Abstract
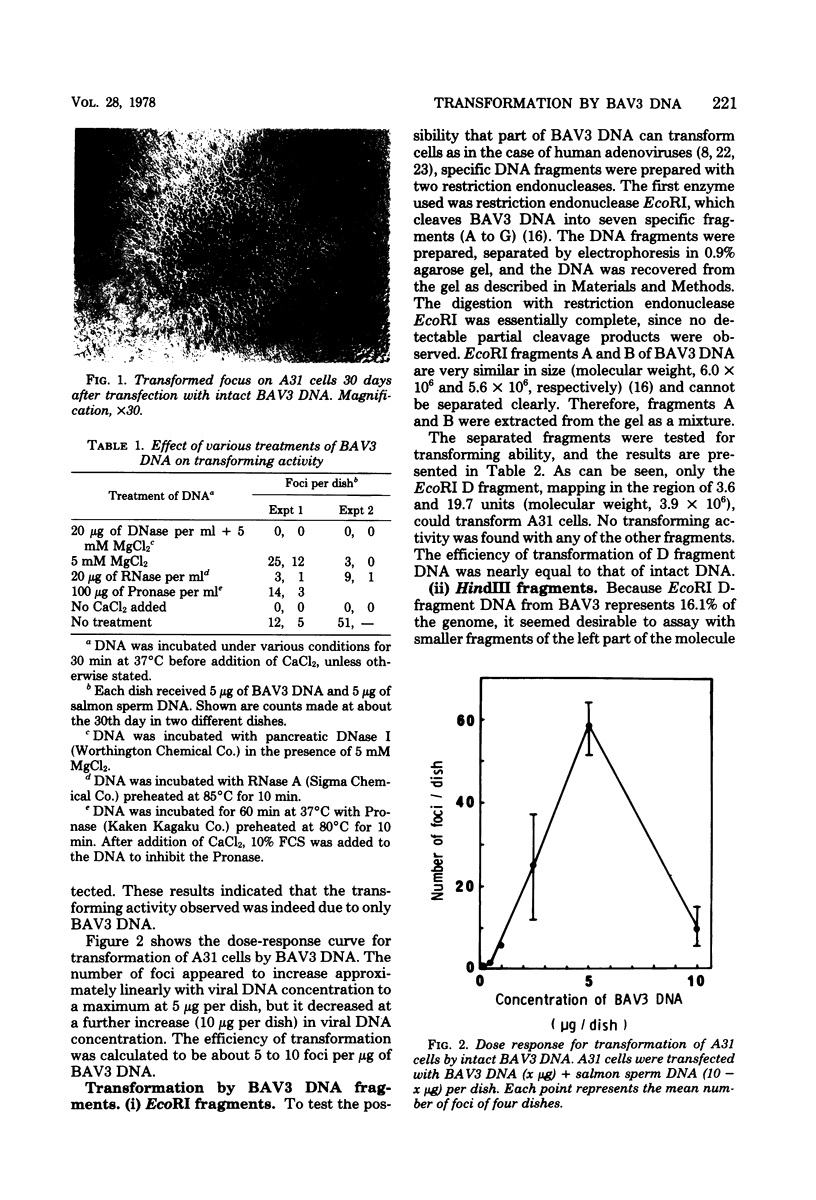
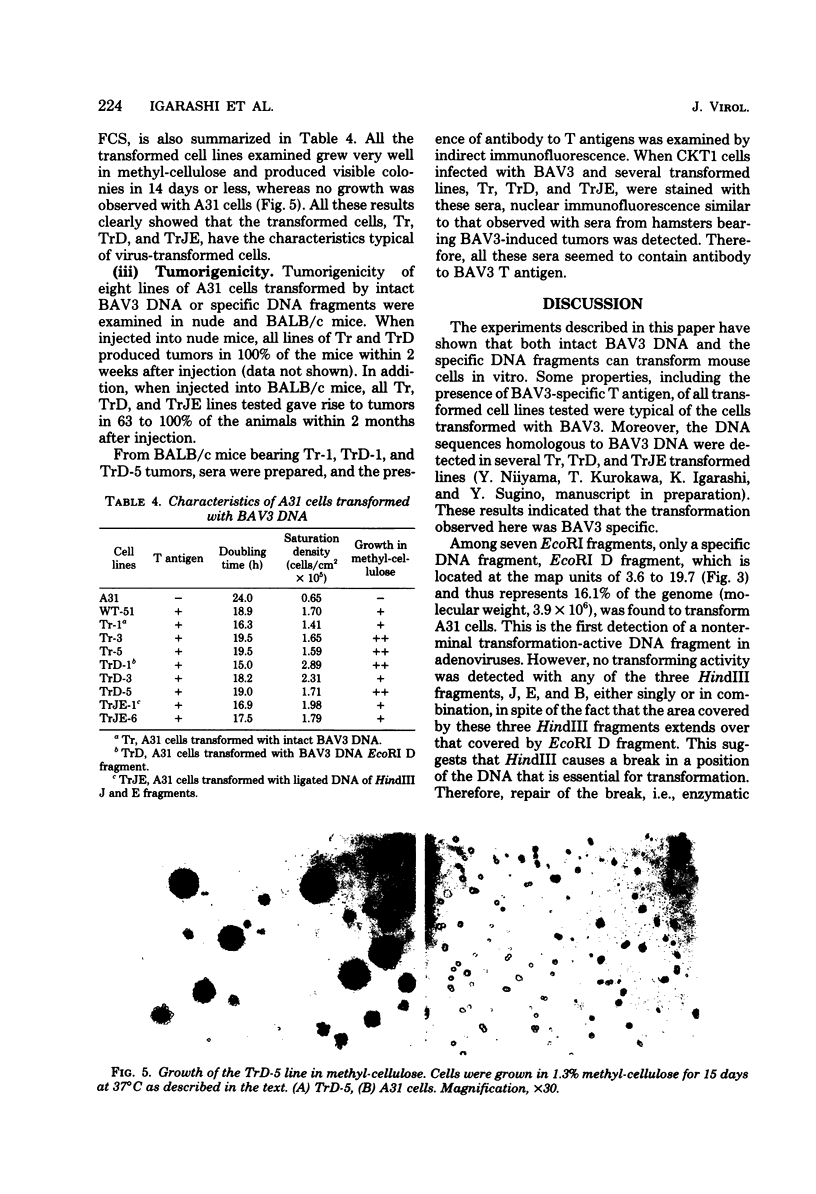
By the calcium technique, intact DNA of bovine adenovirus type 3 (BAV3) was found to transform A31 cells, a clone of BALB/3T3. Transforming activity was resistant to RNase and Pronase but sensitive to DNase. The efficiency of transformation was approximately 5 to 10 foci per μg of DNA. Attempts were also made to test for transforming activity of BAV3 DNA fragments prepared with restriction endonucleases EcoRI and HindIII. The activity was found to associate exclusively with the EcoRI D fragment mapped in the region of 3.6 and 19.7 units (molecular weight, 3.9 × 106). No transformation could be obtained with three HindIII fragments, J, E, and B, located at the left-hand end of the BAV3 genome. However, the enzymatic joining of J and E fragments (0 to 11.9 map units) with a ligase restored the transforming activity. These results suggest that all the genetic information of BAV3 required for transformation is located in the region between 3.6 and 11.9 units on the viral genome. Some properties of A31 cells transformed by BAV3 DNA EcoRI D fragment (TrD) and the ligated DNA of HindIII J and E fragments (TrJE), as well as those transformed by whole BAV3 DNA (Tr), were examined. As compared to untransformed A31 cells, all the transformed cell lines tested showed rapid growth, high saturation densities, and anchorage-independent growth. Moreover, they contained BAV3-specific T antigen and induced tumors in adult nude and BALB/c mice. These properties of Tr, TrD, and TrJE lines were similar to those of BAV3-transformed cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams P. J., Mulder C., Van De Voorde A., Warnaar S. O., van der Eb A. J. Transformation of primary rat kidney cells by fragments of simian virus 40 DNA. J Virol. 1975 Oct;16(4):818–823. doi: 10.1128/jvi.16.4.818-823.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Cohn R. H., Lowry J. C., Kedes L. H. Histone genes of the sea urchin (S. purpuratus) cloned in E coli: order, polarity, and strandedness of the five histone-coding and spacer regions. Cell. 1976 Sep;9(1):147–161. doi: 10.1016/0092-8674(76)90060-x. [DOI] [PubMed] [Google Scholar]
- Darbyshire J. H. Oncogenicity of bovine adenovirus type 3 in hamsters. Nature. 1966 Jul 2;211(5044):102–102. doi: 10.1038/211102a0. [DOI] [PubMed] [Google Scholar]
- Flint J. The topography and transcription of the adenovirus genome. Cell. 1977 Feb;10(2):153–166. doi: 10.1016/0092-8674(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J., Heijneker H. L. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. doi: 10.1038/251687a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973 Aug;54(2):536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Niiyama Y., Tsukamoto K., Kurokawa T., Sugino Y. Biochemical studies on bovine adenovirus type 3. II. Incomplete virus. J Virol. 1975 Sep;16(3):634–641. doi: 10.1128/jvi.16.3.634-641.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Kamogashira T., Ota F., Fukui K., Yoshida N. Growth of bovine adenovirus type 3 in cells cloned from a cell line of calf kidney. Tokushima J Exp Med. 1971 Oct;18:33–37. [PubMed] [Google Scholar]
- Kurokawa T., Igarashi K., Sugino Y. Biochemical studies on bovine adenovirus type 3. III. Cleavage maps of viral DNA by restriction endoncleases EcoRI, BamHI, and HindIII. J Virol. 1978 Oct;28(1):212–218. doi: 10.1128/jvi.28.1.212-218.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niiyama Y., Igarashi K., Tsukamoto K., Kurokawa T., Sugino Y. Biochemical studies on bovine adenovirus type 3. I. Purification and properties. J Virol. 1975 Sep;16(3):621–633. doi: 10.1128/jvi.16.3.621-633.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Botchan M., Gallimore P., Ozanne B., Pettersson U., Williams J., Sharp P. A. Viral DNA sequences in cells transformed by simian virus 40, adenovirus type 2 and adenovirus type 5. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):615–632. doi: 10.1101/sqb.1974.039.01.075. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Handa H., Shimojo H., Yano S., Ojima S., Fujinaga K. Establishment and characterization of rat cell lines transformed by restriction endonuclease fragments of adenovirus 12 DNA. Virology. 1977 Oct 15;82(2):462–471. doi: 10.1016/0042-6822(77)90019-8. [DOI] [PubMed] [Google Scholar]
- Yano S., Ojima S., Fujinaga K., Shiroki K., Shimojo H. Transformation of a rat cell line by an adenovirus type 12 DNA fragment. Virology. 1977 Oct 1;82(1):214–220. doi: 10.1016/0042-6822(77)90044-7. [DOI] [PubMed] [Google Scholar]