to be associated with disease progression [2]. Subclinical hypothyroid subjects are usually asymptomatic. Clinical manifestations differ based on age, duration, and severity of hormone shortage and are usually non-specific [2, 3].
There are some concerns regarding prolonged levothyroxine treatment such as increased risk of osteoporosis, atrial fibrillation, and coronary artery disease. However, it has been shown that bone metabolism and body composition are not altered by levothyroxine treatment [4, 5]. Furthermore, the cardiovascular benefits of levothyroxine therapy outweigh its possible risks [6]. In addition, the role of thyroid hormone in cognitive organization has been proved [7]. Frank hypothyroidism is known to induce neurological and mental dysfunction but the effect of subclinical hypothyroidism on cognition and mental function is controversial. Cognition refers to mental processes that include storage, remembering, and using information.
Cognitive disorders can affect various activities such as receiving, resembling, memory, persuading, problem solving, decision making, and language [8]. Subclinical hypothyroidism may be associated with a defect in verbal memory and executive function which is due to abnormal function of the hippocampus [7, 9]. Wechsler's memory test has been used as a valid tool to evaluate memory. The Wechsler Memory Scale (WMS) is a neuropsychological test designed to measure different memory functions. It is appropriate for studying logical memory, mental control, visual reproduction, associate learning, working memory and immediate memory. This test is simple and rapid and provides reliable information regarding organic and functional memory disorders. Wechsler's memory test considers the different memory power at different ages. The final score is computed according to the WMS manual which yields the percentile-rank score adjusted for age [10]. In this study, changes of subtests of the Wechsler Memory Scale and memory quotient were investigated in subjects with subclinical hypothyroidism following treatment with levothyroxine.
Material and methods
This study was a randomized double blind placebo-controlled clinical trial. Sixty subjects (51 females and 9 males) referring to the outpatient thyroid clinics with subclinical hypothyroidism between 18 and 64 years old with non-specific symptoms of hypothyroidism such as weight gain, dry skin, fatigue, muscle cramp, hoarseness, constipation, and irregular menstruation were enrolled in this study. Subjects who had a history of endocrine or autoimmune diseases other than hypothyroidism, myxedema coma, proven psychiatric disorder, pregnancy or lactation, intake of thyroid hormones or corticosteroids in the previous 2 months were excluded from the study. All eligible subjects agreed and signed the consent form after full explanation of the purpose and nature of all procedures used. They were randomly allocated to two groups using a simple randomization method. The control group comprised 25 females and 5 males (mean age: 36.07 years, range: 21-58 years) and the intervention group consisted of 26 females and 4 males (mean age: 32.37 years, range: 19-53 years). Memory quotient of the participants was evaluated at the beginning of the study and three months after enrollment using Wechsler's memory test. Subjects were submitted to the Wechsler Memory Scale; their ratings on the neurobehavioral tests and their thyroid hormone profile were compared to the age-or sex-matched controls. Cognitive functioning was evaluated using the Wechsler Memory Scale, designed to assess learning, memory and working memory. Seven subtests are included in the test: information, orientation, mental control, logic memory, digits forward and backward, visual reproduction and associate learning. The WMS provides a total "memory quotient" (MQ) that accounts for age-related mnemonic variability. Subclinical hypothyroidism was defined as serum TSH level between 4.5 mU/l and 10 mU/l in the presence of normal free-T4 (0.8-2 ng/dl) and positive anti-TPO-Ab. Subjects who had diabetes mellitus, heart failure, chronic liver or pulmonary disorder, history of head trauma, seizure, known psychological or mental disorders and pregnancy were excluded. All eligible subjects filled out the Wechsler Memory Scale at the beginning of the study. The intervention group received 100 µg of levothyroxine (Iran Hormone Product) and the control group received placebo for 3 months. The participants were asked to bring the remaining tablets to assess compliance. The subjects were re-evaluated by the Wechsler Memory Scale at the end of the study.
This project was accepted by the ethical committee of Tehran University of Medical Sciences; ethical code: 13/2/1390.
Statistical analysis
Data were analyzed using SPSS software version 18. For comparison of mean age, TSH, FT4 and T4 between two groups Mann-Whitney U test was used. T-test was applied to compare mean memory quotient between groups and paired T-test to compare these values before and after intervention. Independent sample T-test was used for comparison of mean memory quotient variable. Simple Pearson regression test was used to investigate the relationship between memory quotient variable and TSH variability.
Results
In this study, 60 subclinical hypothyroid subjects were divided randomly into two equal groups. The mean age of the participants was 34 ±10 years and 85% of them were female (51). Forty-three subjects were married. There were no statistical differences in WMS subtests and memory quotient (p = 0.31) between the two groups at the beginning of the study. Demographic variables and MQ scores were compared. Baseline characteristics of the participants are shown in Table I. Thyroid hormone parameters were similar in both groups at the beginning of the study, as shown in Table I. The changes in biochemical parameters before and after the treatment are shown in Table II. Table III shows the clinical symptomatology of subclinical hypothyroidism in both groups before and after treatment. Each individual's score on memory was computed according to the WMS manual, which yields the percentile-rank score adjusted for age.
Table I.
Baseline characteristics of participants
| Variables | Intervention group* | Control group* | Value of p |
|---|---|---|---|
| Age [years] | 32.37 ±11.35 | 36.07 ±11.35 | 0.4 |
| TSH [ml/l] | 8.29 ±4.9 | 8.12 ±3.12 | 0.9 |
| FT4 [ng/dl] | 1.38 ±0.26 | 1.37 ±0.29 | 0.83 |
| T4 [mg/dl] | 7.38 ±1.37 | 7.41 ±1.48 | 0.92 |
Data are shown as mean ± SD
Table II.
Biochemical parameters before and after treatment
| Variables | Intervention group | Control group | ||||
|---|---|---|---|---|---|---|
| Baseline* | Final* | Value of p | Baseline* | Final* | Value of p | |
| TSH [ml/l] | 8.3907 ±4.091 | 2.0070 ±1.34 | < 0.001 | 8.1203 ±3.20 | 7.8270 ±5.17 | 0.733 |
| T4 [mg/dl] | 7.3880 ±1.375 | 8.6077 ±2.058 | 0.004 | 7.4703 ±1.48 | 8.1363 ±1.98 | 0.093 |
Data are shown as mean ± SD
Table III.
Frequency of non-specific symptoms of subclinical hypothyroidism in the intervention and control groups
| Symptoms | Intervention group | Control group | ||||
|---|---|---|---|---|---|---|
| Before treatment* | After treatment* | Value of p | Before treatment* | After treatment* | Value of p | |
| Weight gain | 17 (56.7%) | 10 (33.3%) | 0.118 | 16 (53%) | 9 (30%) | 0.065 |
| Dry skin | 13 (43.3%) | 9 (30%) | 0.344 | 14 (46.7%) | 11 (36.7%) | 0.45 |
| Fatigue | 25 (83.3%) | 23 (76.7%) | 0.4 | 25 (83.3%) | 24 (80%) | 1 |
| Muscle cramp | 19 (63.3%) | 15 (50%) | 0.34 | 17 (56.7%) | 15 (50%) | 0.77 |
| Hoarseness | 5 (16.7%) | 3 (10%) | 0.6 | 4 (13.3%) | 4 (13.3%) | 1 |
| Constipation | 7 (23.3%) | 4 (13.3%) | 0.5 | 10 (33.3%) | 7 (23.3%) | 0.3 |
| Irregular menstruation | 11 (36.7%) | 9 (30%) | 0.7 | 24 (80%) | 24 (80%) | 1 |
Data are shown as n (%)
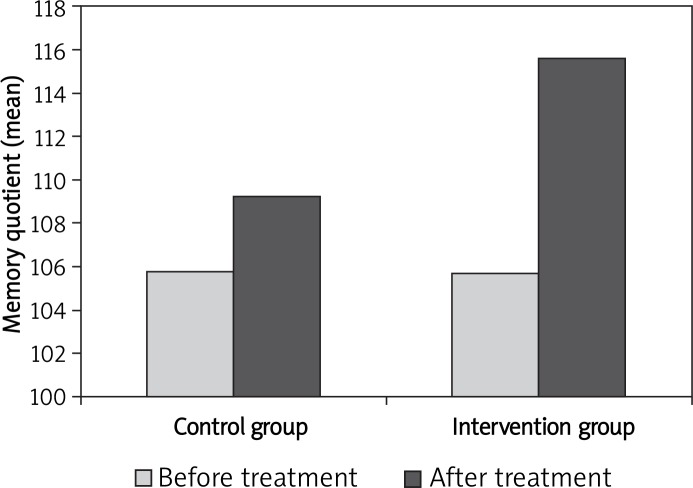
At the end of the study, TSH levels were normalized in all subjects in the intervention group (Table II). Moreover, there was no TSH suppression indicating subclinical hyperthyroidism in this group. In addition, the memory quotient rate increased from 105 ±2.8 to 115 ±2.6 (ΔMQ = 9.9 ±1.46) in the intervention group and from 105.87 ±2.09 to 109 ±2.5 (ΔMQ = 3.2 ±1.39) in the control group (p = 0.002). In Figure 1, comparison of the memory quotient mean is illustrated for the intervention and control groups before and after treatment.
Figure 1.
Comparison of memory quotient mean in the intervention and control groups
The result for the subtests of WMS in the intervention group showed that the mental control (p = 0.002), logical memory (p < 0.001), associate learning (p = 0.014), age-corrected score (p = 0.002), and memory quotient (p < 0.001) improved significantly after treatment. Scores of Wechsler Memory Scale subtests of the intervention group at baseline and after levothyroxine treatment are shown in Table IV.
Table IV.
Baseline and final scores of Wechsler Memory Scale subtests for the intervention and control groups
| WMS Subtests | Intervention group | Control group | ||||
|---|---|---|---|---|---|---|
| Baseline score* | Final score* | Value of p | Baseline score* | Final score* | Value of p | |
| Information | 5.88 ±0.34 | 5.78 ±0.49 | 0.26 | 5.7 ±0.54 | 5.7 ±0.54 | 1.00 |
| Orientation | 4.53 ±0.67 | 4.66 ±0.6 | 0.25 | 4.4 ±0.7 | 4.3 ±0.73 | 0.62 |
| Mental control | 8.03 ±0.99 | 7.35 ±1.33 | 0.002 | 6.4 ±0.97 | 7.04 ±1.2 | 0.024 |
| Logical memory | 10.83 ±2.94 | 12.31 ±2.32 | < 0.001 | 9.5 ±1.9 | 10.2 ±2.6 | 0.14 |
| Digits forward and backward | 8.9 ±1.44 | 8.48 ±1.6 | 0.11 | 8.18 ±1.7 | 8.18 ±1.5 | 1.00 |
| Associate learning | 7.1 ±2.47 | 12.9 ±1.53 | 0.014 | 16.8 ±2.1 | 16.7 ±2.5 | 0.76 |
| Visual reproduction | 12.9 ±1.53 | 12.37 ±1.62 | 0.13 | 13.3 ±1.3 | 12.03 ±1.6 | 0.003 |
| Age-corrected score | 105 ±2.8 | 115 ±2.6 | 0.002 | 101.27 ±6.9 | 100.6 ±6.6 | 0.4 |
| Memory quotient | 101.45 ±7.34 | 107.03 ±7.44 | < 0.001 | 106.4 ±12.1 | 105.4 ±12.06 | 0.5 |
Data are shown as mean ± SD
A significant inverse relationship was detected between TSH level and memory quotient (R = –0.39). Memory quotient significantly improved in subjects after levothyroxine treatment in comparison with subjects taking placebo (p = 0.002).
Discussion
This study indicated that memory quotient improved significantly in subclinical hypothyroid subjects after treatment with levothyroxine. Meanwhile, there was also significant improvement in some Wechsler Memory Scale subtests such as mental control (p = 0.002), logical memory (p < 0.001), and associate learning (p = 0.014). The improvement of memory quotient remained significant after adjustment of the scores for age (p < 0.001).
Thyroid hormone plays a major role as a regulator of nervous system myelination, growth, puberty, metabolism, and organ functions [8]. Thyroid function disorder is a graded phenomenon [11]. Subclinical hypothyroidism represents an early stage of thyroid disease which progresses to overt hypothyroidism by 3-18% per year, especially in the elderly [12, 13]. The risk factors of progression are the presence of antithyroid antibodies, serum TSH of more than 20 µU/ml, history of radioiodine therapy or external radiation and lithium therapy [14]. There is increasing evidence suggesting that mild (subclinical) thyroid disorders may be potential contributors to significant clinical conditions [11]. The overall prevalence of SCH has been reported as 7-26% in the elderly [15] and 3% to 7% in the middle aged population [16]. There has been considerable controversy regarding the clinical significance of this condition and its management. Due to considerable morbidity, the general consensus is to treat selected subjects with early thyroid failure [17]. The degree to which subclinical hypothyroidism affects mood and cognitive functions and whether these symptoms respond to treatment remains controversial. Most studies support a relationship between thyroid function and cognition, particularly poor learning, reduced information processing speed, and efficiency in executive functions [18].
Three cross-sectional studies confirmed mild functional learning disorder and memory problems in young subjects with subclinical hypothyroidism [19–21]. In addition, association of Alzheimer disease and subclinical hypothyroidism has been reported in a population-based study [22]. Improvements of memory performance, frontal executive functions, and some aspects of cognitive performance have been observed after treatment of subclinical hypothyroidism subjects with levothyroxine [23–25].
Quijano et al. conducted a survey that included 15 subclinical hypothyroid subjects and 15 mild clinically hypothyroid subjects. At enrollment, mild clinically hypothyroid subjects showed worse cognitive status in comparison with the subclinical hypothyroid group but after treatment both groups showed normal cognitive status [19]. In another study performed by Monzani et al. comparison was carried out in SCH between pretreatment ratings and those obtained following 6-month L-thyroxin treatment which indicated a significant improvement in subjects’ memory skills as evaluated by Wechsler Memory Scale (memory quotient (MQ) = 99.9 ±4.0; p = 0.002 (treated vs. untreated)) [26]. Improvement of MQ following levothyroxine treatment has been reported in small series of subclinical hypothyroidism subjects as well [7, 27].
On the other hand, some studies have indicated that levothyroxine treatment has no beneficial effect on cognitive function in subclinical hypothyroidism, either in the total memory scale or in the subtests of WMS. Parle et al. [28] examined the effect of LT4 treatment in 94 subjects with subclinical hypothyroidism in a randomized placebo-controlled blinded clinical trial. They used standard tests of cognition at baseline and at 6 and 12 months after treatment and found no difference in cognitive function between the 2 groups. However, the major limitations of this study were the type of cognitive assessment measures which detect gross impairment of cognition, and the low number of placebo-treated subjects who completed the study. In another study conducted by Jorde et al. [29], detailed cognitive testing was done at baseline and 12 months after treatment with LT4 or placebo. There was no difference between the groups at baseline. Furthermore, LT4 treatment had no effect on the outcome measures. This study was limited by the relatively mild degree of subclinical hypothyroidism.
In another double blind clinical trial, on 23 subclinical hypothyroid subjects, immediate and late memory, and psychomotor activity did not improve after treatment. However, the Kaflen test was used for evaluation of cognitive function in the study [30].
It should be mentioned that the discrepancies among the studies regarding the effect of levothyroxine on cognitive function of SCH subjects might be due to different tools used for the assessment of cognitive function as well as the small sample size of the populations being studied [24, 25].
Considering Wechsler Memory Scale subtests, our study showed significant improvement in mental control, logical memory, and associate learning. However, information, orientation, digits forward and backward, and visual reproduction did not change significantly. Considering the improvement of memory quotient in this study, it seems that mental control, logical memory, and associate learning might have a more influential effect on cognitive function. In another study conducted by Jenovsky et al., significant improvement of verbal (p < 0.01), visual (p < 0.05), and total memory (p < 0.01), after levothyroxine treatment compared to placebo in SCH subjects was observed [16]. Nystrom et al. found that four of 17 women with subclinical hypothyroidism exhibited significant improvement on at least two of the neuropsychological measures following treatment with levothyroxine (150 µg/day) in a placebo-controlled, cross-over, 2 × 6 month study [31]. Administration of levothyroxine (100-150 µg/day for 6 months) has also been shown to have beneficial effects on verbal and visual recall in 14 subjects with subclinical hypothyroidism [26].
There are some impressive strengths of this study. All the recruited subjects completed the trial. Moreover, TSH levels in all subjects in the intervention group were normalized (TSH < 4.5 mU/l) by the end of the study and no TSH suppression was observed in the levothyroxine group.
There are, however, some limitations to the study. The study might not have sufficient ability to detect the differences between the groups in certain subscales of cognitive function due to the small sample size. We were also unable to analyze the effect of race/ethnicity or social economic status due to the limited sample size and limitation of facilities.
In conclusion, this study showed the efficacy of levothyroxine on cognitive function of subjects with subclinical hypothyroidism, including mental control, logical memory, associate learning and visual reproduction.
Acknowledgments
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. This study was funded and supported by Tehran University of Medical Sciences (TUMS); Grant No. 90-01-122-12951.
The authors wish to thank the staff who greatly helped us to complete the project. In addition, we appreciate all the people who contributed to this study.
References
- 1.Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol. 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 2.Osterweil D, Syndulko K, Cohen SN, et al. Cognitive function in non-demented older adults with hypothyroidism. J Am Geriatr Soc. 1992;40:325–35. doi: 10.1111/j.1532-5415.1992.tb02130.x. [DOI] [PubMed] [Google Scholar]
- 3.Matuszek B, Strawa-Zakościelna K, Pyzik A, Duma D, Nowakowski A, Paszkowski T. The analysis of the clinical picture of hypothyroidism in menopausal women. Przegl Menopauz. 2010;6:390–6. [Google Scholar]
- 4.Salama HM, El-Dayem SA, Yousef H, Fawzy A, Abou--Ismail L, El-lebedy D. The effects of L-thyroxin replacement therapy on bone minerals and body composition in hypothyroid children. Arch Med Sci. 2010;3:407–13. doi: 10.5114/aoms.2010.14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appetecchia M. Effects on bone mineral density by treatment of benign nodular goiter with mildly suppressive doses of L-thyroxine in a cohort women study. Horm Res. 2005;64:293–8. doi: 10.1159/000089489. [DOI] [PubMed] [Google Scholar]
- 6.Klein I, Danzi S. Cardiovascular involvement in general medical conditions. Thyroid disease and the heart. Circulation. 2007;116:1725–35. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- 7.Correia N, Mullally S, Cooke G, et al. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J Clin Endocrinol Metab. 2009;94:3789–97. doi: 10.1210/jc.2008-2702. [DOI] [PubMed] [Google Scholar]
- 8.Smith JW, Evans AT, Costall B, Smythe JW. Thyroid hormones, brain function and cognition: a brief review. Neurosci Biobehav Rev. 2002;26:45–60. doi: 10.1016/s0149-7634(01)00037-9. [DOI] [PubMed] [Google Scholar]
- 9.Brooks DN. Wechsler Memory Scale performance and its relationship to brain damage after severe closed head injury. J Neurol Neurosurg Psychiatry. 1976;39:593–601. doi: 10.1136/jnnp.39.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wechsler DA. Standardized memory scale for clinical use. J Psychology. 1945;19:87–95. [Google Scholar]
- 11.Hoogendoorn EH, Heijer MD, Dijk AJ, Hermus AR. Subclinical hyperthyroidism: to treat or not to treat? Postgrad Med J. 2004;80:394–8. doi: 10.1136/pgmj.2003.017095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pale JV, Franklyn JA, Cross KW, et al. Prevalence and follow up of abnormal thyrotophin (TSH) concentration in the elderly in the United Kingdom. Clin Endocrinol. 1991;34:77–83. doi: 10.1111/j.1365-2265.1991.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 13.Kabadi UM. Subclinical hypothyroidism. Natural course of syndrome during a prolonged follow-up study. Arch Intern Med. 1993;153:957–61. doi: 10.1001/archinte.153.8.957. [DOI] [PubMed] [Google Scholar]
- 14.Huber G, Mitrache C, Guglielmetti M, Huber P, Staub JJ. Predictors of overt hypothyroidism and natural course: a long term follows up study in impending thyroid failure; 71st Annual Meeting of the American Thyroid Association; Oregan 1998 Abstract 109. [Google Scholar]
- 15.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–34. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 16.Jenovsky J, Ruzick E, Spackova N, Hejdukova B. Changes of event related potential and cognitive processes in patients with subclinical hypothyroidism after thyroxin treatment. Endocrine Regulations. 2002;36:115–22. [PubMed] [Google Scholar]
- 17.Micheal T, Mcdermot T, Chester R. Subclinical hypothyroidism is mild thyroid failure and should be treated. J Clin Endocrinol Metab. 2001;86:4585–90. doi: 10.1210/jcem.86.10.7959. [DOI] [PubMed] [Google Scholar]
- 18.Davis JD, Tremont G. Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinol. 2007;32:49–65. [PubMed] [Google Scholar]
- 19.delSerQuijano T, Delgado C, Martínez Espinosa S, Váz-quez C. Cognitive deficiency in mild hypothyroidism. Neurologia. 2000;15:193–98. [PubMed] [Google Scholar]
- 20.Cook SE, Nebes RD, Halligan EM, et al. Memory impairment in elderly individuals with a mildly elevated serum TSH: the role of processing resources, depression and cerebrovascular disease. Aging, Neuropsychology and Cognition. 2002;9:175–83. [Google Scholar]
- 21.Manciet G, Dartigues JF, Decamps A, et al. The PAQUID survey and correlates of subclinical hypothyroidism in elderly community residents in the southwest of France. Age Ageing. 1995;24:235–41. doi: 10.1093/ageing/24.3.235. [DOI] [PubMed] [Google Scholar]
- 22.Tan ZS, Beiser A, Vasan RS, et al. Thyroid function and the risk of Alzheimer Disease. Arch Intern Med. 2008;168:1514–20. doi: 10.1001/archinte.168.14.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldini IM, Vita A, Mauri MC. Psychological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:925–35. doi: 10.1016/s0278-5846(97)00089-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhu DF, Wang ZX, Zhang DR, et al. fMRI revealed neural substrate for reversible working memory dysfunction subclinical hypothyroidism. Brain. 2006;129:2923–30. doi: 10.1093/brain/awl215. [DOI] [PubMed] [Google Scholar]
- 25.Baldini M, Colasanti A, Orsatti A, Airaghi L, Mauri MC, Cappellini MD. Neuropsychological functions and metabolic aspects in subclinical hypothyroidism: the effects of L-thyroxin. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:854–9. doi: 10.1016/j.pnpbp.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Monzani F, Del Guerra P, Caraccio N. Subclinical hypothyroidism: neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin Investig. 1993;71:367–71. doi: 10.1007/BF00186625. [DOI] [PubMed] [Google Scholar]
- 27.Bégin ME, Langlois MF, Lorrain D, Cunnane SC. Thyroid function and cognition during aging. Curr Gerontol Geriatr Res. 2008;2008:474868. doi: 10.1155/2008/474868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid Study. J Clin Endocrinol Metabol. 2010;95:83623–32. doi: 10.1210/jc.2009-2571. [DOI] [PubMed] [Google Scholar]
- 29.Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metabol. 2006;91:145–53. doi: 10.1210/jc.2005-1775. [DOI] [PubMed] [Google Scholar]
- 30.Moradi S, Bahrainian AM, Azizi F. The effects of L-thyroxin treatment on cognitive and psychiatric aspects of subclinical hypothyroidism: a randomized double blinded clinical trial. Journal of Iran University of Medical Sciences. 2007;14:167–73. [Google Scholar]
- 31.Nystrom E, Caidahl K, Fager G, Wikkelso C, Lundberg PA, Lindstedt GA. Double-blind cross-over 12 month study of women with subclinical hypothyroidism. Clin Endocrinol. 1988;29:63–75. doi: 10.1111/j.1365-2265.1988.tb00250.x. [DOI] [PubMed] [Google Scholar]