Abstract
The novel heavy/light chain (HLC) assay was used for the detection and measurement of monoclonal immunoglobulins, response evaluation and prognostication. This test allows identification and quantification of the different light chain types of each immunoglobulin class (for example, IgGκ and IgGλ) and enables calculation of ratios of monoclonal/polyclonal immunoglobulin (HLC ratio). Sequential sera of 156 patients with IgG or IgA myeloma started on first-line therapy and followed for a median of 46.1 months were analyzed. Results were compared with those obtained with conventional techniques (serum protein electrophoresis (SPEP), immunofixation electrophoresis (IFE), nephelometry (NEPH), and the free light chain test (FLC)). Our data show that the HLC assay allowed quantification of monoclonal proteins not accurately measurable by SPEP or NEPH. When both HLC and FLC testing were applied for response assessment, clonal excess was noted in 14/31 patients with complete response (CR). HLC ratio indicated presence of disease in 8/31 patients who achieved CR and, in sequential studies indicated evolving relapse in three patients before IFE became positive. Highly abnormal HLC ratios at presentation were significantly associated with shorter overall survival (40.5 months vs median not reached, P=0.016). Multivariate analysis revealed HLC ratio (P=0.03) and β2-microglobulin (P<0.01) as independent risk factors for survival.
Keywords: heavy/light chain assay, multiple myeloma, monoclonal immunoglobulin, response evaluation and prognostication
Introduction
In multiple myeloma (MM), precise quantification of monoclonal immunoglobulin (M-Ig) and its variations during the course of the disease, discrimination between monoclonal and polyclonal isotype particularly at low concentrations of M-Igs,1 information about the ratio of clonal and polyclonal plasma cells2 and about the amount of immunosuppression3 is important for prognostication, response evaluation and treatment selection. International guidelines recommend the use of serum protein electrophoresis (SPEP) and nephelometry (NEPH) for quantification of monoclonal immunoglobulins (M-Ig) in MM,4 with immunofixation electropheresis (IFE) being used to confirm complete response (CR). However, the usefulness of SPEP is limited at low M-Ig levels, when the M-Igs co-migrate with other serum proteins, as commonly observed in IgA and less frequently in IgG MM, and when the M-Ig fails to resolve as a discrete band. Total immunoglobulin measurements with NEPH can resolve some of these issues. However, they tend to overestimate the amount of M-Ig at high concentrations, particularly in IgG and IgM paraproteinemias,5, 6 but do not discriminate between M-Ig and normal polyclonal immunoglobulins, which becomes particularly relevant at low M-Ig concentrations.
Novel antibodies have been produced (heavy/light chain (HLC) antibodies), which separately identify the different light chain types of each immunoglobulin class, that is, IgGκ, IgGλ, IgAκ, and IgAλ.7 They allow accurate quantification of the involved and uninvolved immunoglobulin of the patient's affected isotype. Furthermore, a ratio of the monoclonal and the polyclonal immunoglobulin of the same isotype, that is, of IgGκ/IgGλ, can be calculated (HLC ratio) in the same manner as serum-free light chain (FLC) κ/λ ratios. Measurement of FLC has become a standard technique for response assessment.8 Preliminary studies employing the HLC assay have shown that HLC ratios may be of use in screening,9 monitoring and risk stratifying of patients with MM,10 AL amyloidosis,11 Waldenström's macroglobulinemia12 and MGUS (monoclonal gammopathy of undetermined significance).13 In addition, they may provide the only sensitive marker of disease progression in some patients.14
Here, we compare the use of IgGκ/IgGλ and IgAκ/IgAλ HLC assays with conventional measurements for quantification of M-Igs and for response assessment, and evaluate the advantages and limitations of the new test. In addition, we assess the prognostic relevance of the HLC ratio in patients started on first-line therapy and followed by sequential serum measurements.
Materials and methods
In all, 156 previously untreated patients with MM and measurable disease as defined by the International Myeloma Working Group criteria15 were included. Patients had been treated with one of the following treatment protocols: Thalidomide-Dexamethasone (27), Melphalan–Prednisolone (15), Vincristine–Adriamycin–Dexamethasone, followed by autologous stem cell transplantation (23), Vincristine–Melphalan–Cyclophosphamide–Prednisolone (19), Bortezomib–Thalidomide–Dexamethasone with or without Cyclophosphamide (42) and Lenalidomide–Dexamethasone (30). Median age was 66 years (range 32–94). All patients gave written informed consent for therapy, and their characteristics are shown in Table 1. The IMWG criteria15 were applied to classify very good partial response (VGPR) and CR, and the European Group for Blood and Marrow Transplantation (EBMT) criteria16 were used to categorize partial response (PR), minor response (MR), stable disease (SD) and progressive disease (PD). In addition, the response class nCR (IFE-positive CR), was used to denote those patients who had normalization of the isotype concentration of their involved immunoglobulin class, but still a monoclonal band detectable on IFE.
Table 1. Patient characteristics.
| Number of patients | All | IgG patients | IgA patients |
|---|---|---|---|
| 156 | 100 | 56 | |
| Age (years), median (range) | 66 (32–94) | 66 (37–86) | 66 (32–94) |
| Sex (male/female) | 82/74 | 51/49 | 31/25 |
| β2 microglobulin (median, range (mg/l)) | 3.7 | 3.7 | 3.9 |
| Albumin (median, range (g/dl)) | 3.8 (1.6–5.1) | 3.8 | 3.8 |
| FLC ratio | 24.8 (0.7–41.100) | 26.1 (0.7–41.100) | 18.1 (0.88–10100) |
| HLC ratio | 38.4 | 29.4 | 85.3 |
| IgG (g/l) | 15.0 (0.9–112.0) | 36.2 (3.2–112.0) | 4.8 (0.9–15.6) |
| IgA (g/l) | 0.72 (0.1–117.2) | 0.3 (0.1–4.7) | 27.1 (2.8–117.2) |
| IgM (g/l) | 0.14 (0.01–2.98) | 0.16 (0.01–2.98) | 0.10 (0.01–1.24) |
| M protein migration in β region | 31 | 4 | 27 |
| ISS stage I | 59 | 39 | 20 |
| ISS stage II | 63 | 35 | 28 |
| ISS stage III | 34 | 26 | 8 |
| IgGκ/IgGλ–IgAκ/IgAλ | 64/36–33/23 | 64/36 | 33/23 |
| Median follow-up (months; range) | 46.1 (0.5–157.8) | 56.2 (0.5–157.8) | 25.3 (0.5–125.9) |
| Median overall survival (months 95% CI) | 53.5 (47.3–59.6) | 61.8 (54.1–69.6) | 38.5 (29.4–47.5) |
Abbreviations: FLC, free light chain test; HLC, heavy/light chain; ISS, International Staging System.
Immunoglobulin HLC pairs (IgGκ/IgGλ and IgAκ/IgAλ) were assessed using polyclonal antibodies targeted at unique junctional epitopes between heavy-chain and light-chain constant regions of intact Ig (Hevylite, Binding Site, Birmingham, UK) on a Binding Site SPAPLUS Analyzer. Measurements of these parameters were used to derive IgGκ/IgGλ and IgAκ/IgAλ ratios, which were compared with reference ranges.17, 18 HLC ratios outside of the reference range were considered to be indicative of a clonal process. Tests were run independently in Birmingham and Vienna, and results were read by experienced qualified clinical chemists. Immunoelectropheresis was run on Sebia Hydrasys (Sebia, Evry, France) in both laboratories and results were compared. Concentrations of conventional parameters such as IgA, IgG, β2-microglobulin (β2-M), FLC, lactate dehydrogenase, and creatinine were assessed by standard techniques.
Patients were categorized using HLC ratio and FLC ratio prognostic values, which were broadly identified using receiver operator characteristic (ROC) analysis with final cut-offs being identified by trial and error. Patients were stratified into different treatment-induced response groups, and distribution of HLC values at baseline according to response category was assessed by the Welch test.19 Kaplan–Meier survival curves were compared using the log-rank test,20 whereas univariate and multivariate analysis were performed by Cox proportional regression analysis21 (SPSS version 18, IBM, Armonk, NY, USA).
Results
Patient characteristics at baseline are shown in Table 1. Patients were followed up for a median of 46.1 months (range: 0.5–157.8). Previous assessment of HLC concentrations in normal blood donors established median HLC ratios and 95% ranges of 1.74 (1.12–3.21) for IgGκ/IgGλ17 and of 1.18 (0.78–1.94) for IgAκ/IgAλ.18
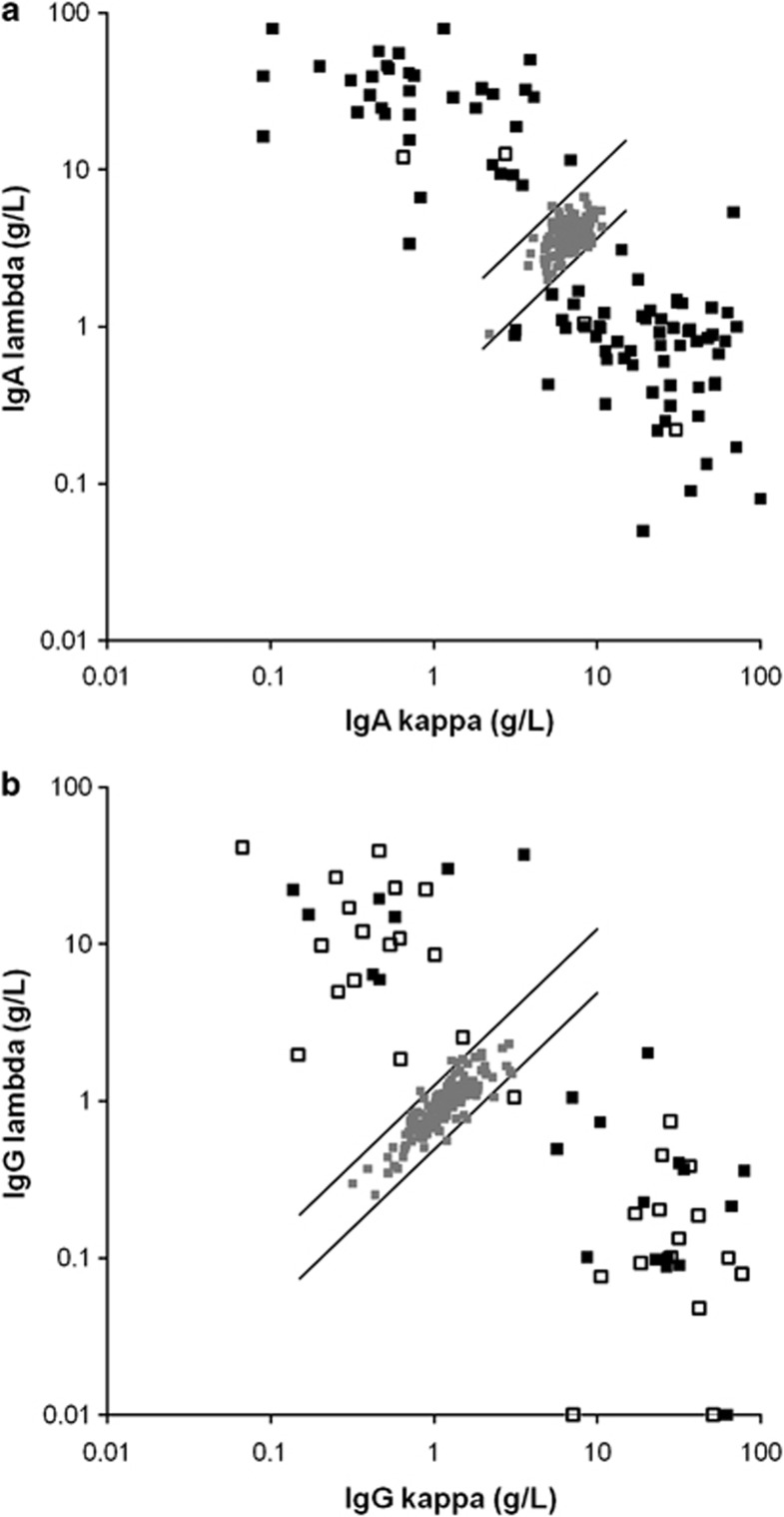
Results of HLC testing revealed 5% of control sera lying outside but very close to the verge of the normal range, and an abnormal HLC ratio in all patients of this study (Figures 1a and b, respectively) including 18 patients with oligosecretory disease (<10 g/l, 7 IgG and 11 IgA).
Figure 1.
Scatter graph of the HLC values. All individual values of the 100 patients with IgG (a) and of the 56 patients with IgA M-Ig (b) were outside the normal range. The solid squares denote patients with quantifiable M-Igs by SPEP, whereas open squares denote patients with anodal migration of their M-Igs, which made accurate quantification impossible.
Accurate measurement of M-Ig by SPEP densitometry was not possible in 46% (26/56) IgA and 4% (4/100) IgG patients because the M-Ig had been obscured by other proteins, mostly because of migration toward the β region. Quantitative HLC measurements were possible in all patients, even in those with M-Ig difficult to be quantified by SPEP. Summated Ig′κ+Ig′λ correlated well to total Ig for each isotype measures; IgG (r2=0.93), IgA (r2=0.94).
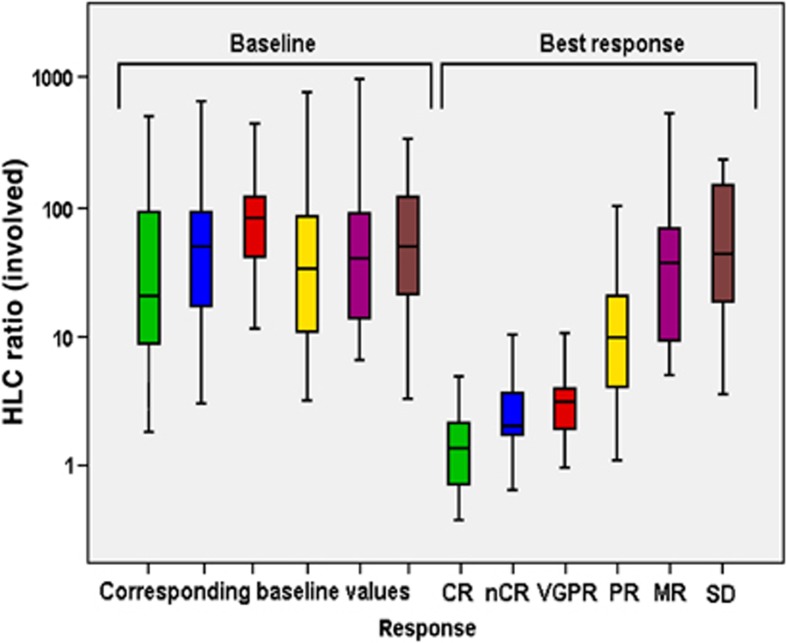
Myeloma responses were assessed after induction therapy by conventional techniques. CR and nCR were noted in 31 (20.8%) and 17 (11.4%) of all patients; 18 (12.1%) were categorized as VGPR, 46 (30.9%) as PR and 18 (12.1%) as minor response, respectively, yielding an overall response rate (CR–PR) of 75.2%. Eight (5.4%) patients presented with stable disease and 11 (8%) with progressive disease. At time of best response, exact quantification by SPEP was not possible in two patients with minor response, seven patients with PR, and one patient with VGPR. HLC ratios could be determined in all of those patients and were abnormal in all. The distribution HLC ratios at baseline was similar in patients with subsequent different response categories (P=0.451), thus baseline HLC levels did not predict the quality of response after induction therapy (Figure 2). Measurement of HLC ratio at best response yielded significantly different results in the various response groups (P<0.006).
Figure 2.
Box and whisker representation of HLC ratios of the involved monoclonal immunoglobulin at baseline and maximal response for patients classified according to the IMWG and EBMT (minor response) criteria. HLC ratio at baseline did not differ between patients with different response categories.
Table 2 shows the results of HLC, FLC, IFE and SPEP testing in patients at time of best response. Of note, an abnormal HLC ratio was seen in 8 of the 31 patients in CR; four of them had abnormal HLC ratio only, whereas in the other four both an abnormal HLC and FLC ratio were noted. In the 17 patients with nCR and the 18 patients with VGPR, an abnormal HLC ratio was observed in 9 and 14 patients, respectively, with 8 and 10 each having both abnormal HLC and FLC ratio.
Table 2. Results of HLC, FLC, IFE and SPEP testing in patients with different response categories.
| Test | Result | CR (n=31) | nCR (n=17) | VGPR (n=18) | PR (n=46) | MR (n=18) | SD (n=8) | PD (n=11) |
|---|---|---|---|---|---|---|---|---|
| HLC ratio | Normal | 23 | 8 | 4 | 0 | 0 | 0 | 0 |
| Abnormal | 8 | 9 | 14 | 46 | 18 | 8 | 11 | |
| FLC ratio | Normal | 16 | 6 | 5 | 9 | 1 | 1 | 3 |
| Abnormal | 15 | 11 | 13 | 37 | 17 | 7 | 8 | |
| HLC– & FLC ratio | Normal (HLC– & FLC ratio) | 13 | 5 | 1 | 0 | 0 | 0 | 0 |
| Abnormal (HLC– & FLC ratio) | 4 | 8 | 10 | 37 | 17 | 7 | 8 | |
| Abnormal (HLC– or FLC ratio) | 14 | 4 | 7 | 9 | 1 | 1 | 3 | |
| IFE | Negative+oligo | 26+5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Positive | 0 | 17 | 18 | 46 | 18 | 8 | 11 | |
| M–Spike (SPEP) | No | 31 | 17 | 0 | 0 | 0 | 0 | 0 |
| Yes | 0 | 0 | 18 | 46 | 18 | 8 | 11 |
Abbreviations: CR, complete response; FLC, free light chain; HLC, heavy/light chain; IFE, immunofixation; MR, minor response; nCR, near complete response; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response.
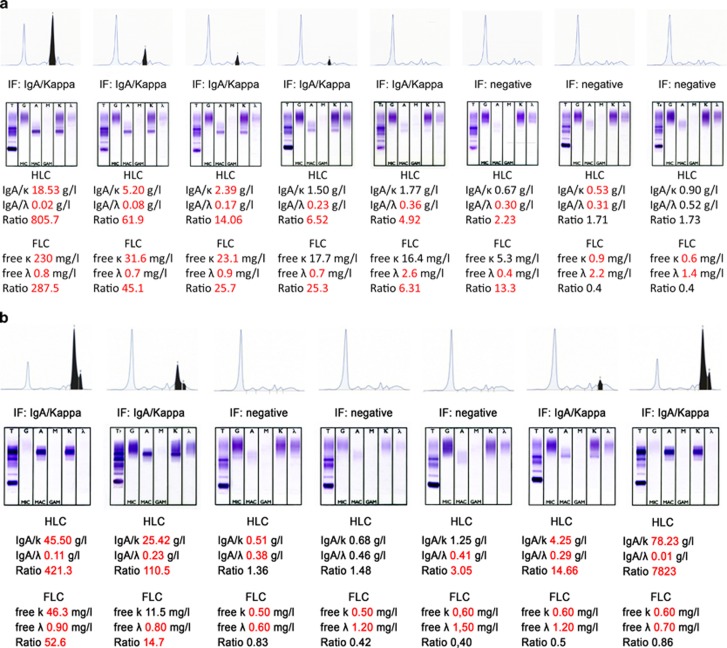
Figures 3a and b show a typical follow-up of individual patients with sequential testing and the additional use of HLC ratios. Patient A achieved a CR established by SPEP and IFE, but an abnormal HLC ratio indicated the presence of M-Ig at a time when an M-Ig was no longer detectable by IFE (Figure 3a). With further follow-up and continued CR, the HLC ratio became normal. In this patient, the kinetics of FLC ratio corresponded with the changes of the HLC ratio during the entire follow-up. Patient B already achieved CR after induction therapy. At the time of first documentation of CR, which occurred before the start of high-dose melphalan, both HLC ratio and FLC ratio also became normal (Figure 3b). The patient remained in IFE-negative CR for 14 months, but the HLC ratio became abnormal 7 months after the first achievement of a normal HLC ratio, indicating an evolving relapse. At that time, total IgA and IgAκ were still within the normal range. During subsequent testing, IFE became positive 5.5 months after the HLC ratio had changed from normal to abnormal. Later, SPEP became positive, with further increase in the HLC ratio. With marked delay, clinical relapse also became apparent. These patterns indicated the presence of M-Ig, while IFE already had become negative or pointing to imminent relapse while IFE was still negative, have been observed in three patients each.
Figure 3.
Sequential SPEP, IFE, HLC ratios, and FLC ratios in two patients with IgAκ myeloma. In patient A (a), IFE became negative at a time when abnormal HLC ratio and FLC ratio still indicated presence of residual disease. Both later tests became normal with further follow-up. The kinetics of FLC ratio corresponded with the changes of the HLC ratio during the entire follow-up. In patient B (b), both IFE and HLC ratio became normal at the same time. The HLC ratio became abnormal indicating relapse when IFE was still normal. IFE remained normal for further 5.5 months. Thereafter, laboratory relapse was confirmed by IFE; later clinical relapse was noted.
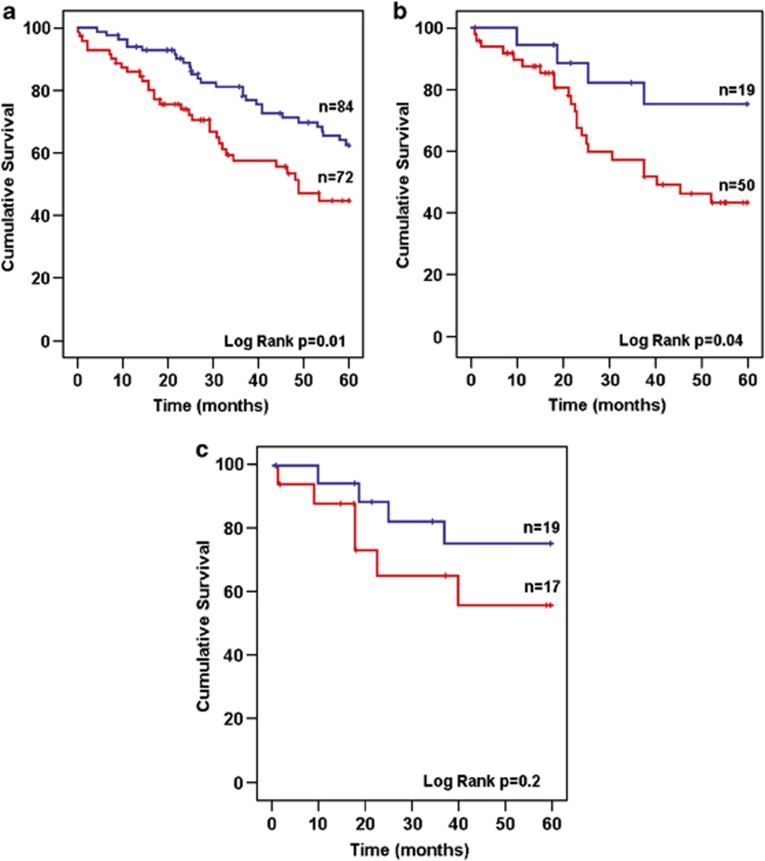
Median survival of the entire patient cohort was 53.5 months. When patients were stratified according to either abnormal (0.022–45) or highly abnormal (<0.022 or >45) presentation HLC ratio values, survival was significantly shorter in those with highly abnormal ratios (median 40.5 months vs median not reached, hazards ratio (HR): 2.07, confidence interval (CI): 1.15–3.75, P=0.016) (Figure 4a). The survival rates at 5 years were 33.4% for the former and 58.9% for the latter group (P=0.01). For patients with a highly abnormal FLC ratio (<0.1 or >30), a statistically not significant tendency for shorter survival was noted (40.8 months vs median not reached, HR: 1.72, CI: 0.93–3.17, P=0.08) compared with those with less abnormal FLC ratios (0.1–30).
Figure 4.
Overall survival in patients stratified by HLC ratio. Median survival was 40.5 months in patients with highly abnormal HLC ratio (red line) and was not reached in those with less abnormal HLC ratio (blue line, P=0.016) (a). Patients achieving a PR or better were assessed at best response (b). In this group, patients with a normal HLC ratio (blue line) had a significantly longer survival from maximum response (P=0.04) compared with those with an abnormal HLC ratio (red line). Similarly, patients achieving a VGPR or better were compared (c). Patients with a normal HLC ratio (blue line) had a tendency for longer overall survival compared with those with an abnormal HLC ratio (red line) although significance was not reached.
When survival was analyzed from time of first achievement of maximal response (PR or better), patients with HLC ratios remaining abnormal had a significantly shortened survival as compared with those achieving a normal HLC ratio (40.5 months; CI: 17–65 vs median not reached, HR: 2.8, CI: 0.99–8.3, P<0.03) (Figure 4b). A similar, but statistically not significant tendency was noted in the patients who achieved VGPR or better (median not reached, HR: 2.1, CI: 0.6–7.5, P=0.2) (Figure 4c).
Univariate Cox analysis associated an increasingly abnormal HLC ratio (<0.022, >45) and a β2-microglobulin concentration of >5.5mg/l at presentation with shorter survival (HR 1.88, CI: 1.1–3.1, P=0.015 and HR 2.2, CI: 1.3–3.9, P=0.016, respectively), whereas for the other parameters tested (FLC ratio, albumin >35g/l, lactate dehydrogenase >248 U/l) no correlation was found (Table 3). Results of multivariate Cox analysis proved a highly abnormal HLC ratio and highly abnormal β2-microglobulin as parameters independently associated with survival (HR: 1.94, CI: 1.1–3.3, P=0.016, and HR: 2.01, CI: 1.1–3.6, P=0.016, respectively).
Table 3. Univariate and Cox regression multivariate analysis of prognostic parameters with survival.
| Variable | Significance | Hazard ratio |
|---|---|---|
| Univariate analysis | ||
| HLC ratio <0.02, >40 | 0.015 | 1.9 |
| B2M>5.5 mg/l | 0.016 | 2.2 |
| FLC ratio <0.03, >32 | 0.105 | 0.6 |
| Age>75 years | 0.305 | 1.47 |
| Albumin >35 g/l | 0.439 | 1.29 |
| B2M>3.5mg/l | 0.562 | 1.20 |
| LDH>248 UI/l | 0.642 | 1.25 |
| Multivariate analysis | ||
| HLC ratio <0.02, >40 | 0.016 | 1.94 |
| β2M >5.5 mg/l | 0.016 | 2.0 |
Abbreviations: FLC, free light chain test; HLC, heavy/light chain; LDH, lactate dehydrogenase.
Discussion
Our study shows that the HLC assay can overcome an important limitation of measuring M-Igs with conventional techniques. The M-Igs of patients with IgA myelomas frequently migrate into the β region of the SPEP, where they are obscured by proteins such as transferrin, β lipoprotein and C3, which precludes quantification by SPEP. This problem occurred in 46% of our IgA and in 4% of our IgG patients. A similar observation was previously reported by Wang et al.,22 and recently by Avet-Loiseau et al.,23 who were unable to quantify IgA levels by SPEP in 57% and 33% of their patients, respectively. Changes in HLC ratios in these patients reflected the disease course. Similarly, in patients with oligosecretory disease (M-Ig <10g/l), characterized by subtly abnormal HLC ratio, changes in HLC ratio reflected response and relapse, and therefore could be used as an additional monitoring tool in difficult-to-quantify patients.
A further important advantage of the HLC assay is its ability to detect clonality at low M-Ig concentrations. This cannot be achieved with total immunoglobulin measurements because these assays are unable to distinguish between the involved and uninvolved immunoglobulin of the respective isotype. SPEP, particularly capillary SPEP, may provide more reliable measurements, but has important limitations in assigning the M-Ig fraction to a specific band, for example, when the M-Ig is polymerized and therefore spread over a larger distance in the electrophoresis curve.24
Table 2 shows discordance between HLC and FLC ratios seen in several patients with CR; it shows that both tests are needed to exclude presence of minimal amounts of M-Ig. Persistent disease was indicated by an abnormal HLC ratio in 8/31 patients who achieved CR with four of them showing abnormal HLC, but normal FLC ratios (Table 2). The demonstration of abnormal HLC ratios in patients with IFE-negative CR suggests a higher sensitivity for the detection of residual M-Ig, and this is corroborated by the association of abnormal HLC ratios with shorter survival times from maximal response. Somewhat contradictory to this assumption is the observation of a normal HLC ratio in 12/35 IFE-positive patients achieving nCR or VGPR. Patients responding to therapy will have an increased polyclonal immunoglobulin production which, in the presence of low levels of monoclonal immunoglobulin, may yield a normal ratio. The low-level M-Ig could be due to a prolonged half-life, which has previously been reported,25 or be due to the persistence of a small clone that has not been cleared by treatment. The latter is unlikely in light of the observation that irrespective of IFE positivity a normal HLC ratio predicts a good patient outcome in this study.
The importance of true-clonal remission has been indicated in recent studies.26, 27 Paiva et al.27 reported superior prognostic relevance of immunophenotypically defined response over stringent CR (sCR). The achievement of sCR was not associated with improved survival compared with patients achieving CR only, but patients with immunophenotypically defined CR had significantly improved survival. This concurs with our data showing significantly better survival from onset of ⩾PR in patients with normal HLC ratio (Figure 4b). For patients who achieved ⩾VGPR, a similar, albeit statistically not significant, observation was made (Figure 4c). Normalization of the FLC ratio, in contrast, did not correlate with overall survival, neither in the entire patient group nor in those achieving ⩾PR, or⩾VGPR.
Results of a recent transplant study are in line with our observations.28 Patients with >VGPR and an HLC normalization after induction therapy before transplant had a lower risk of treatment failure and a longer progression free survival, whereas for normalization of FLC ratios no such association was noted. On the basis of the available evidence, normalization of the HLC ratio seems to be of greater predictive value than FLC normalization. Further studies should compare immunophenotyping with HLC measurements, which can be performed repetitively without the need for bone marrow aspiration or biopsy.
Sequential testing in our patients (Figures 3a and b) showed that patients who are deemed having achieved CR by conventional testing (SPEP, NEPH and IFE) may still have residual disease by HLC testing. Vice versa, conversion of a normal to an abnormal HLC ratio indicated evolving relapse, weeks or months before relapse became evident by conventional methods. FLC ratios frequently concurred with the HLC ratios, but in some patients results were discordant. This finding underlines the variability in clonal evolution during the course of MM and highlights the emergence of various clones. The original clone may persist as single clone, but probably more often subclones with truncated ability for production of intact immunoglobulins do emerge.29, 30 They may produce light chains, or as shown in some patients in our study, intact immunoglobulins (heavy and light chains) only, and may coexist with the original population of intact molecule secreting myeloma cells. Thus, the HLC assay likely will provide additional information on the clonal evolution in myeloma.
The finding of a significant association of baseline HLC ratios with overall survival is remarkable. This was true when HLC ratios were considered as linear variable (P=0.016) or stratified according to the extremity of the HLC ratio (<0.022 or >45 vs 0.022–45 excluding normal ranges). An association of extreme HLC ratios with significantly shorter progression-free survival has already previously been reported by Avet-Loiseau et al.23 Notably, for highly abnormal FLC ratios only, a tendency for shorter survival was noted in our cohort, which is at variance with some studies showing prognostic significance of abnormal FLC ratios.31, 32, 33, 34
The results of univariate and Cox multivariate regression analysis confirmed the prognostic relevance of the HLC ratio together with β2-microglobulin, whereas for FLC ratio, lactate dehydrogenase and albumin no significant association was noted.
One of the limitations of this report is that we did not analyze the relevance of the IgA and IgG HLC ratio separately, because of the limited number of IgA patients. Also we did not include patients with oligosecretory MM or light-chain MM. Furthermore, our patients have been studied retrospectively in order to enable long-term follow-up for survival analysis, but studies need to be designed to prospectively assess the value of the assay.
In conclusion, the findings presented here indicate a considerable potential of the HLC assay. Alongside clinical evaluation this test likely will be used to confirm and quantify M-Igs, identify patient responses, early relapse and aid in the recognition of minimal residual disease in patients with MM and several other paraproteinemic disorders.
Acknowledgments
The study has been supported in part by the Austrian Forum Against Cancer.
Author Contributions
HL and SJH designed the study, analyzed the data and wrote the manuscript; DM performed the HLC and the other assays in Vienna, JMF conducted the statistical analysis; and NZ, WH and ARB were involved in writing and reviewing the manuscript.
HL has received honorarium from Binding Site, and SJH and JMF are employed by Binding Site. ARB has been owner of Binding Site and now serves as consultant.
References
- Harousseau JL, Attal M, Avet-Loiseau H, Margit G, Caillot D, Mohty M, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28:4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- Paiva B, Almeida J, Pérez-Andrés M, Mateo G, López A, Rasillo A, et al. Utility of flow cytometry immunophenotyping in multiple myeloma and other clonal plasma cell-related disorders. Cytometry B Clin Cytom. 2010;78:239–252. doi: 10.1002/cyto.b.20512. [DOI] [PubMed] [Google Scholar]
- Jacobson DR, Zolla-Pazner S. Immunosuppression and infection in multiple myeloma. Semin Oncol. 1986;13:282–290. [PubMed] [Google Scholar]
- Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV. San Miguel J, Chanan-Khan A et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117:4701–4705. doi: 10.1182/blood-2010-10-299529. [DOI] [PubMed] [Google Scholar]
- Murray DL, Ryu E, Snyder MR, Katzmann JA. Quantitation of serum monoclonal proteins: relationship between agarose gel electropheresis and immunonephelometry. Clin Chem. 2009;55:1523–1529. doi: 10.1373/clinchem.2009.124461. [DOI] [PubMed] [Google Scholar]
- Riches PG, Sheldon J, Smith AM, Hobbs JR. Overestimation of monoclonal immunoglobulin by immunochemical methods. Ann Clin Biochem. 1991;28:253–259. doi: 10.1177/000456329102800310. [DOI] [PubMed] [Google Scholar]
- Bradwell AR, Harding SJ, Fourrier NJ, Wallis GL, Drayson MT, Carr-Smith HD, et al. Assessment of monoclonal gammopathies by nephelometric measurement of individual immunoglobulin kappa/lambda ratios. Clin Chem. 2009;55:1646–1655. doi: 10.1373/clinchem.2009.123828. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment in multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharam A, Mirbahai L, Lovatt T, Macwhannell A, Jacob A, Handa S, et al. Heavy/light chain ratios provide a rapid and sensitive alternative to IFE in identifying haematological malignancies. 13th International Myeloma Workshop, Paris 2011 Haematologica 201196P80 [Google Scholar]
- Avet-Loiseau H, Young P, Mathiot C, Attal M, Moreau P, Harousseau J, et al. Multiple myeloma outcome is associated with polyclonal immunoglobulin suppression of the same isotype as the tumour. Haematologica. 2011;96:382. [Google Scholar]
- Wechalekar A, Faint J, Bradwell S, Lachmann H, Hawkings P, Harding S, et al. Significance of abnormal serum immunoglobulin heavy/light chain ratios (Hevylite) in 294 patients with systemic AL amyloidosis. Haematologica. 2011;96:392. [Google Scholar]
- Leleu X, Koulieris E, Maltezas D, Itzykson R, Xie W, Manier S, et al. Novel M-component based biomarkers in Waldenström's macroglobulinemia. Clin Lymphoma Myeloma Leuk. 2011;11:164–167. doi: 10.3816/CLML.2011.n.039. [DOI] [PubMed] [Google Scholar]
- Katzmann JA, Clark R, Dispenzieri A, Kyle R, Landgren O, Bradwell A, et al. Isotype-specific heavy/light chain (HLC) suppression as a predictor of myeloma development in monoclonal gammopathy of undetermined significance (MGUS) Blood (ASH Annual Meeting Abstracts) 2009;114:1788. [Google Scholar]
- Donato LJ, Zeldenrust SR, Murray DL, Katzmann JA. A-71-year-old woman with multiple myeloma status after stem cell transplantation. Clin Chem. 2011;57:1645–1648. doi: 10.1373/clinchem.2011.163766. [DOI] [PubMed] [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International Myeloma Working Group. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- Bladé J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- Kaur A, Snelus T, Mitchell F, Showell PJ, Drayson MT, Bradwell AR, et al. Turbidimetric immunoassays for IgAκ and IgAλ quantification for the assessment of patients with multiple myeloma Clin Chem 201056B 170a. [Google Scholar]
- Alvi AJ, Fourrier NJ, Shemar M, Bradwell AR, Harding SJ.Turbidimetric immunoassays for IgGκ and IgGλ quantification for the assessment of patients with IgG multiple myeloma Clin Chem 201157A 26a. [Google Scholar]
- Reed JF, Stark DB. Robust alternatives to traditional analysis of variance: Welch W, James J I, James J II, Brown-Forsythe BF. Comput Methods Programs Biomed. 1988;26:233–237. doi: 10.1016/0169-2607(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–481. [Google Scholar]
- Cox D. Regression models and life tables. J Royal Stat Soc. 1972;34:187–220. [Google Scholar]
- Wang H, Gao C, Xu L, Yang Z, Hao W, Kong X. Laboratory characterizations on 2007 cases of monoclonal gammopathies in East China. Cell Mol Immunol. 2008;5:293–298. doi: 10.1038/cmi.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avet-Loiseau H, Mirbahai L, Harousseau JL, Moreau P, Mathiot C, Facon T, et al. Serum immunoglobulin heavy/light chain ratios are independent risk factors for predicting progression free survival in multiple myeloma. Haematologica. 2010;95 (395:0953. [Google Scholar]
- Chandy KG, Stockley RA, Leonard RC, Crockson RA, Burnett D, MacLennan IC. Relationship between serum viscosity and intravascular lgA polymer concentrations in lgA myeloma. Clin Exp Immunol. 1981;46:653–661. [PMC free article] [PubMed] [Google Scholar]
- Waldmann TA, Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- Ladetto M, Pagliano G, Ferrero S, Cavallo F, Drandi D, Santo L, et al. Major tumor shrinking and persistent molecular remissions after consolidation with bortezomib, thalidomide, and dexamethasone in patients with autografted myeloma. J Clin Oncol. 2010;28:2077–2084. doi: 10.1200/JCO.2009.23.7172. [DOI] [PubMed] [Google Scholar]
- Paiva B, Martinez-Lopez J, Vidriales MB, Mateos MV, Montalban MA, Fernandez-Redondo E, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol. 2011;29:1627–1633. doi: 10.1200/JCO.2010.33.1967. [DOI] [PubMed] [Google Scholar]
- Hari P, Pasquini MC, Logan BR, Stadmauer EA, Krishnan A, Howard A, et al. Immunglobulin free light chain (FLC) and heavy chain/light chain (HLC) assays – comparison with electrophoretic responses in multiple myeloma (MM) Blood. 2011;118:2877. [Google Scholar]
- Fernández de Larrea C, Tovar N, Cibeira MT, Aróstegui JI, Rosiñol L, Elena M, et al. Emergence of oligoclonal bands in patients with multiple myeloma in complete remission after induction chemotherapy: association with the use of novel agents. Heamatologica. 2011;96:171–173. doi: 10.3324/haematol.2010.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson PJ, Loken MR, Panem S, Schreiber H. Clonal evolution of myeloma cells leads to quantitative changes in immunoglobulin secretion and surface antigen expression. Proc Natl Acad Sci USA. 1979;76:2937–2941. doi: 10.1073/pnas.76.6.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snozek CL, Katzmann JA, Kyle RA, Dispenzieri A, Larson DR, Therneau TM, et al. Prognostic value of the serum free light chain ratio in newly diagnosed myeloma: proposed incorporation into the international staging system. Leukemia. 2008;22:1933–1937. doi: 10.1038/leu.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrtsonis MC, Vassilakopoulos TP, Kafasi N, Sachanas S, Tzenou T, Papadogiannis A, et al. Prognostic value of serum free light chain ratio at diagnosis in multiple myeloma. Br J Heamatol. 2007;137:240–243. doi: 10.1111/j.1365-2141.2007.06561.x. [DOI] [PubMed] [Google Scholar]
- van Rhee F, Bolejack V, Hollmig K, Pineda-Roman M, Anaissie E, Epstein J, et al. High serum-free light chain levels and their rapid reduction in response to therapy define an aggressive multiple myeloma subtype with poor prognosis. Blood. 2007;110:827–832. doi: 10.1182/blood-2007-01-067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dispenzieri A, Zhang L, Katzmann JA, Snyder M, Blood E, Degoey, et al. Appraisal of immunoglobulin free light chain as a marker of response. Blood. 2008;111:4908–4915. doi: 10.1182/blood-2008-02-138602. [DOI] [PMC free article] [PubMed] [Google Scholar]