Abstract
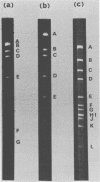
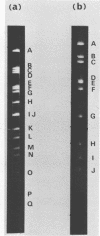
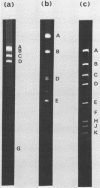
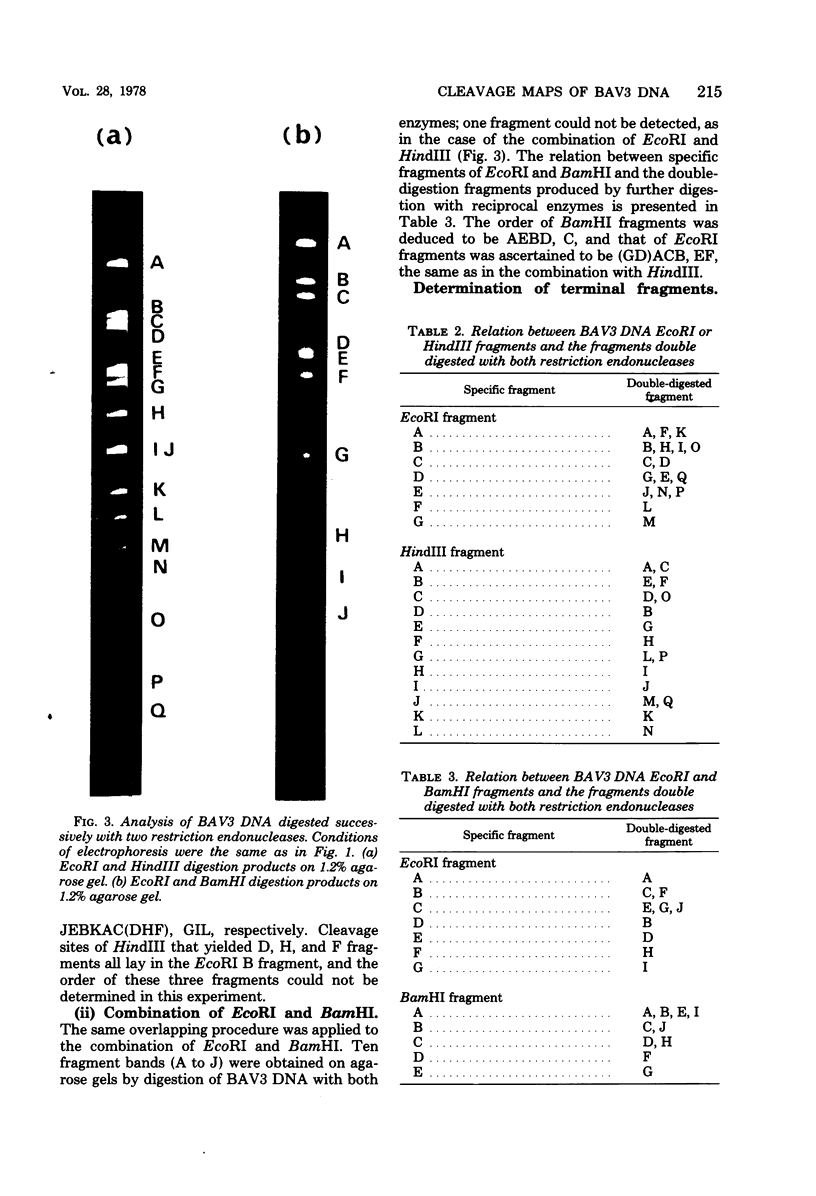
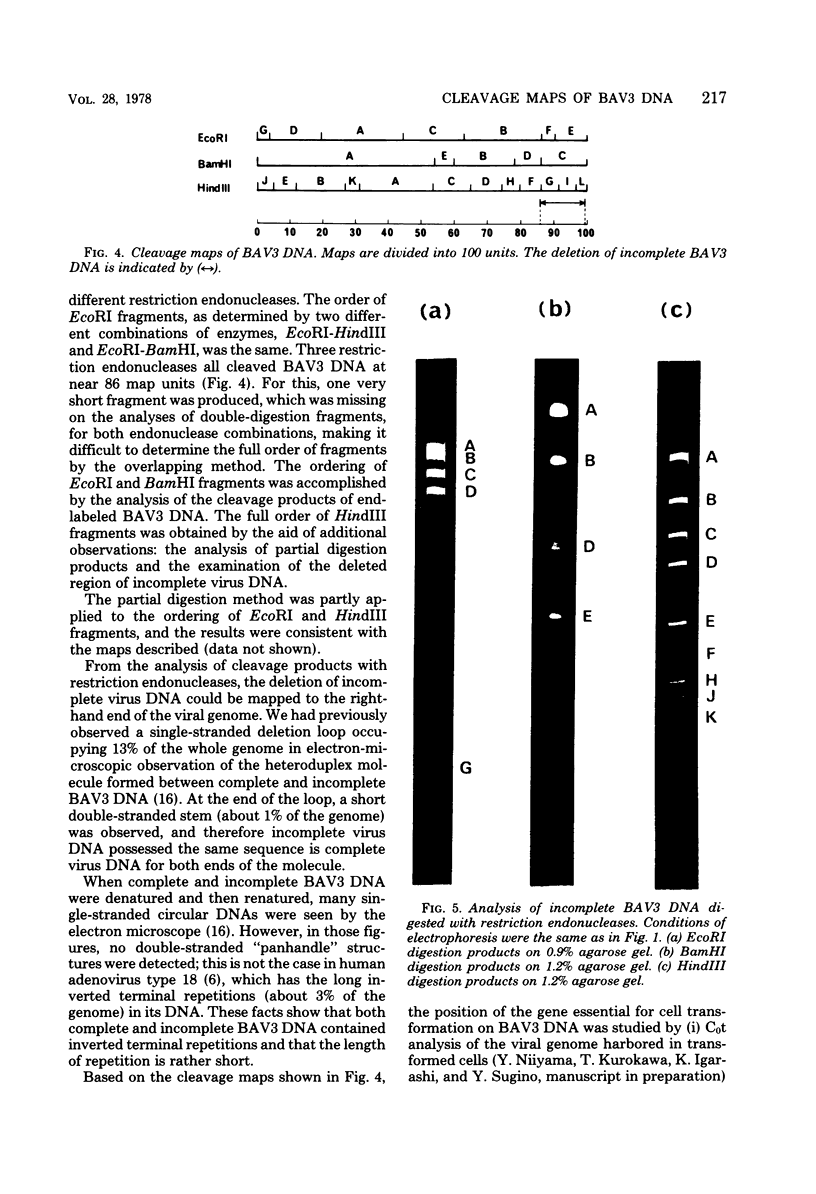
Cleavage of bovine adenovirus type 3 (BAV3) DNA by restriction endonucleases EcoRI, BamHI, and HindIII yielded 7 (A to G), 5 (A to E), and 12 (A to L) fragments, respectively. The order of these fragments has been determined to be GDACBFE for EcoRI fragments, AEBDC for BamHI fragments, and JEBKACDHFGIL for HindIII fragments, and cleavage sites of these enzymes have been mapped on the genome of BAV3. BAV3 preparation contains incomplete virus whose genome has a deletion of about 13% of complete virus genome. Restriction endonuclease digestion of the incomplete virus DNA revealed that EcoRI E and F, BamHI C and HindIII G, I, and L fragments were deleted. Therefore, the deleted region of incomplete virus DNA is located near the right-hand end of the BAV3 DNA molecule, a result consistent with our previous electron-microscopic observations on heteroduplex molecules formed between complete and incomplete BAV3 DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adenovirus strand nomenclature: a proposal. J Virol. 1977 Jun;22(3):830–831. doi: 10.1128/jvi.22.3.830-831.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Darbyshire J. H. Oncogenicity of bovine adenovirus type 3 in hamsters. Nature. 1966 Jul 2;211(5044):102–102. doi: 10.1038/211102a0. [DOI] [PubMed] [Google Scholar]
- Dunn A. R., Hassell J. A. A novel method to map transcripts: evidence for homology between an adenovirus mRNA and discrete multiple regions of the viral genome. Cell. 1977 Sep;12(1):23–36. doi: 10.1016/0092-8674(77)90182-9. [DOI] [PubMed] [Google Scholar]
- Englund P. T. Analysis of nucleotide sequences at 3' termini of duplex deoxyribonucleic acid with the use of the T4 deoxyribonucleic acid polymerase. J Biol Chem. 1971 May 25;246(10):3269–3276. [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. Arrangement of sequences in the inverted terminal repetition of adenovirus 18 DNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3039–3043. doi: 10.1073/pnas.72.8.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Niiyama Y., Tsukamoto K., Kurokawa T., Sugino Y. Biochemical studies on bovine adenovirus type 3. II. Incomplete virus. J Virol. 1975 Sep;16(3):634–641. doi: 10.1128/jvi.16.3.634-641.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Sasada R., Kurokawa T., Niiyama Y., Tsukamoto K., Sugino Y. Biochemical studies on bovine adenovirus type 3. IV. Transformation by viral DNA and DNA fragments. J Virol. 1978 Oct;28(1):219–226. doi: 10.1128/jvi.28.1.219-226.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Nathans D. The genome of simian virus 40. Adv Virus Res. 1977;21:85–173. doi: 10.1016/s0065-3527(08)60762-9. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Deletion mutants of simian virus 40 generated by enzymatic excision of DNA segments from the viral genome. J Mol Biol. 1974 Oct 15;89(1):179–193. doi: 10.1016/0022-2836(74)90169-7. [DOI] [PubMed] [Google Scholar]
- Larsen S. H., Nathans D. Mouse adenovirus: growth of plaque-purified FL virus in cell lines and characterization of viral DNA. Virology. 1977 Oct 1;82(1):182–195. doi: 10.1016/0042-6822(77)90041-1. [DOI] [PubMed] [Google Scholar]
- Mulder C., Arrand J. R., Delius H., Keller W., Pettersson U., Roberts R. J., Sharp P. A. Cleavage maps of DNA from adenovirus types 2 and 5 by restriction endonucleases EcoRI and HpaI. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):397–400. doi: 10.1101/sqb.1974.039.01.051. [DOI] [PubMed] [Google Scholar]
- Niiyama Y., Igarashi K., Tsukamoto K., Kurokawa T., Sugino Y. Biochemical studies on bovine adenovirus type 3. I. Purification and properties. J Virol. 1975 Sep;16(3):621–633. doi: 10.1128/jvi.16.3.621-633.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin J., Scheidtmann K. H., Greenberg R., Westphal M., Doerfler W. Transcription of the genome of adenovirus type 12. III. Maps of stable RNA from productively infected human cells and abortively infected and transformed hamster cells. J Virol. 1976 Nov;20(2):355–372. doi: 10.1128/jvi.20.2.355-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Williams J., Sharp P. A., Grodzicker T. Physical mapping of temperature-sensitive mutations of adenoviruses. J Mol Biol. 1975 Sep 25;97(3):369–390. doi: 10.1016/s0022-2836(75)80046-5. [DOI] [PubMed] [Google Scholar]
- Sekikawa K., Fujinaga K. Cleavage maps of human adenovirus type 7 DNA by restriction endonuclease HindIII and EcoRI. Virology. 1977 Oct 15;82(2):509–512. doi: 10.1016/0042-6822(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Tibbetts C. Physical organization of subgroup B human adenovirus genomes. J Virol. 1977 Nov;24(2):564–579. doi: 10.1128/jvi.24.2.564-579.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Isolation of a sequence-specific endonuclease (BamI) from Bacillus amyloliquefaciens H. J Mol Biol. 1975 Sep 5;97(1):123–125. doi: 10.1016/s0022-2836(75)80028-3. [DOI] [PubMed] [Google Scholar]