Abstract
Failure to efficiently induce apoptosis contributes to cisplatin resistance in non-small-cell lung cancer (NSCLC). Although BCL-2-associated X protein (BAX) and BCL-2 antagonist killer (BAK) are critical regulators of the mitochondrial apoptosis pathway, their requirement has not been robustly established in relation to cisplatin. Here, we show that cisplatin can efficiently bypass mitochondrial apoptosis block caused by loss of BAX and BAK, via activation of the extrinsic death receptor pathway in some model cell lines. Apoptosis resistance following cisplatin can only be observed when both extrinsic and intrinsic pathways are blocked, consistent with redundancy between mitochondrial and death receptor pathways in cisplatin-induced apoptosis. In H460 NSCLC cells, caspase-8 cleavage was shown to be induced by cisplatin and is dependent on death receptor 4, death receptor 5, Fas-associated protein with death domain, acid sphingomyelinase and ceramide synthesis. In contrast, cisplatin-resistant cells fail to activate caspase-8 via this pathway despite conserving sensitivity to death ligand-driven activation. Accordingly, caspase-8 activation block acquired during cisplatin resistance, can be bypassed by death receptor agonism.
Keywords: Apoptosis, BAX, BAK, non-small-cell lung cancer, cisplatin resistance, caspase-8
Lung cancer is the leading cause of cancer mortality with over one million new cases diagnosed each year.1 The most common type is non-small-cell lung cancer (NSCLC), which accounts for 80% of all primary lung cancer.2 The majority of patients present with advanced disease, whereby surgical resection is of no benefit and systemic therapy forms the mainstay of treatment.3 Platinum-based therapy is the mainstay of first line therapy but is associated with high failure with response rates in the region of 20% observed.4, 5 In recent years, our understanding of how to treat NSCLC has undergone a paradigm shift initiated by the identification of somatic activating mutations in the epidermal growth factor receptor (EGFR).6, 7 Lethality relies on the efficient induction of apoptosis involving the BH3-only domain containing protein, BIM.8, 9, 10, 11 Similarly, ELK4-ALK (anaplastic lymphoma kinase) translocation12 is associated with extreme preclinical sensitivity to respective ALK kinase inhibition in both preclinical and clinical settings. Additional molecular subclasses with associated somatic gene alterations have been discovered, predominantly in lung adenocarcinomas and include mutations of BRAF,13 human epidermal growth factor receptor 214 and phosphoinositide-3-kinase, catalytic, alpha polypeptide.15 Despite these advances the majority of patients still fall outside these subclasses and rely on standard cisplatin-based treatment.
Induction of apoptosis is important in mediating tumour response to platinum-based chemotherapy. Defective apoptosis underpins drug resistance and is a hallmark of cancer.16, 17 Apoptosis can be initiated through two principle core signalling pathways, which are death receptor- or mitochondria-dependent. The mitochondrial apoptotis pathway is regulated by the multidomain pro-apoptotic B-cell lymphoma-2 (BCL-2) family proteins, BCL-2-associated X protein (BAX) and BCL-2 antagonist killer (BAK). These induce permeabilization of the outer mitochondrial membrane upstream of effector caspases. Double knockout of these proteins is sufficient to confer multidrug resistance in mouse embryonic fibroblasts, however, their requirement for the action of cisplatin is less well defined.18, 19
Altered expression of BAX and BAK has been frequently reported in NSCLC with BAX expression lost in 34–47% and BAK expression lost in 42–59% of surgically resected NSCLC tumours.20, 21, 22, 23, 24, 25 Although these studies failed to demonstrate any prognostic effect of loss of these proteins, analysis was carried out for each gene in isolation and the majority of patients did not receive adjuvant therapy. Given that BAX and BAK are frequently lost in NSCLC, we hypothesised that loss of both BAX and BAK in NSCLC could potentially mediate profound chemoresistance. In contrast, however, our findings reveal that BAX and BAK are dispensable for cisplatin-mediated apoptosis in some model systems, where generation of ceramide can induce apoptosis via caspase-8. Moreover, in cisplatin-resistant NSCLC cells, although both the intrinsic apoptotic pathway and caspase-8 activation are impaired in response to cisplatin, sensitivity to death receptor activation is still conserved.
Results
Silencing BAX and BAK in H460 and H1299 cells results in mitochondrial apoptosis block
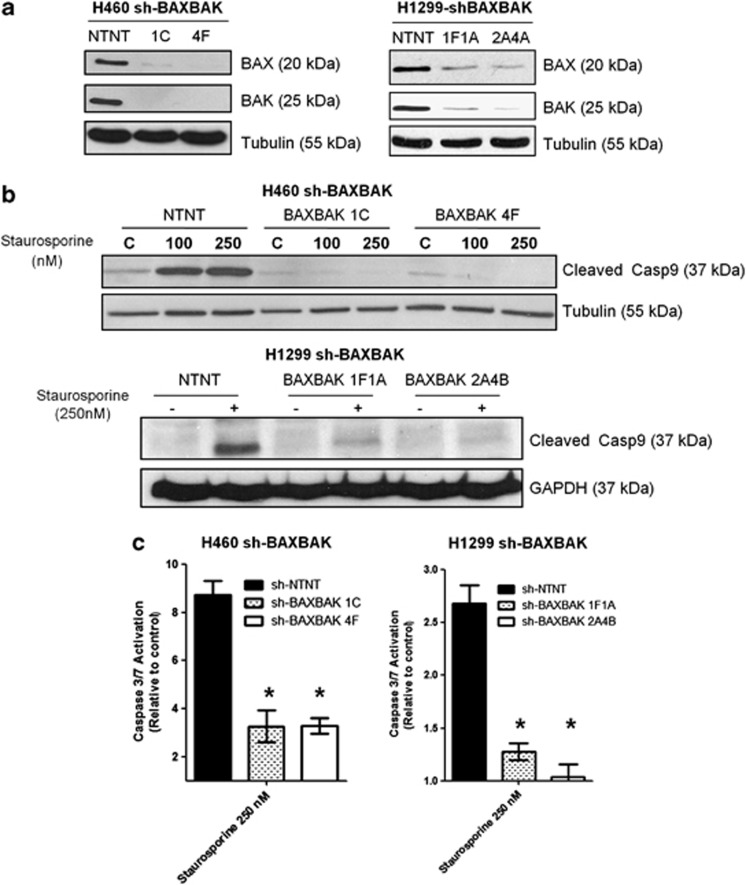
In order to determine if mitochondrial apoptosis block would confer resistance to cisplatin-induced apoptosis in NSCLC cells, we generated H460 and H1299 clones expressing short hairpin RNAs (shRNAs) simultaneously targeting BAX and BAK. Pairs of clones with different BAX and BAK targeting sequences were generated (sh-BAXBAK) and comparisons made with stably expressing non-targeting (NT) short hairpin RNA (shRNA) clones (sh-NTNT). BAX and BAK protein expression in H460 clones sh-BAXBAK 1C and 4F was undetectable and in H1299 clones sh-BAXBAK 1F1A and 2A4B was considerably reduced as assessed by western blotting (Figure 1a).
Figure 1.
BAXBAK silenced NSCLC cells have mitochondrial apoptosis block. (a) Western blot showing BAX and BAK protein expression in H460 and H1299 sh-BAXBAK clones compared with corresponding sh-NTNT controls. (b) Western blot for caspase-9 following 24 h treatment with indicated doses of staurosporine in sh-NTNT and sh-BAXBAK clones from H460 and H1299 cells. (c) Luminescent DEVD-ase assay measuring caspase-3-like activity in sh-NTNT and sh-BAXBAK cells from H460 and H1299 shown relative to untreated control. Data are expressed as mean±S.D. (*P<0.001)
We next confirmed that the level of BAX and BAK silencing in our clones was sufficient to block mitochondrial apoptosis. All sh-BAXBAK clones showed inhibition of caspase-9 cleavage to the p37 form following 24 h treatment with staurosporine, versus sh-NTNT controls (Figure 1b). Caspase-3 activation was also significantly attenuated after staurosporine in the sh-BAXBAK clones of both H460 and H1299 cells compared with the respective sh-NTNT controls (Figure 1c). The H460 sh-BAXBAK clones also exhibited resistance to pro-apoptotic peptides corresponding to the BH3 domain of the pro-apoptotic protein BID (BH3BID) both at isolated mitochondria and whole-cell level, as we recently reported.26 Together these data show that stable knockdown of BAX and BAK using short hairpin RNA interference in H460 and H1299 cells results in effective suppression of mitochondrial apoptotic signalling.
Cisplatin efficiently induces apoptosis in H460 sh-BAXBAK cells but not H1299 sh-BAXBAK cells
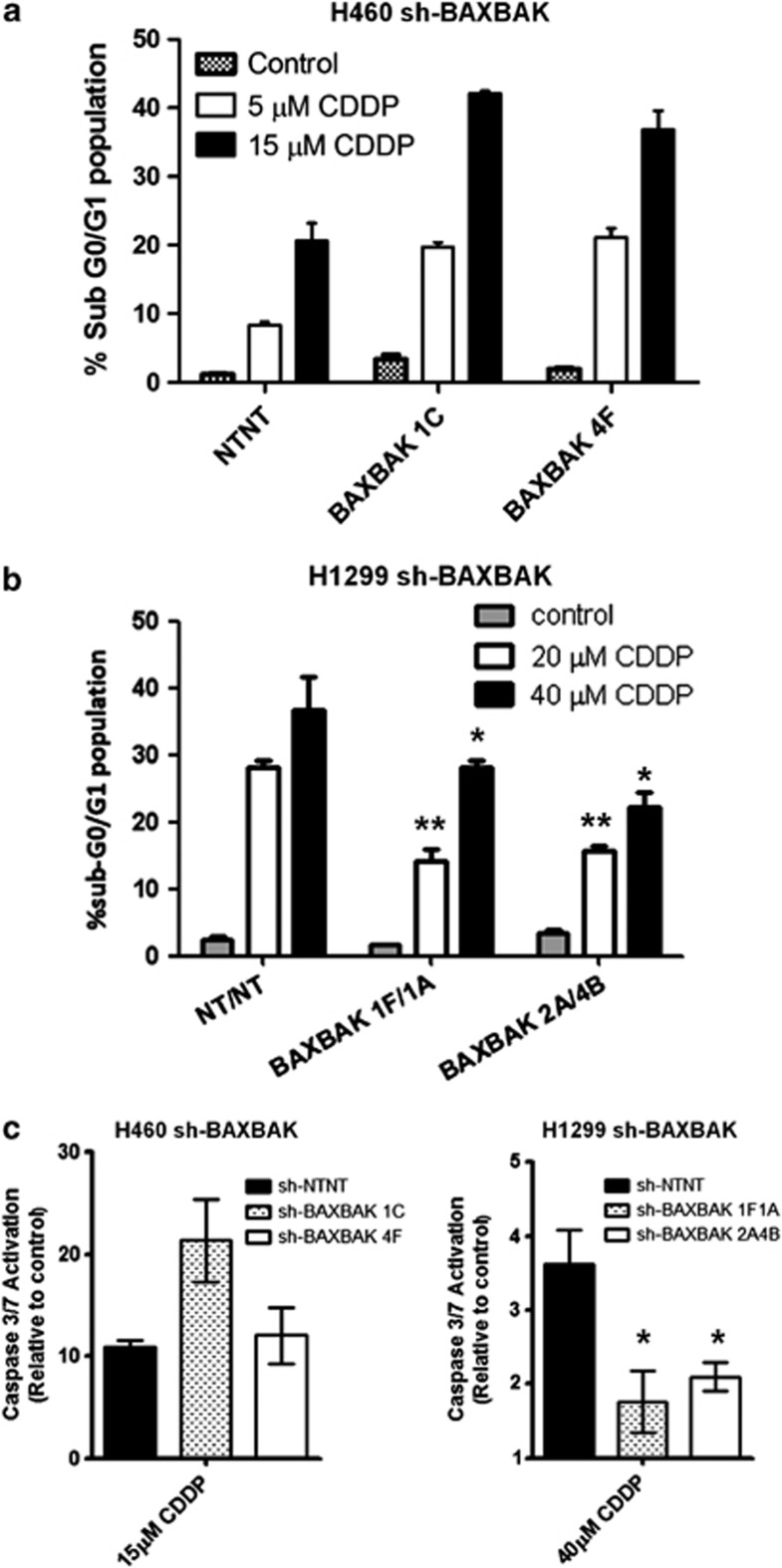
Having demonstrated mitochondrial apoptosis block in both cell lines in response to staurosporine (Figures 1b and c) and R8BID,26 we wanted to study the effect that this block would have on cisplatin-induced apoptosis. H460 sh-BAXBAK cells showed no attenuation in apoptosis after treatment with cisplatin, as evidenced by sub-G0/G1 fraction (Figure 2a). In contrast, H1299 sh-BAXBAK clones showed statistically significant attenuation of apoptosis induction, as evidenced by sub-G0/G1 fraction, compared with sh-NTNT control cells (Figure 2b). These results were confirmed by immunoblot detection of poly (ADP-ribose) polymerase (PARP) cleavage (Supplementary Figure S1). To understand what could be the reason for this difference in apoptotic response, we assessed activation of caspase-3. H460 sh-BAXBAK cells showed no attenuation in caspase-3 activity after cisplatin treatment, whereas a significant reduction was observed in H1299 sh-BAXBAK clones (Figure 2c). Together, these data show that cisplatin can induce apoptosis independent of BAX and BAK in H460 cells, however, in H1299 cells, cisplatin-induced apoptosis is BAX/BAK-dependent.
Figure 2.
Cisplatin induces apoptosis in H460 sh-BAXBAK cells but not H1299 sh-BAXBAK cells. (a) Flow cytometry of PI-stained H460 clones after 48 h treatment with cisplatin. Apoptotic cells are indicated by increase in sub-G0/G1 population. (b) Flow cytometry of of PI-stained H1299 clones after 48 h treatment with cisplatin. Apoptotic cells are indicated by increase in sub-G0/G1 population (*P<0.001, **P<0.01). Data are expressed as mean±S.D. (c) Luminescent DEVD-ase assay measuring caspase-3-like activity in sh-NTNT and sh-BAXBAK cells from H460 and H1299 after 24 h treatment with cisplatin, shown relative to untreated controls. Data are expressed as mean±S.D. (*P<0.001)
Cisplatin induces caspase-8 cleavage in a panel of NSCLC cell lines and H460 sh-BAXBAK clones, but not in H1299 cells
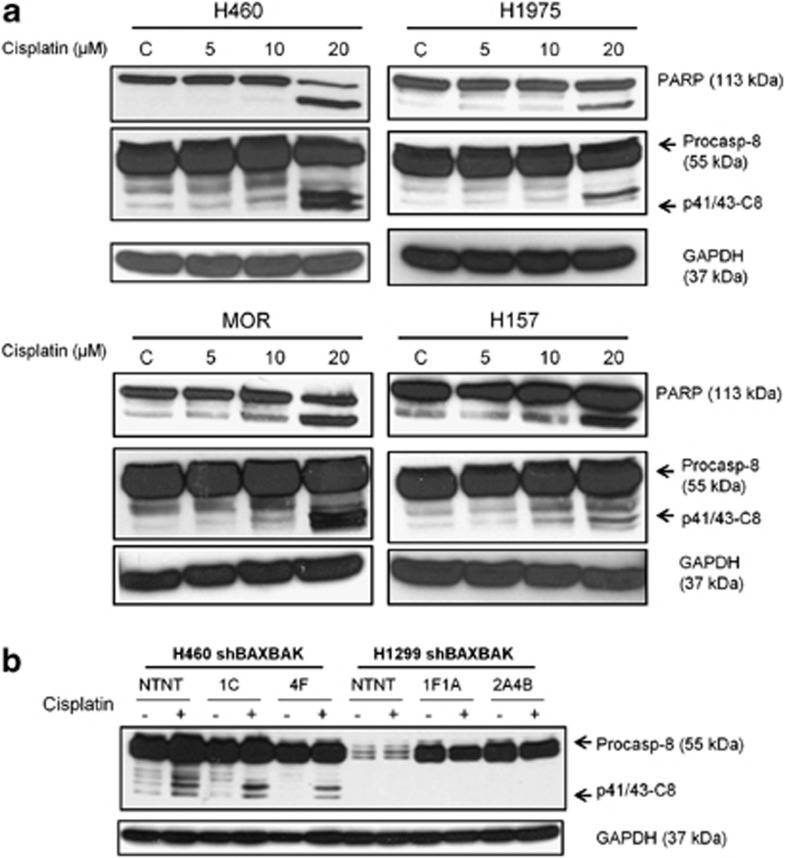
It has been shown previously that chemotherapy treatment has effects on the death receptor pathway by sensitising a number of different cell types to TNF receptor-related apoptosis inducing ligand (TRAIL).27, 28 It has also been reported that cisplatin leads to Fas receptor clustering and activation independent of ligand binding.29 This led us to hypothesise that cisplatin induces apoptosis independently of BAX and BAK through its effects on caspase-8, the initiator caspase of the death receptor pathway. Accordingly, caspase-8 cleavage in response to cisplatin was assessed in a panel of NSCLC lines. Cleavage of procaspase-8 to p41/43-caspase-8 was detected by western blotting in all four cell lines studied, in a dose-dependent manner, after 24 h exposure to cisplatin (Figure 3a). To validate these data, caspase-8 activity assays were performed, showing caspase-8 activity in these cells in response to cisplatin (Supplementary Figure S8). Together these data suggest that cisplatin-induced caspase-8 activity is a common feature in NSCLC cell lines.
Figure 3.
Cisplatin induces caspase-8 cleavage in a panel of NSCLC cells but not in H1299 cells. (a) Western blots showing processing of PARP and caspase-8 in a panel of NSCLC cell lines with increasing doses of cisplatin for 24 h. (b) Western blot for caspase-8 in H460 and H1299 sh-BAXBAK cells with matched sh-NTNT controls after 24 h treatment with cisplatin (H460 15 μM, H1299 40 μM)
Next, we assessed caspase-8 cleavage following cisplatin treatment in the sh-BAXBAK and sh-NTNT clones as a possible explanation for the difference observed in caspase-3 activity in sh-BAXBAK cells. In H460 cells, caspase-8 was cleaved in both sh-NTNT and sh-BAXBAK clones. Caspase-8 cleavage, however, was not detected in any of the H1299 clones (Figure 3b). Again these data were supported by similar findings using the caspase-8 activity assay (Supplementary Figure S9).
Caspase-8 silencing rescues H460 sh-BAXBAK cells but not sh-NTNT control cells from cisplatin-induced apoptosis
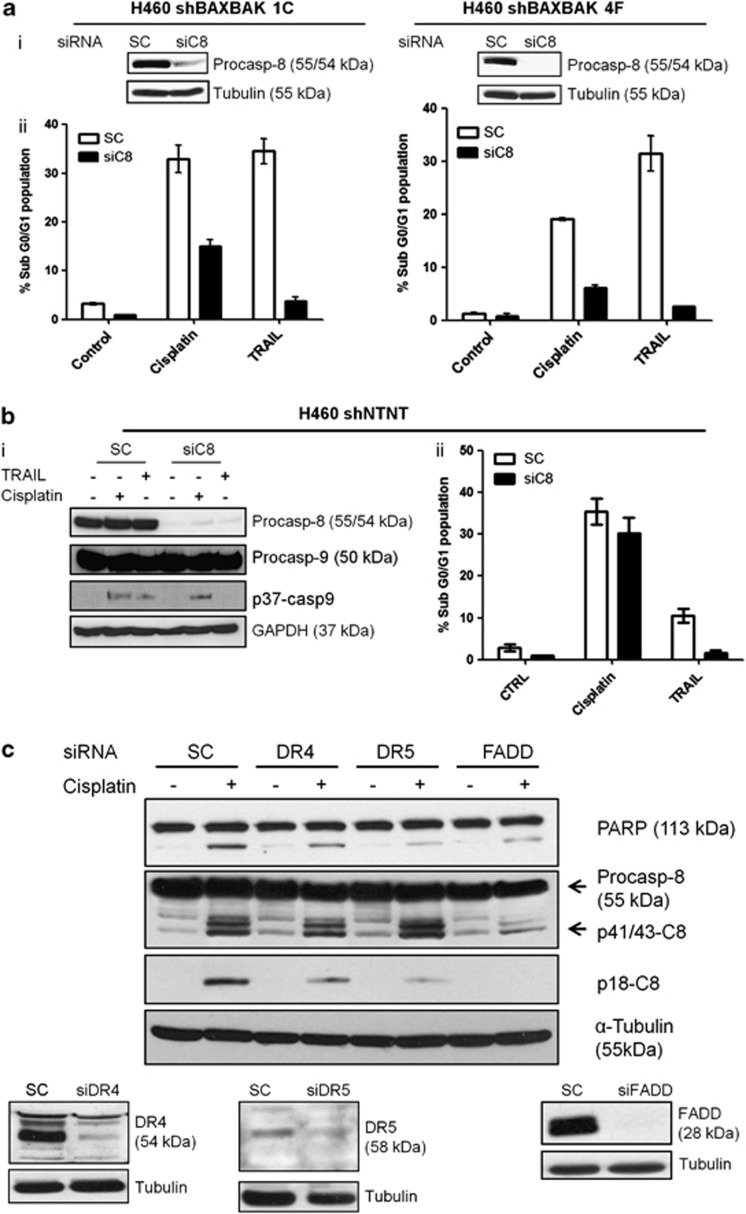
Cisplatin-induced caspase-8 cleavage in H460 cell line was sufficient to drive apoptosis independent of BAX and BAK, contrary to H1299 cells, where caspase-8 cleavage was not seen after cisplatin treatment, implicating BAXBAK dependence. To confirm whether caspase-8 was required for apoptosis signalling in the absence of BAXBAK, we silenced caspase-8 expression in H460 sh-BAXBAK clones using transient siRNA transfection. TRAIL treatment was included as a positive control to confirm the level of caspase-8 silencing was sufficient to block death ligand/death receptor-driven apoptosis. Significant attenuation in cisplatin-induced apoptosis was noted in both H460 sh-BAXBAK clones studied (1C and 4F) in the caspase-8-silenced cells compared with scrambled control siRNA (Figure 4a). Attenuation in caspase-3 activation was also detected (data not shown). However, in control sh-NTNT cells silencing of caspase-8 did not prevent sh-NTNT cells and apoptosis induction (Figure 4b). These data show that redundancy can exist between intrinsic and extrinsic apoptosis pathways in response to cisplatin. In H1299 cells, the death receptor pathway is dysfunctional as evidenced by their resistance to rhTRAIL.30 This was associated with reduced procaspase-8, death receptor 4 (DR4) and death receptor 5 (DR5) expression as compared with H460 cells (Figure 3b and Supplementary Figure S2).
Figure 4.
Caspase-8 mediates cisplatin-induced apoptosis in H460 sh-BAXBAK cells but not sh-NTNT control cells. (a) (i) Western blot showing siRNA-mediated silencing of caspase-8 in H460 sh-BAXBAK clones. (ii) Flow cytometry of PI-stained H460 sh-BAXBAK clones, transfected for 48 h with caspase-8 siRNA (siC8) or scrambled control (SC) and treated with cisplatin (5 μM) or TRAIL (10 ng/ml) for 48 h. Apoptotic cells are indicated by increase in sub-G0/G1 population. Data are expressed as mean±S.D. (b) (i) Western blot for caspase-8 and caspase-9 in H460 sh-NTNT cells after transfection for 48 h with C8 siRNA and treatment with cisplatin or TRAIL. Caspase-8 silencing attenuates TRAIL induced but not cisplatin-induced caspase-9 cleavage. (ii) Flow cytometry of PI-stained H460 sh-NTNT cells, transfected for 48 h with caspase-8 siRNA (siC8) or scrambled control (SC) and treated with cisplatin (15 μM) or TRAIL (10 ng/ml) for 48 h. Data are expressed as mean±S.D. (c) Western blots for PARP, caspase-8, DR4, DR5, FADD. Caspase-8 and PARP cleavage, induced by cisplatin, is attenuated after siRNA-mediated silencing of DR4, DR5 and FADD in H460 sh-BAXBAK 1C
To determine if cisplatin-induced caspase-8 cleavage was dependent on formation of the death inducing signalling complex (DISC), its components were silenced in H460 sh-BAXBAK cells and cisplatin-induced caspase-8 cleavage was assessed (Figure 4c). Silencing of the death receptors, DR4 and DR5 did not affect generation of p41/43-caspase-8, but resulted in partial attenuation of p18-caspase-8 generation after cisplatin. This correlated with attenuation in PARP cleavage. Furthermore, silencing Fas-associated protein with death domain (FADD) resulted in marked attenuation of p41/43-caspase-8 and no detectable p18-caspase-8 after 24 h cisplatin treatment. Again this correlated with attenuation of PARP cleavage (Figure 4c). Additionally, caspase-3/7 activation was reduced following silencing of either one of the DISC components (Supplementary Figure S3). Similar results were also observed in both H460 sh-BAXBAK 1C and sh-BAXBAK4F clones (not shown). In contrast, Fas silencing had no effect on cisplatin-induced apoptosis (Supplementary Figure S4).
Cisplatin-induced procaspase-8 cleavage is acid sphingomyelinase (ASM)-dependent
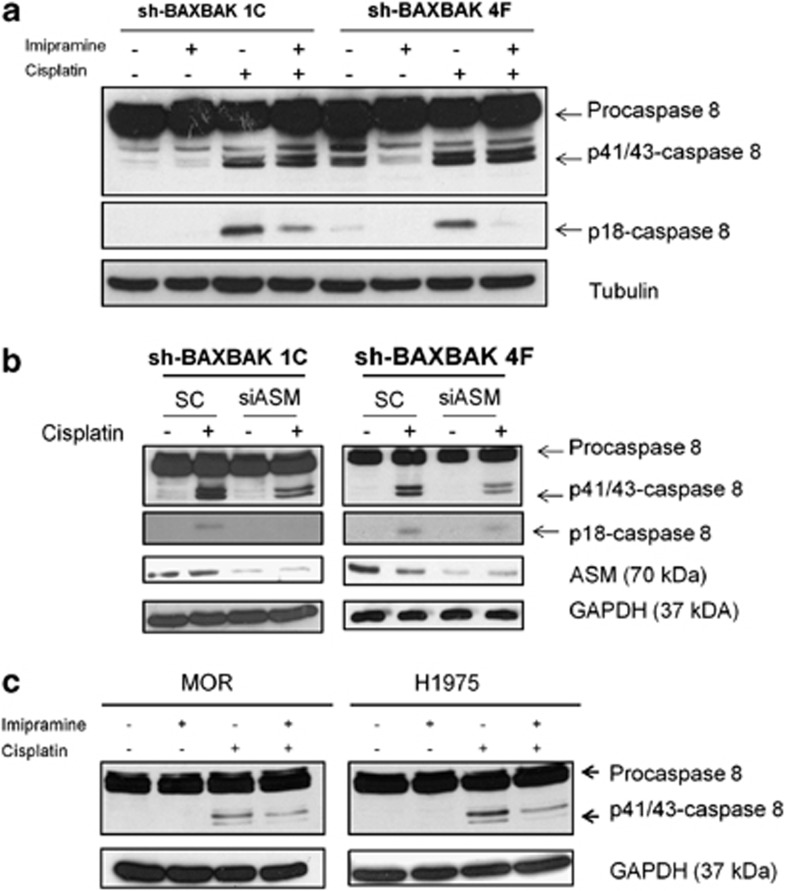
It has been shown previously that cisplatin can induce ceramide generation.31 We therefore investigated the role of ASM in controlling caspase-8 activation by cisplatin in H460 sh-BAXBAK clones. Pre-treatment of cells with imipramine, a known inhibitor of ASM, resulted in an attenuation of p18-caspase-8 generation following treatment with cisplatin (Figure 5a). Similar results were seen when ASM expression was silenced using siRNA, as assessed by western blotting (Figure 5b). To establish whether cisplatin-induced caspase-8 cleavage was ASM-dependent, we studied this effect in other NSCLC cell lines. In MOR and H1975 cells pretreated with imipramine, we observed reduced p41/43-caspase-8 following cisplatin treatment; p18-caspase-8 was not detectable in these cell lines (Figure 5c). In addition, cisplatin-induced caspase-8 activity was suppressed with imipramine in H460 MOR and H157 cells (Supplementary Figure S10) Ceramide clusters were observed 24 h after cisplatin treatment in H460 cells, but these were abolished following pre-treatment with imipramine (Supplementary Figure S5). Together these data confirm that ASM, responsible for ceramide generation following cisplatin treatment, induces caspase-8 activation in both parental and shBAX/BAK cell lines. Accordingly, imipramine strongly attenuated caspase-3 activation following cisplatin treatment in shBAX/BAK cells (Supplementary Figure S6).
Figure 5.
Cisplatin-induced caspase-8 cleavage is acid sphingomyelinase (ASM)-dependent. (a) Western blot for caspase-8 after 2 h treatment with imipramine (50 μM) and 24 h cotreatment with cisplatin (5 μM) in H460 sh-BAXBAK cells. (b) Western blot for caspase-8 and ASM. (c) Western blot for caspase-8 from MOR and H1975 treated as in (a) showing imipramine attenuates cisplatin-induced caspase-8 cleavage in these NSCLC cell lines
In cisplatin-resistant cells, caspase-8 can be activated by TRAIL, but not by cisplatin
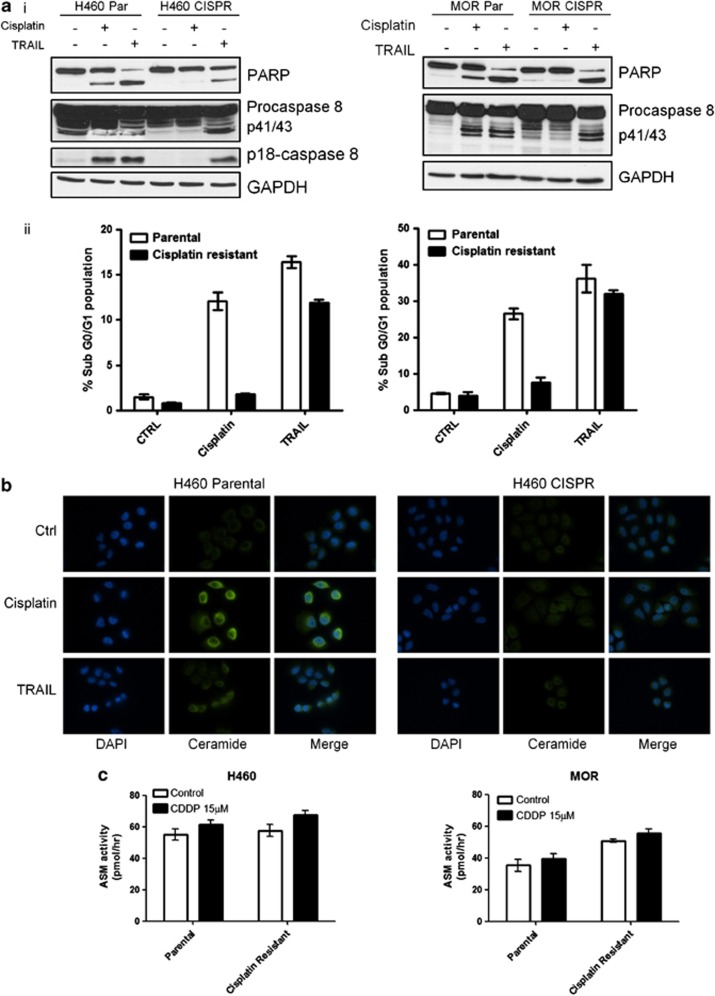
We have previously shown that cisplatin-resistant cells harbour defective mitochondrial signalling in response to cisplatin, owing to suppression of BH3 death signals, whereas there is conservation of BAX/BAK sensitivity to exogenous BH3 domains.32 We therefore decided to investigate whether an analogous dysregulation of the death receptor pathway signalling occurs in cisplatin-resistant NSCLC. In both H460 cisplatin-resistant (CISPR) cells developed in our laboratory (as described previously by Crawford et al.32) and MOR CISPR cells,33 no caspase-8 cleavage was observed following cisplatin treatment, although caspase-8 cleavage and apoptosis could be efficiently induced by TRAIL (Figure 6a). Thus, the death receptor apoptosis machinery is present in H460 CISPR and MOR CISPR cells and remains functional in response to receptor activation. Indeed, ceramide expression was shown to increase in response to cisplatin in the H460 parental but not the H460 cisplatin-resistant cells (Figure 6b). This was also observed in the MOR CISPR cells (not shown). However, this block in ceramide generation was not due to a block in ASM enzyme activity per se, as we detected no difference in either basal, or cisplatin-induced ASM activity between resistant and parental cells (Figure 6c). This suggests that defective ASM is not responsible for failure of caspase-8 cleavage in response to cisplatin in resistant cells.
Figure 6.
Caspase-8 activation is blocked in cisplatin-resistant cells but can be activated by TRAIL. (a) (i) Western blot for PARP and caspase-8 in H460 and MOR paired parental (Par) and cisplatin-resistant (CISPR) cells after 24 h cisplatin (15 μM) or TRAIL (10 ng/ml) treatment. (ii) Flow cytometry of PI-stained H460 and MOR paired parental (Par) and cisplatin-resistant (CISPR) cells after 24 h cisplatin (15 μM) or TRAIL(10 ng/ml) treatment. Data are expressed as mean±S.D. (b) Representative images of cells stained for ceramide (green) in H460 Par and CISPR cells. Cells were treated for 24 h with cisplatin (15 μM) or 4 h with TRAIL (10 ng/ml). (c) Fluorescent ASM activity assay shows PAR and CISPR cells for H460 and MOR have equivalent baseline levels of ASM activity with a small increase after 24 h cisplatin treatment
Discussion
BAX and BAK are key regulators of the mitochondrial apoptosis pathway as evidenced by knockout of BAX and BAK in mouse embryonic fibroblasts.19 Their frequency of loss in NSCLC prompted us to investigate whether functional mitochondrial signalling was required for activity of cisplatin, the most commonly used drug in the treatment of NSCLC. To achieve this, we studied the effect of cisplatin in BAX/BAK-silenced NSCLC cell lines and observed two different phenotypes. In H460, caspase-8 cleavage was observed in response to cisplatin, sufficient to induce apoptosis independent of BAX and BAK. In contrast, H1299 cells showed no evidence of caspase-8 cleavage in response to cisplatin and so were unable to bypass mitochondrial apoptosis block induced by loss of BAX and BAK. This is in agreement with other reports showing that H1299 cells are resistant to ligand stimulation of the death receptor pathway.30 Blocking death receptor-mediated apoptosis, through depletion of caspase-8 by RNA interference, was sufficient to rescue H460 sh-BAXBAK, but not sh-NTNT cells from cisplatin, demonstrating redundancy between the two apoptotic pathways following cisplatin treatment.
This is the first report to show that cisplatin can induce apoptosis through a BAX/BAK-independent, ASM/caspase-8/FADD-dependent fashion. This is contrary to our initial hypothesis based on previous reports in BAX/BAK knockout MEFs. Moreover, we found that sh-BAXBAK H460 cells were more sensitive to cisplatin than parental cells. This could be because of an impairment of mitochondrial network dynamics and metabolism occurring after BAX/BAK depletion (for review see Auret and Martin34), that could in turn lead to increased sensitivity to toxic effect of the drug despite block of caspase-9 activation following staurosporine. Although bypass of the mitochondrial pathway was only observed in the H460 sh-BAXBAK model, treatment of a panel of NSCLC cell lines showed caspase-8 activation following cisplatin in all, except H1299, suggesting that this is a potentially common process in NSCLC. This is not surprising given our previous report that procaspase-8 is overexpressed in 85% of NSCLC compared with matched normal lung tissue.35
The finding that BAX and BAK are not essential for effective apoptosis induction, led us to investigate the mechanism regulating the mitochondrial bypass pathway and its potential alteration in resistance to cisplatin. In both cell lines studied, H460 and MOR, cisplatin-induced caspase-8 cleavage and ceramide accumulation were abrogated in cisplatin-resistant (CISPR) cells, but not in parental cells. However, the death receptor pathway apparatus remained functional in both cisplatin-resistant daughter cell lines, as evidenced by caspase-8 cleavage, PARP cleavage and G0/G1 fraction after treatment with rhTRAIL. This would suggest absence of an upstream death signal initiating apoptosis via the death receptor pathway. The importance of ceramide generation via ASM for both cisplatin-induced apoptosis and death receptor-dependent apoptosis has been demonstrated previously.36, 37, 38 Our data confirm generation of ceramide in response to cisplatin in parental cells, but not in cisplatin-resistant cells and this is not due to dysfunctional ASM in the resistant cells. Although ASM has been shown to regulate cisplatin-induced ceramide generation, production of ceramide requires auxiliary factors. It is possible that an as yet undefined functional defect in one or more of these factors may explain lack of cisplatin-induced ceramide generation in cisplatin-resistant NSCLC cells. From a translational perspective, our data suggest that in cisplatin-resistant cells, despite the absence of ceramide generation and subsequent caspase-8 signalling in response to cisplatin, DISC activation can still be achieved by ligand stimulation of the death receptors with rhTRAIL.
In conclusion, cisplatin resistance requires a block in both mitochondrial and a parallel, independent pathway involving ASM/FADD/caspase-8. Importantly, selection for cisplatin resistance leads to a block in death receptor apoptosis. These cells, however, do not exhibit cross-resistance to death receptor-targeted therapies such as TRAIL and silencing of cFLIP (data not shown). These findings therefore support a rationale for targeting the death receptor pathway, as a strategy for bypassing platinum resistance.
Materials and Methods
Reagents, cytotoxics
Recombinant human TRAIL was purchased from Calbiochem (Gibbstown, NJ, USA), Imipramine and staurosporine were purchased from Sigma-Aldrich (St Louis, MO, USA); cisplatin was obtained from Mayne Pharma, Adelaide, SA, Australia.
Cell culture
ATCC NCI-H1975, NCI-H460, NCI-H1299 and MOR non-small-cell lung cancer (NSCLC) cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% foetal bovine serum and 50 μg/ml penicillin/streptomycin (Invitrogen, Paisley, UK). NCI-H157 NSCLC cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum, 1 mM sodium pyruvate and 50 μg/ml penicillin/streptomycin. All cell lines were maintained in a 5% CO2 incubator at 37 oC.
Generation of BAX and BAK-silenced NSCLC cell lines
Plasmids encoding shRNA sequences to BAX, BAK and NT shRNA (SA Biosciences, Frederick, MD, USA) were transfected into H460 and H1299 NSCLC cells using FuGene 6 reagent (Roche, Burgess Hill, West Sussex, UK) according to manufacturer's protocol. BAX plasmids with G418 resistance selection marker, BAK plasmids with puromycin resistance selection marker and separate NT plasmids bearing either G418 or puromycin resistance selection markers were used. Cells were transfected cells in six-well plates and were incubated with either G418 (H460–0.4 mg/ml, H1299–0.8 mg/ml) (Sigma-Aldrich) or 2 μg/ml puromycin (Calbiochem). Discrete colonies were selected and screened by western blotting. Cells stably expressing shRNA targeting BAX were then re-transfected with shRNA targeting BAK to generate clones with stable knockdown of both BAX and BAK.
siRNA transfections
The non-silencing control (SC) siRNA, caspase-8-targeted (C8), FADD-targeted, DR5-targeted and DR4-targeted siRNA sequences were the same as previously described.35, 39 siRNAs for in vitro transfection were obtained from Dharmacon (Chicago, IL, USA). SMARTpool ON-TARGET plus ASM (SMPD1) siRNA was obtained from Dharmacon. Reverse siRNA transfections were carried out using LipofectamineRNAi max reagent (Invitrogen) according to the manufacturer's instructions.
Western blotting
Twenty-five micrograms of cell lysate were loaded and resolved by SDS-PAGE as previously described.40 Primary antibodies used were: anti-caspase-8 (Alexis Biochemicals, San Diego, CA, USA), anti-PARP (eBioscience, San Diego, CA, USA), anti-GAPDH (Serotec, Kidlington, UK), anti-ASM, anti-α-tubulin (Abcam, Cambridge, MA, USA), anti-BAX, anti-BAK, anti cleaved caspase-9 (Cell Signalling Technology Inc., Danvers, MA, USA) anti-DR4 (Calbiochem), anti-DR5 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and FADD (BD Bioscience Oxford, Oxfordshire, UK). Secondary antibodies used were: goat anti-mouse HRP (DAKO P0447, DAKO Ely, Cambridgeshire, UK) and goat anti-rabbit HRP (DAKO P0448).
Caspase activity assay (8 and 3/7)
For caspase activity assay, cells were seeded at 5000/well in a white-walled 96-well plate 24 h before start of experiment. Media was then replaced with drug concentrations as shown in the text. Imipramine was added 2 h before cisplatin, where required. DEVD-ase activity was determined after 24 h incubation with Caspase-glo luminescent assay (Promega, Southampton, UK) according to manufacturer's instructions, using a Berthold luminometer with 1 s integration time.
Ceramide immunostaining
Cells were seeded onto a glass coverslip inside a six-well plate at 150 000 cells per well, 24 h before start of experiment. Cisplatin and/or imipramine were applied as described previously, and after 24 h cells were fixed in 2% paraformaldehyde. Coverslips were incubated with anti-ceramide antibody (Alexis) followed by goat anti-mouse AlexaFluor488 secondary antibody (Invitrogen) and mounted on slides with Pro-long gold mountant with DAPI (Invitrogen). Images were captured on a Leica DM microscope (Leica Microsystems, Wetzlar, Germany) using a × 40 objective lens.
Flow cytometry
Cells were seeded 48 h post transfection with siRNA as described, at a density of 1 × 105 cells per well in six-well plates. Cells were treated after 24 h and allowed to grow for a further 48 h. Following treatment with cisplatin, DNA content was evaluated by propidium iodide staining of cells using a FACS Calibur Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), in the FL-2 channel to determine percentage of cells with DNA content <2N.
Surface expression of death receptors was assessed following live cell staining with one of the phycoerythrin-conjugated monoclonal antibodies: DR4, DR5, Fas, DcR2 (all from eBioscience) or TNFR1 antibody (R&D Systems, Minneapolis, MN, USA). In all experiments, isotype control (mouse IgG1 from eBioscience) was used.
ASM activity assay
ASM activity was determined using an ASM activity assay kit (Echelon Biosciences Inc., Salt Lake City, UT, USA) according to the manufacturer's protocol. Cells were disrupted using a microprobe sonicator (Sonics Vibra-Cell VC 130 Ultrasonic Homogeniser) with 10 pulses (2 s on, 1 s off) at 30% output. 30 μg of protein was used to determine ASMase activity. Reaction was stopped after incubation at 37 °C for 2 h and analysed using a fluorescence plate reader at 260 nm excitation and 460 nm emission.
Statistical analysis
Statistical analyses were conducted using Microsoft Office Excel 2007. Results were expressed as mean value±S.D. Differences between groups were evaluated using unpaired two-tailed Student's t-test.
Acknowledgments
IP was a recipient of Northern Ireland HSC R&D Office doctoral fellowship. IS was funded by British Lung Foundation. NC, FM and BW were funded by the Department for Education and Learning. AC, SB and NM were funded by Cancer Research UK. DF is a recipient of CR-UK Clinician Scientist Fellowship.
Glossary
- ALK
anaplastic lymphoma kinase
- ASM
acid sphingomyelinase
- BAK
BCL-2 antagonist killer
- BAX
BCL-2-associated X protein
- BCL-2
B-cell lymphoma-2
- BID
BCL-2 interacting domain death agonist
- BIM
BCL2-interacting mediator of cell death-associated recruitment domain
- cFLIP
cellular FLICE inhibitory protein
- DR4
death receptor 4
- DR5
death receptor 5
- EML4
echinoderm microtubule-associated protein-like 4
- FADD
Fas-associated protein with death domain
- FLICE
FADD-like interleukin-1β-converting enzyme
- HER2
human epidermal growth factor receptor 2
- PARP
poly (ADP-ribose) polymerase
- PIKC3A
phosphoinositide-3-kinase, catalytic, alpha polypeptide
- R8BID
polyarginine tagged BID
- RNA
ribose nucleic acid
- shRNA
short hairpin RNA
- TRAIL
TNF receptor-related apoptosis inducing ligand
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Stephanou
Supplementary Material
References
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- Fennell DA. Caspase regulation in non-small cell lung cancer and its potential for therapeutic exploitation. Clin Cancer Res. 2005;11:2097–2105. doi: 10.1158/1078-0432.CCR-04-1482. [DOI] [PubMed] [Google Scholar]
- Klastersky J, Awada A. Milestones in the use of chemotherapy for the management of non-small cell lung cancer (NSCLC) Crit Rev Oncol Hematol. 2012;81:49–57. doi: 10.1016/j.critrevonc.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Belani CP, Langer C. First-line chemotherapy for NSCLC: an overview of relevant trials. Lung Cancer. 2002;38 (Suppl 4:13–19. doi: 10.1016/s0169-5002(02)00394-x. [DOI] [PubMed] [Google Scholar]
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Deng J, Shimamura T, Perera S, Carlson NE, Cai D, Shapiro GI, et al. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res. 2007;67:11867–11875. doi: 10.1158/0008-5472.CAN-07-1961. [DOI] [PubMed] [Google Scholar]
- Costa DB, Halmos B, Kumar A, Schumer ST, Huberman MS, Boggon TJ, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations PLoS Med 200741669–1679.discussion 1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A.Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics PLoS Med 200741681–1689.discussion 1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- Cappuzzo F, Bemis L, Varella-Garcia M. HER2 mutation and response to trastuzumab therapy in non-small-cell lung cancer. N Engl J Med. 2006;354:2619–2621. doi: 10.1056/NEJMc060020. [DOI] [PubMed] [Google Scholar]
- Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431:525–526. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 4;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolinario RM, van der Valk P, de Jong JS, Deville W, van Ark-Otte J, Dingemans AM, et al. Prognostic value of the expression of p53, bcl-2, and bax oncoproteins, and neovascularization in patients with radically resected non-small-cell lung cancer. J Clin Oncol. 1997;15:2456. doi: 10.1200/JCO.1997.15.6.2456. [DOI] [PubMed] [Google Scholar]
- Groeger AM, Esposito V, De Luca A, Cassandro R, Tonini G, Ambrogi V, et al. Prognostic value of immunohistochemical expression of p53, bax, Bcl-2 and Bcl-xL in resected non-small-cell lung cancers. Histopathology. 2004;44:54. doi: 10.1111/j.1365-2559.2004.01750.x. [DOI] [PubMed] [Google Scholar]
- Shabnam MS, Srinivasan R, Wali A, Majumdar S, Joshi K, Behera D. Expression of p53 protein and the apoptotic regulatory molecules Bcl-2, Bcl-XL, and Bax in locally advanced squamous cell carcinoma of the lung. Lung Cancer. 2004;45:181. doi: 10.1016/j.lungcan.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Yaren A, Oztop I, Kargi A, Ulukus C, Onen A, Sanli A, et al. Bax, bcl-2 and c-kit expression in non-small-cell lung cancer and their effects on prognosis. Int J Clin Pract. 2006;60:675. doi: 10.1111/j.1368-5031.2006.00742.x. [DOI] [PubMed] [Google Scholar]
- Berrieman HK, Smith L, O'Kane SL, Campbell A, Lind MJ, Cawkwell L. The expression of Bcl-2 family proteins differs between nonsmall cell lung carcinoma subtypes. Cancer. 2005;103:1415. doi: 10.1002/cncr.20907. [DOI] [PubMed] [Google Scholar]
- Borner MM, Brousset P, Pfanner-Meyer B, Bacchi M, Vonlanthen S, Hotz MA, et al. Expression of apoptosis regulatory proteins of the Bcl-2 family and p53 in primary resected non-small-cell lung cancer. Br J Cancer. 1999;79:952. doi: 10.1038/sj.bjc.6690152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul I, Savage KI, Blayney JK, Lamers E, Gately K, Kerr K, et al. PARP inhibition induces BAX/BAK-independent synthetic lethality of BRCA1-deficient non-small cell lung cancer. J Pathol. 2011;224:564–574. doi: 10.1002/path.2925. [DOI] [PubMed] [Google Scholar]
- Ganten TM, Haas TL, Sykora J, Stahl H, Sprick MR, Fas SC, et al. Enhanced caspase-8 recruitment to and activation at the DISC is critical for sensitisation of human hepatocellular carcinoma cells to TRAIL-induced apoptosis by chemotherapeutic drugs. Cell Death Differ. 2004;11 (Suppl 1:S86–S96. doi: 10.1038/sj.cdd.4401437. [DOI] [PubMed] [Google Scholar]
- Lacour S, Micheau O, Hammann A, Drouineaud V, Tschopp J, Solary E, et al. Chemotherapy enhances TNF-related apoptosis-inducing ligand DISC assembly in HT29 human colon cancer cells. Oncogene. 2003;22:1807–1816. doi: 10.1038/sj.onc.1206127. [DOI] [PubMed] [Google Scholar]
- Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, et al. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. 2004;64:3593–3598. doi: 10.1158/0008-5472.CAN-03-2787. [DOI] [PubMed] [Google Scholar]
- Stegehuis JH, de Wilt LH, de Vries EG, Groen HJ, de Jong S, Kruyt FA. TRAIL receptor targeting therapies for non-small cell lung cancer: current status and perspectives. Drug Resist Updat. 2010;13:2–15. doi: 10.1016/j.drup.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Rebillard A, Tekpli X, Meurette O, Sergent O, LeMoigne-Muller G, Vernhet L, et al. Cisplatin-induced apoptosis involves membrane fluidification via inhibition of NHE1 in human colon cancer cells. Cancer Res. 2007;67:7865–7874. doi: 10.1158/0008-5472.CAN-07-0353. [DOI] [PubMed] [Google Scholar]
- Crawford N, Chacko AD, Savage KI, McCoy F, Redmond K, Longley DB, et al. Platinum resistant cancer cells conserve sensitivity to BH3 domains and obatoclax induced mitochondrial apoptosis. Apoptosis. 2011;16:311–320. doi: 10.1007/s10495-010-0561-1. [DOI] [PubMed] [Google Scholar]
- Twentyman PR, Fox NE, Wright KA, Bleehen NM. Derivation and preliminary characterisation of adriamycin resistant lines of human lung cancer cells. Br J Cancer. 1986;53:529–537. doi: 10.1038/bjc.1986.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autret A, Martin SJ. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell. 2009;36:355–363. doi: 10.1016/j.molcel.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Wilson TR, Redmond KM, McLaughlin KM, Crawford N, Gately K, O'Byrne K, et al. Procaspase 8 overexpression in non-small-cell lung cancer promotes apoptosis induced by FLIP silencing. Cell Death Differ. 2009;16:1352–1361. doi: 10.1038/cdd.2009.76. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- Grassme H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22:5457–5470. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- Gulbins E. Regulation of death receptor signaling and apoptosis by ceramide. Pharmacol Res. 2003;47:393–399. doi: 10.1016/s1043-6618(03)00052-5. [DOI] [PubMed] [Google Scholar]
- Wilson TR, McLaughlin KM, McEwan M, Sakai H, Rogers KM, Redmond KM, et al. c-FLIP: a key regulator of colorectal cancer cell death. Cancer Res. 2007;67:5754–5762. doi: 10.1158/0008-5472.CAN-06-3585. [DOI] [PubMed] [Google Scholar]
- McCoy F, Hurwitz J, McTavish N, Paul I, Barnes C, O'Hagan B, et al. Obatoclax induces Atg7-dependent autophagy independent of beclin-1 and BAX/BAK. Cell Death Dis. 2010;1:e108. doi: 10.1038/cddis.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.