Abstract
Background
Milk thistle (MT) is often used for the treatment of chemotherapy associated hepatotoxicity despite limited preclinical and clinical investigations. Limited treatment options exist for chemotherapy-related hepatoxicity. Given the wide use of MT, we investigated MT in both the laboratory and clinical setting.
Methods
In a double-blind study, children with ALL with hepatic toxicity were randomized to MT or placebo orally for 28 days. Liver function tests were evaluated during the study period. To assess MT in vitro, we evaluated supratherapeutic concentrations in an ALL cell line.
Results
50 children were enrolled. No significant differences in the frequency of side effects, incidence and severity of toxicities, or infections were observed between groups. There were no significant changes in mean AST, ALT, or TB at day 28. At day 56, the MT group had a significantly lower AST (p=0.05) and a trend towards a significantly lower ALT (p=0.07). Although not significantly different, chemotherapy doses were reduced in 61% of the MT group, compared to 72% of the placebo group. In vitro experiments revealed no antagonistic interactions between MT and vincristine or L-asparaginase in CCRF-CEM cells. A modest synergistic effect with vincristine was observed.
Conclusions
In children with ALL with liver toxicity, MT was associated with a trend towards significant reductions in liver toxicity. MT does not antagonize the effects of chemotherapy agents used for the treatment of ALL. Future study is needed to determine the most effective dose and duration of MT and its effect on hepatotoxicity and leukemia-free survival.
Keywords: Acute Lymphoblastic Leukemia, Hepatotoxicity, Milk Thistle, Complementary Medicine, Childhood Cancer
Background
Over the past two decades, there has been an increased interest towards understanding the mechanisms and clinical applications of the herbal plant, milk thistle (Silybum marianum).(1–3) Prior studies have found milk thistle (MT) has both hepato- and nephro-protectant activity suggesting its application as a supportive care agent.(4) MT is available in the United States as a dietary supplement and most often is used for its effects on the liver. Clinical studies have investigated MT for the prevention or treatment of liver damage in patients with hepatitis and cirrhosis.(3;5) A case report suggests a beneficial role for the treatment of chemotherapy-induced hepatotoxicity. (6)
In the treatment of children with acute lymphoblastic leukemia (ALL), the administration of chemotherapy agents is frequently interrupted for liver toxicity, especially during the maintenance phase of treatment. Schmiegelow, et al found that children with ALL, who experience a cumulative withdrawal of methotrexate or 6-mercaptopurine of greater than 10% of the prescribed therapy, have an increased risk of bone marrow relapse (Methotrexate: Complete Hematologic Remission (CHR) 45%±12% versus 78%±5%, p=0.009; 6-mercaptopurine:CHR 31%±12% versus 77%±5%p<0.00001.(7) Hepatotoxicity was the main reason for cumulative long-term drug withdrawals. More recent investigations have found that 66.5% of children with ALL encountered Grade 2 or higher liver toxicity at some point during therapy.(8)
Currently, there are no substitute chemotherapy agents that provide the same effectiveness against ALL, yet preserve liver function. There are also no hepatoprotective medications that allow chemotherapy to continue to be administered while preserving liver function. Thus, adjunctive agents that may enable optimal doses of chemotherapy to be administered without necessitating a decrease in the recommended doses of chemotherapy are of clinical significance and may further improve survival in children with ALL. We present the results from a multicenter pilot study that evaluated the safety and feasibility of MT for the treatment of hepatotoxicity in children with ALL receiving maintenance phase chemotherapy.
Methods
In vitro antileukemic cytotoxicity assay
MT (Siliphos®) is a 1:2 mixture of silibinin, the most active fraction of milk thistle, to soy phosphatidylcholine, a formulation reported to improve the bioavailability of milk thistle.(9) Silibinin is a mixture of two diastereoisomeric compounds, silibin A and B. Silibinin was investigated for potential antagonism of the in vitro cytotoxic effects of selected agents used in ALL treatment regimens. CCRF-CEM T-cell ALL cells were concurrently exposed to a dose-range of either vincristine or L-asparaginase in the presence of a constant concentration of 0, 10, or 30 µM silibinin for 72 hours. These silibinin concentrations were chosen to represent at least a 10-fold higher concentration than the anticipated Cmax in the clinical trial. The described in vitro experiments used previously described methodology.(10) Mean percent survival (+ standard error of the mean) for each treatment group was calculated relative to the mean absorbance of vehicle-treated controls.
Clinical Trial
Eligibility
This study was approved by the Institutional Review Boards of all participating institutions. Children with ALL between the ages of 1 and 21, treated per Children’s Cancer Group or Children’s Oncology Group(11;12), or Dana Farber Cancer Institute ALL Consortium protocols, and were in the maintenance phase of therapy (Table I), were eligible.
Table 1.
Demographics by Randomized Group
| Variable | Milk Thistle (n=24) |
Placebo (n=26) |
|---|---|---|
| Age, y | ||
| Mean | 8.7 ± 5.1 | 7 ± 3.2 |
| Median | 7.8 | 6.2 |
| Range | 1.7–18.9 | 2–14.3 |
| Gender | ||
| Male | 14 | 15 |
| Female | 10 | 11 |
| Race | ||
| Caucasian | 15 | 19 |
| Black, Not Hispanic | 1 | 0 |
| Hispanic | 6 | 4 |
| Other | 2 | 3 |
| Risk Group | ||
| Standard risk | 15 | 17 |
| High risk | 9 | 9 |
| Eligibility (elevated) | ||
| AST | 0 | 0 |
| ALT | 19 | 19 |
| AST and ALT | 3 | 4 |
| TB | 1 | 1 |
| AST, ALT, TB | 1 | 2 |
ALT indicates amino alaine transferase; AST, aspartate amino transferase; TB, total bilirubin.
During the maintenance phase of therapy, patients are routinely evaluated for liver toxicity at the beginning of each cycle of chemotherapy. Children were eligible for participation if they had hepatic toxicity of grade 2 or greater (National Cancer Institute, Common Toxicity Criteria (Version 2.0)),(13) on any one of the three tests, amino alanine transferase (ALT), aspartate amino transferase (AST), or total bilirubin (TB). Patients with extrahepatic biliary obstruction, severe hepatic/kidney failure, gastrointestinal obstruction, or malabsorption syndromes were excluded from participation.
Study Design
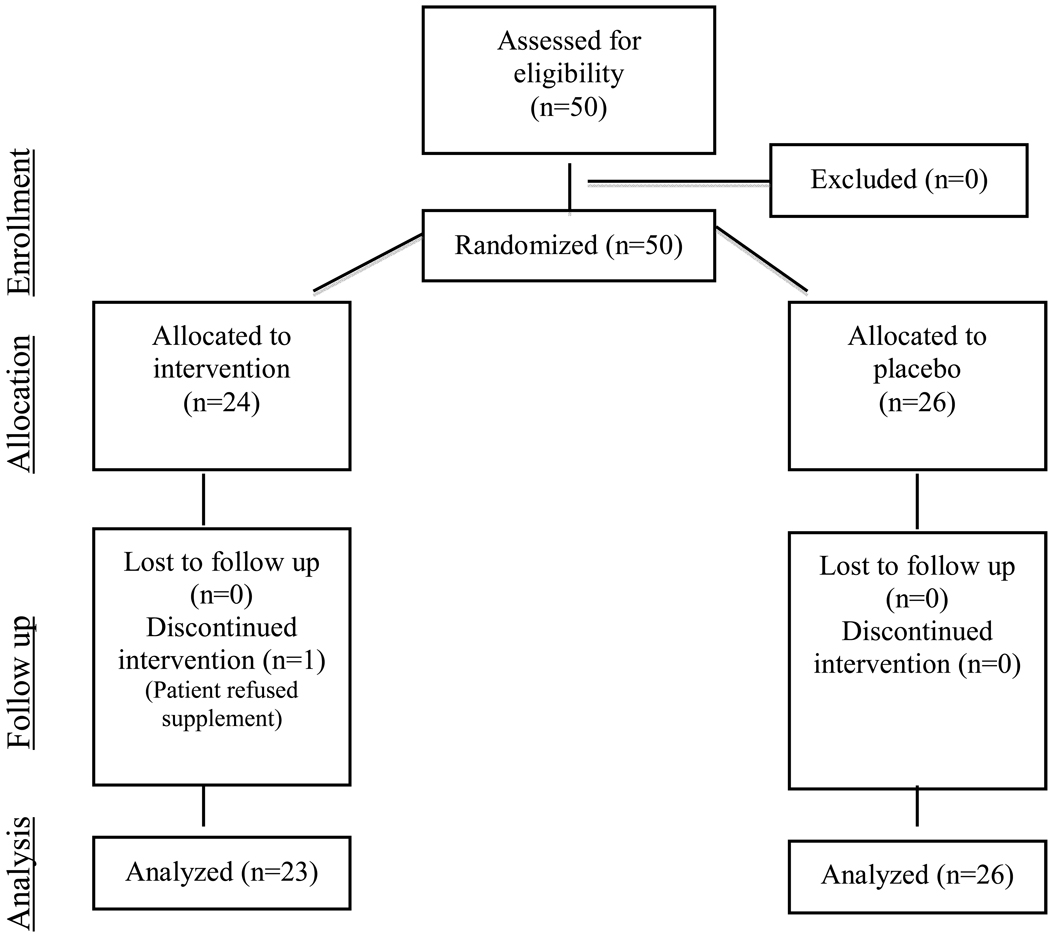
The study was a randomized, double-blind, placebo-controlled trial (Figure I). Upon informed consent and assent, subjects were randomized to receive the study drug, milk thistle, or placebo, by mouth daily for 28 days. Supplementation began the day following administration of intravenous chemotherapy. Hepatic toxicity was measured at Day 0, Day 28, and Day 56. To monitor adherence, subjects were contacted weekly by telephone interviews and were asked to returned medication containers. Adherence was defined as completed at least 80% of the assigned drug/placebo.
Figure I.
Study Design
Safety Monitoring
The safety of MT was monitored by patient visits, chart reviews, and patient reports. We monitored for published side-effects associated with administration of MT. (14) A safety monitoring rule was incorporated into the statistical analysis to screen for rates of Grade 3 and 4 toxicities. We also monitored for any unexpected side-effects, related to milk thistle, through weekly telephone contact by our research assistant using a standardized questionnaire that was administered to the subject or primary guardian during the intervention period.
Milk Thistle
Each MT capsule contained 240 mg of milk thistle, standardized to 80 mg of silibinin (Silibin A and B). The target dose of silibinin was 5.1mg/kg/day. Given the capsule sizes and wide range in body weight of study participants, the following dose ranges were prescribed: 15–20 kg = 80 mg/day; 21–40 kg= 160 mg/day; 41–60 kg= 240 mg/day; 61–70kg = 320mg/day.
Each subject was instructed to take the capsule or its contents by mouth. Each subject was instructed to take the capsule or its contents by mouth. Patients who opened capsules were instructed to take the contents either mixed with liquid or food, using a spoon for administration. The placebo was identical in appearance and odor to the active agent. The placebo was identical in appearance and odor to the active agent. The study agent and placebo were stored and administered by the research pharmacy. Only the research pharmacist had knowledge of the randomization assignment.
Purity and Stability Analysis
MT (Siliphos®) and placebo were donated by Thorne Research, Inc. (Sandpoint, ID). A certificate of analysis accompanied each shipment of MT and placebo. To ensure purity, content, and stability, MT and placebo capsules were analyzed independently at the Natural Products Laboratory at the Research Triangle Institute (Research Triangle Park, NC), using previously described methodology.(10;15) MT was assessed at the initiation, and at midpoint of the study. A slight but consistent overfill of each MT capsule was observed (281.6 ± 3.7 mg), with each MT capsule containing a total of 97.0 mg (± 5.5 mg), silibinin presented as 42.4 mg (± 2.2 mg) silybin A and 54.6 mg (± 3.2 mg) silybin B. Stability of >98% was observed at 21 months.
Plasma Silibinin
Plasma silibinin levels were evaluated in sub-set of patients who had reported taking the final dose of MT or placebo, within two hours prior to their routine blood draw. Plasma silibinin levels were evaluated at each time point using previous described methodology.(16) The limit of the detection for each spiked sample was 15 ng/mL (0.031 µM) with an average recovery of 52%. The maximum sensitivity of the assay was 0.06 µM for each compound or approximately 0.12 µM for the silibinin mixture.
Statistical methods
Demographic information and eligibility criteria were summarized using descriptive statistics for both the MT and the placebo group. The two groups were compared on these variables using either the two-sample t-test or the chi-square test (or Fisher’s exact test for sparse data).
The main analysis compared groups on liver toxicity, as measured by AST and ALT. A two-sample t-test was used to compare mean AST and ALT levels by group at each time point and to compare the groups on mean change in AST or ALT from baseline to Day 28 and baseline to Day 56. We analyzed the changes in TB by comparing the percentages of patients with greater than 50% reduction in TB in the two groups using chi-square test. The analyses were conducted using SAS version 9.1. A p-value of 0.05 or less is considered as statistically significant.
Sample size justification
The primary analysis endpoint was evidence of hepatic toxicity, measured by the liver function tests, ALT, AST, TB at Day 28 and Day 56. Data from previous studies suggest that liver enzyme measurements are more nearly normally distributed on the logarithmic scale. To detect a mean difference from pre- to post-treatment liver enzymes of 50%, or 0.7 units on the log scale assuming log-scale SD to be 0.7, a two-sample t test has 90% power when there are at least 23 subjects per arm. To account for possible data losses, we enrolled 25 subjects per arm.
Results
In vitro
Silibinin did not antagonize the activity of vincristine or L-asparaginase in vitro using the CCRF CEM cell line as no significant reduction in cytotoxicity was observed. We observed a degree of antileukemic synergy between silibinin and vincristine. Fixed concentration ratio experiments and cell survival data revealed a modest degree of synergism was observed between vincristine and silibinin with Chou-Talalay (17) combination indices ranging from 0.38 to 0.62 (p<0.05) over a 20-fold concentration range of the two agents (<1.0, synergy; 1.0, simple additivity, >1.0, antagonism). No such effect was observed with L-asparaginase and silibinin. Silibinin did not anatagonize vincristine or L-asparaginase-mediated cell kill in T-cell ALL cell culture studies.
Clinical Study
Subjects
Informed consent was obtained for the 50 children enrolled from May 2002 to August 2005, with 26 subjects randomized to the placebo arm and 24 to the MT arm. Of the 50 children, 49 were evaluable. One parent withdrew her child from participation due to the child refusing to take any dose of MT. This patient was excluded from the study analysis.
The baseline characteristics by group assignment are described in Table II. The mean ages were 8.7 and 7.0 years for the MT and placebo group, respectively. 58% were males and more subjects were in the standard risk group (64%) compared to the high risk group. No significant differences for any demographic variables were observed.
Table II.
Demographics by randomized group
| Variable | Milk Thistle | Placebo |
|---|---|---|
| (N=24) | (N=28) | |
| Age | ||
| Mean age (years) | 8.7 ± 5.1 | 7 ± 3.2 |
| Median (years) | 7.8 | 6.2 |
| Range | 1.7–18.9 | 2–14.3 |
| Gender | ||
| Male | 14 | 15 |
| Female | 10 | 11 |
| Race | ||
| Caucasian | 15 | 19 |
| Black, Not Hispanic | 1 | 0 |
| Hispanic | 6 | 4 |
| Other | 2 | 3 |
| Risk Group | ||
| Standard Risk | 15 | 17 |
| High Risk | 9 | 9 |
| Eligibility (elevated) | ||
| AST* | 0 | 0 |
| ALT* | 19 | 19 |
| AST and ALT | 3 | 4 |
| TB* | 1 | 1 |
| AST, ALT, TB | 1 | 2 |
Abbreviations: Amino alanine transferase (ALT), Aspartate amino transferase (AST), Total bilirubin (TB).
Eligibility and Dosing
Most subjects (76%) were enrolled due to elevations in ALT (19 per group). 83% of subjects in the MT group and 96% of subjects in the placebo experienced a grade 2 toxicity; 17% experienced a Grade 3 toxicity in the MT group and 4% in the placebo group. No subjects were eligible for participation due to a Grade 4 toxicity. Four subjects in the MT and 10 subjects in the placebo group received one capsule (80 mg) per day, 10 patients in the MT and 13 patients in the placebo received 2 capsules (160 mg) per day; 5 in MT and 3 in placebo received three capsules (240 mg) per day, and 4 in the MT and 2 in the placebo received four capsules (320 mg) per day.
Silibinin plasma levels
We analyzed plasma silibinin levels in 18 subjects at each timepoint (9 subjects in each group). Plasma samples were processed for quantification of the silibinin components, silybin A and silybin B. While our limit of detection was at least 10-fold below the concentration in the published literature(16), detectable levels of silibinin compounds were not observed in any of the plasma samples.
Evaluation of Liver Toxicity
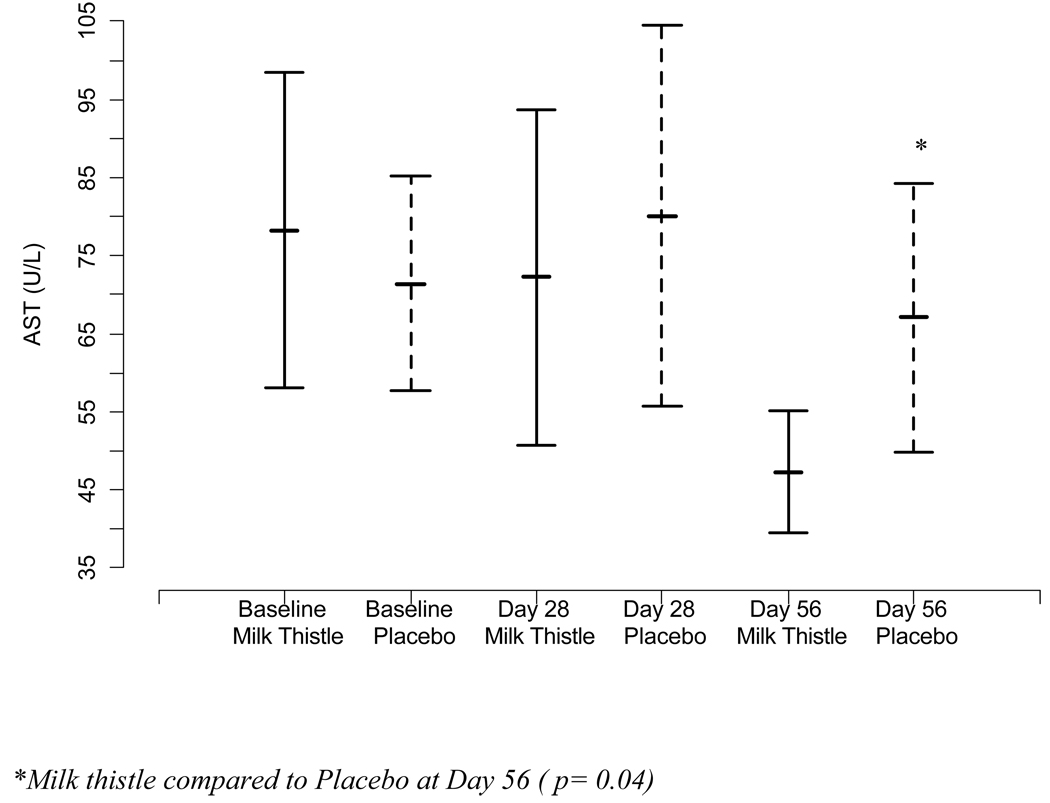
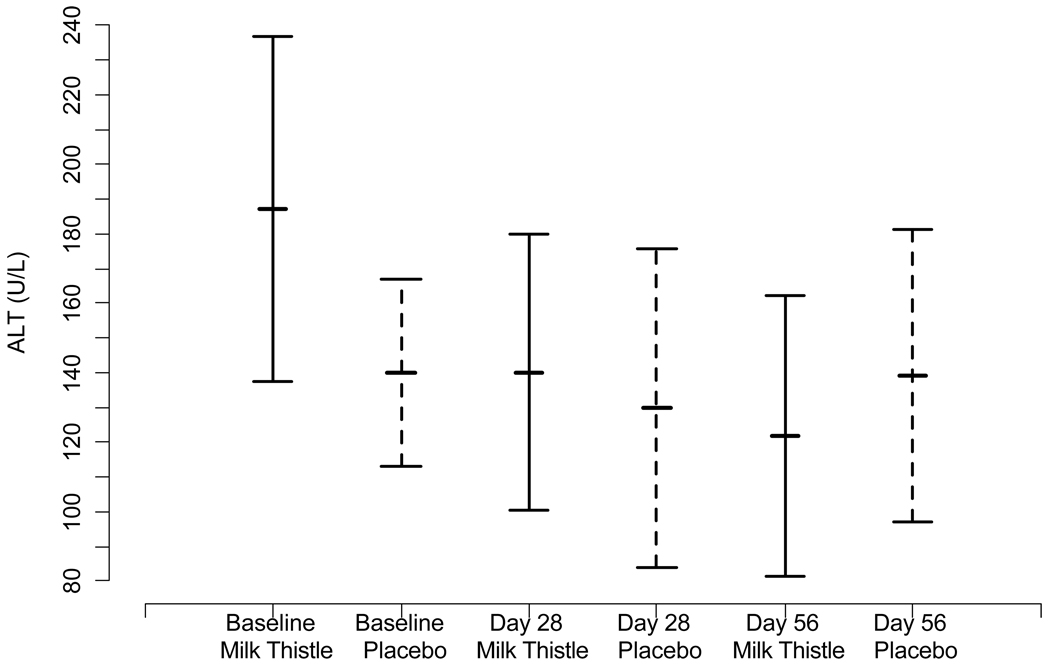
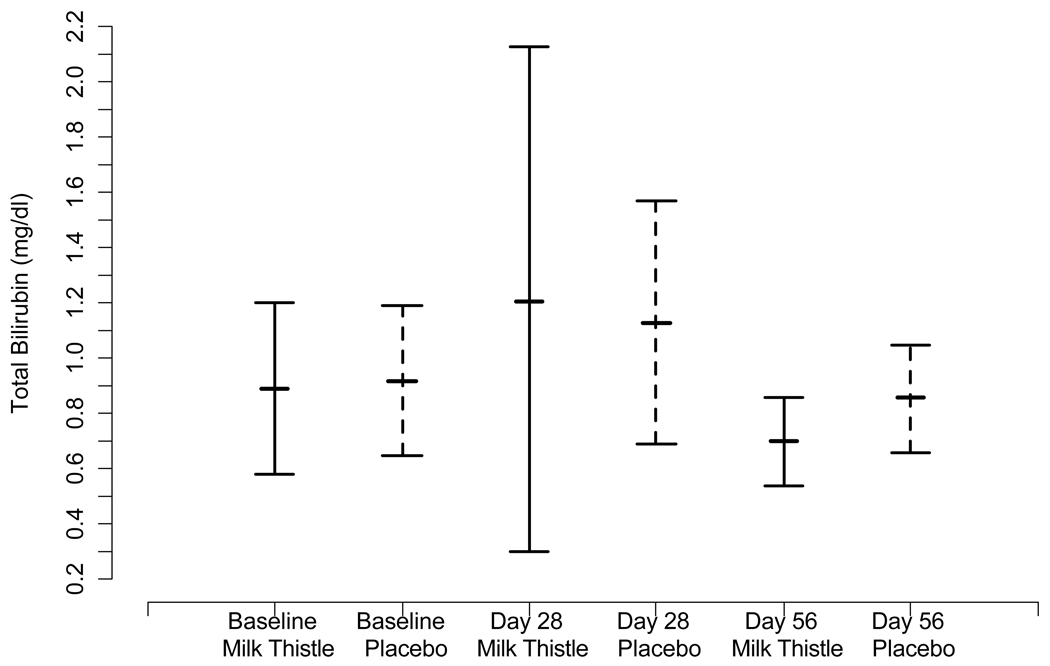
We investigated changes in mean transaminase and bilirubin levels in the MT and placebo group over the course of the study period. The mean levels of AST, ALT, and TB at day 0, day 28, and day 56 for the treatment and placebo groups are presented in Figure IIa–c. Mean baseline values did not differ significantly between the groups for AST, ALT, or TB at baseline or day 28. At day 56, the MT group had lower AST than the placebo group (p= 0.04).
Figure II.
Figure II(a). Mean changes in AST and 95% confidence intervals at each time point by randomized group
Figure II(b). Mean changes in ALT and 95% confidence intervals at each time point by randomized group
Figure II(c). Mean changes in TB and 95% confidence intervals at each time point by randomized group
We evaluated the mean reductions in intra-individual AST, and ALT over time using difference scores from baseline to day 28 and baseline to day 56. No significant differences between MT and placebo in AST or ALT from baseline to day 28 (p= 0.55; p= 0.50) were observed. At day 56, the MT group had a significantly lower AST (p=0.05) and a trend towards a significantly lower ALT (p=0.07) from baseline than the placebo group.
We did not observe a significant difference in mean TB levels at each of the time points. However, at Day 28, 5 patients in the MT group and no patients in the placebo group had greater than a 50% reduction in total bilirubin during the intervention period (p < 0.007).
Administration of Chemotherapy
We investigated the effects of MT on median doses and reductions in doses of chemotherapy, and delays in administration of chemotherapy. The median dose (range) of each chemotherapy agent that was potentially modifiable for hepatoxicity and administered during the intervention period in the MT/placebo groups were: Methotrexate: MT 25 mg/m2/week (10–75mg/m2/week); Placebo 20 mg/m2/week (10–40mg/m2/week)); 6-mercaptopurine: MT 500 mg/m2/week (75–1300 mg/m2/week); Placebo 413 mg/m2/week (235–900 mg/m2/week) and Vincristine MT 1.7 mg/dose (0.6–2 mg/dose) and Placebo 1.4 mg/dose (0.8–2 mg/dose). Four out of 23 and 3 out of 26 patients in the MT and placebo groups, respectively experienced a delay in therapy. Fourteen (61%) of patients in the MT and 19 (73%) of patients in the placebo received a reduction in dose during the four-week intervention period. No significant differences in the doses of chemotherapy administered, reductions of chemotherapy doses, or delays in treatment between the two groups were observed.
Toxicities and Adherence
No significant differences in chemotherapy-related grade 3 or 4 toxicities were observed between the two groups (Table III). Hematologic and infectious toxicities were observed in 6 patients in the MT group and 17 patients in the placebo group. Non-hematologic toxicities were observed in 10 patients in MT and 6 patients in the placebo groups. No significant differences in the number or severity of toxicities, or rates of infection were found.
Table III.
Number of Patients by Group with Grade 3/4 toxicities and Infections
| Hematologic | Neurological | Hepatic | Gastrointestinal | Infection | |
|---|---|---|---|---|---|
| Day 0 to 28 | |||||
| Milk Thistle | 3 | 0 | 5 | 0 | 2 |
| Placebo | 4 | 1 | 3 | 0 | 0* |
| Day 28 to 56 | |||||
| Milk Thistle | 2 | 0 | 8 | 1 | 5 |
| Placebo | 6 | 0 | 5 | 0 | 8† |
Infections during Day 0 to Day 28 were pneumonia, URI.
Infections during Day 28 to Day 56 were PCP-Pneumocystis juroveci pneumonia, Rotovirus, bacteremia, URI-upper respiratory infection, Sinusitis, mucositis,
The patient reported side effects in the MT group were diarrhea (2), flatulence (1), irritability (2), and stomach ache (2). In the placebo group, patient reported side effects were decreased appetite (1), diarrhea (2), stomach ache (2), and soft stools (1). The patient reported side effects were mild and were pre-existing complaints prior to the initiation of treatment with milk thistle. No significant differences in patient reported side effects were found between the two groups.
Adherence to the protocol was 68% for the MT group and 96% for the placebo group (p =0.02). We observed a significant difference in age between patients that adhered to the study protocol (mean age 6.9 ± 3) in comparison to patients with poor adherence (13.1 ± 5.4) (p = 0.01).
Discussion
This is the first randomized, controlled, clinical study to investigate the feasibility and safety of the herbal plant, milk thistle, in combination with the administration of chemotherapy in children undergoing treatment for cancer. We found that a short course of MT can be administered to children in the maintenance phase of treatment for ALL. No unexpected toxicities, reductions in doses of chemotherapy, or delays in therapy were observed during the supplementation period, despite the intervention group receiving slightly higher doses of vincristine and 6-mercaptopurine, and experiencing a lower percentage of chemotherapy dose reductions. Our preclinical data demonstrates that MT does not compromise the anticancer activity of L-asparaginase or vincristine in CCRF-CEM cell lines.
The administration of a 28-day course of MT was associated with a significant reduction in AST and a trend towards a significant reduction in ALT at Day 56, but not immediately after cessation of supplementation. The effect observed on AST and ALT could be due to delayed effects of milk thistle, inadequate dosing, or short duration of supplementation. Evaluation of the clinical literature has reported a wide range of therapeutic doses and duration.(5) At the time of development of this clinical trials for supportive care, Phase 1 studies were not routinely developed for investigation of herbal or nutrition supplements.(18;19) Therefore, we chose a short course of treatment and a conservative dose as this was the first trial conducted among children undergoing treatment for cancer. A recent phase 1 study in men with prostate cancer suggests a dose of 13 grams per day for future trials, thus our dose may have been too conservative.(20) Phase 1 trials are needed in our patient population to determine appropriate dose and duration for both the prevention and treatment of hepatic toxicity.
Our study was strengthened by the product quality analysis, stability testing, and the goal of quantifying plasma levels of silibinin. Although detectable silibinin plasma concentrations were not observed at the dose prescribed, several hypotheses could account for this. Our limit of detection was 15 ng/mL (0.03 µM) for each compound with a mean recovery of 52%. This corresponds to 0.06 µM for each silybin A or silybin B, or 0.12 µM for the silibinin mixture. Previous studies in adults administered a similar dose found total silibinin plasma concentrations were quite variable and near undetectable (0.3 µM ± 0.3 µM or 144 ± 144 ng/mL).(16) Since silibinin analysis is comprised of two compounds (silybin A and B) that equates to roughly 72 ng/mL each, the detectable concentrations of each compound was approximately 36 ng/mL or within two-fold of our limits of detection. Moreover, Hoh et al collected blood samples within 1–4 hours of the final dose.(16) In the current study, we relied primarily on patient reporting on the timing of the last dose and the time to blood draw was outside of the 4 hour range in several cases. When operating near the limits of detection, the timing of blood draws may be particularly crucial and should be closely controlled in future studies. Finally, no information is available on the metabolism of silibinin in pediatric patients compared to adults. Children may metabolize silibinin isomers at rates different from that of adults. Taken together, this combination of confounding variables contributes to the lack of silybin A or silybin B detection in patient plasma samples.
Our study was weakened by a small sample size. Based upon previously published studies, the study was powered to detect a minimum reduction in liver function tests of 50%. However, analysis of the current data (data not shown), found that the reduction was only 30%, which was achieved by AST at Day 56. Therefore, our current study was under powered to detect a significant treatment effect at Day 28. We also found that the MT group had a significantly lower compliance rate compared to the placebo group. We hypothesize that the treatment effect would be more pronounced had the compliance rate been improved in the MT group. Furthermore, assessment of liver toxicity by ALT and particularly AST are limited by their lack of specificity for chemotherapy-induced hepatocellular injury.
Despite the study limitations, the current study provides preliminary evidence that MT may be a safe, effective supportive care agent. Future investigations are needed to determine the appropriate dose and duration, and identify populations that may gain the largest clinical benefit. Possible populations are those undergoing treatment for acute myelogenous leukemia or stem cell transplantation in which hepatotoxicty frequently results in interruptions of treatment. Hepatoprotectant agents could also advance the management of patients with total parenteral nutrition -induced hepatoxicity.
In conclusion, this was the first study to evaluate milk thistle, a commonly used dietary supplement, in a blinded, controlled, trial among children undergoing treatment for ALL with biochemical evidence of elevated liver function tests. Future clinical trials should explore MT in the setting of patients in which hepatic toxicity prevents the provision of the recommended chemotherapy in individuals with cancer.
Acknowledgements
The authors wish to acknowledge the excellent technical assistance of Dr. Yuka Nakanishi for the in vitro cytotoxicity experiments, and Tyler N. Graf for methods development and determination of silibinin stability and plasma analysis . We would also like to thank the following institutions for patients accrual and data collection: Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania: Susan Rheingold, MD and Nancy Sacks, MS, RD; Winthrop University Hospital, Mineola, New York: Mark Weinblatt, MD; Children’s Hospital Medical Center of Akron, Akron, Ohio: Sarah Friebert, MD; Mount Sinai School of Medicine, New York, New York: William Carroll, MD; McMaster Children’s Hospital, Hamilton, Ontario, Canada: Ronald Barr, MD; Children's Hospital and Regional Medical Center, Seattle, Washington: Debra Friedman, MD.
Financial Disclosers: This study was supported by American Institute for Cancer Research #01A047, The Tamarind Foundation, and in part by NCI grant R01 CA104286.
Footnotes
Informed Consent: Informed consent was obtained for each patient recruited to the clinical trial
Reference List
- 1.Greenlee H, Abascal K, Yarnell E, et al. Clinical applications of Silybum marianum in oncology. Integr.Cancer Ther. 2007;6:158–165. doi: 10.1177/1534735407301727. [DOI] [PubMed] [Google Scholar]
- 2.Sagar SM. Future directions for research on Silybum marianum for cancer patients. Integr.Cancer Ther. 2007;6:166–173. doi: 10.1177/1534735407301566. [DOI] [PubMed] [Google Scholar]
- 3.Tamayo C, Diamond S. Review of clinical trials evaluating safety and efficacy of milk thistle (Silybum marianum [L.] Gaertn.) Integr.Cancer Ther. 2007;6:146–157. doi: 10.1177/1534735407301942. [DOI] [PubMed] [Google Scholar]
- 4.Comelli MC, Mengs U, Schneider C, et al. Toward the definition of the mechanism of action of silymarin: activities related to cellular protection from toxic damage induced by chemotherapy. Integr.Cancer Ther. 2007;6:120–129. doi: 10.1177/1534735407302349. [DOI] [PubMed] [Google Scholar]
- 5.Rambaldi A, Jacobs BP, Gluud C. Milk thistle for alcoholic and/or hepatitis B or C virus liver diseases. Cochrane.Database.Syst.Rev. 2007 doi: 10.1002/14651858.CD003620.pub3. CD003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Invernizzi R, Bernuzzi S, Ciani D, et al. Silymarine during maintenance therapy of acute promyelocytic leukemia. Haematologica. 1993;78:340–341. [PubMed] [Google Scholar]
- 7.Schmiegelow K. Prognostic significance of methotrexate and 6-mercaptopurine dosage during maintenance chemotherapy for childhood acute lymphoblastic leukemia. Pediatr.Hematol.Oncol. 1991;8:301–312. doi: 10.3109/08880019109028803. [DOI] [PubMed] [Google Scholar]
- 8.Farrow AC, Buchanan GR, Zwiener RJ, et al. Serum aminotransferase elevation during and following treatment of childhood acute lymphoblastic leukemia. J.Clin.Oncol. 1997;15:1560–1566. doi: 10.1200/JCO.1997.15.4.1560. [DOI] [PubMed] [Google Scholar]
- 9.Barzaghi N, Crema F, Gatti G, et al. Pharmacokinetic studies on IdB 1016, a silybin- phosphatidylcholine complex, in healthy human subjects. Eur.J.Drug Metab Pharmacokinet. 1990;15:333–338. doi: 10.1007/BF03190223. [DOI] [PubMed] [Google Scholar]
- 10.Davis-Searles PR, Nakanishi Y, Kim NC, et al. Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005;65:4448–4457. doi: 10.1158/0008-5472.CAN-04-4662. [DOI] [PubMed] [Google Scholar]
- 11.Matloub Y, Lindemulder S, Gaynon PS, et al. Intrathecal triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared with intrathecal methotrexate: results of the Children's Cancer Group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia, reported by the Children's Oncology Group. Blood. 2006;108:1165–1173. doi: 10.1182/blood-2005-12-011809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2008;111:2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute Common Toxicity Criteria. National Cancer Institute; 2009. [Google Scholar]
- 14.Ladas EJ, Kroll D, Kelly K, Milk Thistle. In: Encyclopedia of Dietary Supplements. 1st ed. Coates P, Levine M, Blackman M, Moss J, Cragg G, White J, editors. New York City: Marcel Dekker, Inc.; 2004. pp. 467–482. [Google Scholar]
- 15.Kim NC, Graf TN, Sparacino CM, et al. Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum) Org.Biomol.Chem. 2003;1:1684–1689. doi: 10.1039/b300099k. [DOI] [PubMed] [Google Scholar]
- 16.Hoh C, Boocock D, Marczylo T, et al. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin.Cancer Res. 2006;12:2944–2950. doi: 10.1158/1078-0432.CCR-05-2724. [DOI] [PubMed] [Google Scholar]
- 17.Chou T-C, Talaly P. Analysis of combined drug effects: a new look at a very old problem. Trends in Pharmacological Sciences. 1983;4:450–454. [Google Scholar]
- 18.Straus SE, Chesney MA. Science and government. Enhanced: in defense of NCCAM. Science. 2006;313:303–304. doi: 10.1126/science.1131608. [DOI] [PubMed] [Google Scholar]
- 19.Parkman CA. The NCCAM and new research priorities. Case.Manager. 2004;15:28–32. doi: 10.1016/j.casemgr.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Flaig TW, Gustafson DL, Su LJ, et al. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 21.Malempati S, Gaynon PS, Sather H, et al. Outcome after relapse among children with standard-risk acute lymphoblastic leukemia: Children's Oncology Group study CCG-1952. J Clin Oncol. 2007;25:5800–5807. doi: 10.1200/JCO.2007.10.7508. %20. [DOI] [PubMed] [Google Scholar]
- 22.Panosyan EH, Seibel NL, Martin-Aragon S, et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children's Cancer Group Study CCG-1961. J Pediatr Hematol.Oncol. 2004;26:217–226. doi: 10.1097/00043426-200404000-00002. [DOI] [PubMed] [Google Scholar]