Abstract
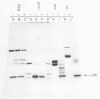
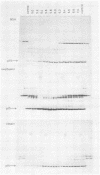
It was observed that the viral structural protein p15 from avian myeloblastosis virus emerges from ion-exchange column chromatography along with a proteolytic activity. p15 is apparently pure, as judged by sodium dodecyl sulfate-polyacryl-amide gel electrophoresis and isoelectric focusing. Increase and decrease in proteolytic activity coincided exactly with increasing and decreasing amounts of p15 during ion-exchange chromatography and during size fractionation by gell filtration. The proteolytic activity cleaved various substrates such as bovine serum albumin, ovalbumin, concanavalin A, and casein after denaturation by sodium dodecyl sulfate and heat. Highest enzyme activity was observed around pH 5.7. As judged from its cleavage pattern and its response to proteolytic inhibitors, the proteolytic activity appears papain-like, and the protease responsible for it may be classified as a thiol protease. If added to immunoprecipitated viral polyprotein precursor Pr76, p15 resulted in cleavage of Pr76,which could be inhibited by antibodies against p15.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcement L. J., Karshin W. L., Naso R. B., Arlinghaus R. B. "gag" polyprotein precursors of Rauscher murine leukemia virus. Virology. 1977 Sep;81(2):284–297. doi: 10.1016/0042-6822(77)90145-3. [DOI] [PubMed] [Google Scholar]
- Eisenman R., Vogt V. M., Diggelmann H. Synthesis of avian RNA tumor virus structural proteins. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1067–1075. doi: 10.1101/sqb.1974.039.01.122. [DOI] [PubMed] [Google Scholar]
- Gallis B. M., Eisenman R. N., Diggelmann H. Synthesis of the precursor to avian RNA tumor virus internal structural proteins early after infection. Virology. 1976 Oct 15;74(2):302–313. doi: 10.1016/0042-6822(76)90337-8. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Hubert E., Trávnícek M., Bolognesi D. P., Burny A., Cleuter Y., Huez G., Kettmann R., Marbaix G., Portetelle D. Frog oocytes synthesize and completely process the precursor polypeptide to virion structural proteins after microinjection of avian myeloblastosis virus RNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3230–3234. doi: 10.1073/pnas.74.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Verma I. M. Studies on the reverse transcriptase of RNA tumor viruses. Structural relatedness of two subunits of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4991–4994. doi: 10.1073/pnas.71.12.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Green R. W., Bolognesi D. P. Isolation of proteins by gel filtration in 6M guanidinium chloride: application to RNA tumor viruses. Anal Biochem. 1974 Jan;57(1):108–117. doi: 10.1016/0003-2697(74)90057-8. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S. Proteolytic enzymes. Annu Rev Biochem. 1960;29:45–72. doi: 10.1146/annurev.bi.29.070160.000401. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Bolognesi D. P., Lewandowski L. J. Isolation of the major viral glycoprotein and a putative precursor from cells transformed by avian sarcoma viruses. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2342–2346. doi: 10.1073/pnas.71.6.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A. C., Green R. W., Bolognesi D. P., Vanaman T. C. Comparative chemical properties of avian oncornavirus polypeptides. Virology. 1975 Apr;64(2):339–348. doi: 10.1016/0042-6822(75)90110-5. [DOI] [PubMed] [Google Scholar]
- Hizi A., Joklik W. K. RNA-dependent DNA polymerase of avian sarcoma virus B77. I. Isolation and partial characterization of the alpha, beta2, and alphabeta forms of the enzyme. J Biol Chem. 1977 Apr 10;252(7):2281–2289. [PubMed] [Google Scholar]
- Hizi A., Leis J. P., Joklik W. K. RNA-dependent DNA polymerase of avian sarcoma virus B77. II. Comparison of the catalytic properties of the alpha, beta2, and alphabeta enzyme forms. J Biol Chem. 1977 Apr 10;252(7):2290–2295. [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Further characterization of intracellular precursor polyproteins of Rauscher leukemia virus. Virology. 1977 May 1;78(1):11–34. doi: 10.1016/0042-6822(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Klenk H. D., Nagai Y., Rott R., Nicolau C. The structure and function of paramyxovirus glycoproteins. Med Microbiol Immunol. 1977;164(1-3):35–47. doi: 10.1007/BF02121300. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewandowski L. J., Smith R. E., Bolognesi D. P., Halpern M. S. Viral glycoprotein synthesis under conditions of glucosamine block in cells transformed by avian sarcoma viruses. Virology. 1975 Aug;66(2):347–355. doi: 10.1016/0042-6822(75)90208-1. [DOI] [PubMed] [Google Scholar]
- Lundblad R. L., Stein W. H. On the reaction of diazoacetyl compounds with pepsin. J Biol Chem. 1969 Jan 10;244(1):154–160. [PubMed] [Google Scholar]
- Moelling K. Characterization of reverse transcriptase and RNase H from friend-murine leukemia virus. Virology. 1974 Nov;62(1):46–59. doi: 10.1016/0042-6822(74)90302-x. [DOI] [PubMed] [Google Scholar]
- Moelling K., Hayami M. Analysis of precursors to the envelope glycoproteins of avian RNA tumor viruses in chicken and quail cells. J Virol. 1977 Jun;22(3):598–607. doi: 10.1128/jvi.22.3.598-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K. Reverse transcriptase and RNase H: present in a murine virus and in both subunits of an avian virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):969–973. doi: 10.1101/sqb.1974.039.01.111. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- Rho H. M., Grandgenett D. P., Green M. Sequence relatedness between the subunits of avian myeloblastosis virus reverse transcriptase. J Biol Chem. 1975 Jul 10;250(13):5278–5280. [PubMed] [Google Scholar]
- Singer S. J. Covalent labeling of active sites. Adv Protein Chem. 1967;22:1–54. doi: 10.1016/s0065-3233(08)60040-6. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of ribonucleic acid tumor viruses. Purification of envelope, core, and internal components. J Biol Chem. 1976 Jan 25;251(2):559–564. [PubMed] [Google Scholar]
- Tang J. Specific and irreversible inactivation of pepsin by substrate-like epoxides. J Biol Chem. 1971 Jul 25;246(14):4510–4517. [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R. Identification of a large polypeptide precursor of avian oncornavirus proteins. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1734–1738. doi: 10.1073/pnas.70.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Murine leukemia virus morphogenesis: cleavage of P70 in vitro can be accompanied by a shift from a concentrically coiled internal strand ("immature") to a collapsed ("mature") form of the virus core. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3446–3450. doi: 10.1073/pnas.74.8.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Partial characterization of a P70 proteolytic factor that is present in purified virions of Rauscher leukemia virus (RLV). Biochem Biophys Res Commun. 1977 May 9;76(1):54–63. doi: 10.1016/0006-291x(77)91667-9. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Properties of a P70 proteolytic factor of murine leukemia viruses. Cell. 1977 Nov;12(3):709–719. doi: 10.1016/0092-8674(77)90271-9. [DOI] [PubMed] [Google Scholar]
- von der Helm K. Cleavage of Rous sarcoma viral polypeptide precursor into internal structural proteins in vitro involves viral protein p15. Proc Natl Acad Sci U S A. 1977 Mar;74(3):911–915. doi: 10.1073/pnas.74.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]